MAVIS Hub Edition 114
Published 5 May 2020
1. Product news
Prednidale 5 mg Tablets - Product defect recall alert
Availability of Lactating Cow Intramammary Antibiotics
Coronavirus (COVID-19) outbreak - availability of veterinary medicines
Known supply problems with animal medicines
Foston 20% w/v Solution for Injection - Product defect recall alert
2. Enforcement
Animal medicine improvement notice: Companion Care Orpington
Animal medicine seizure notice: Gansera Leveque
Animal medicines seizure notices: fish medicines for aquarium and pond fish
Animal medicine seizure notice: Border Force, Newcastle Airport
3. AMR (Antimicrobial Resistance)
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 28th February 2020 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to animal and public health. The group also undertook discussions on the future ambitions of AMR surveillance in the UK. Topical updates were provided by the Livestock Information Programme and Health Protection Scotland. Summary minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
3.2 Communications
The UK’s FAO Reference Centre for AMR was represented at the launch of the SMArt charity at the House of Lords in early March. This charity will provide worldwide regulation of veterinary medicines to ensure their responsible, safe and effective use. The Centre was showcased at the event using banners and leaflets which highlighted the ongoing work of the centre.
3.3 FAO Reference Centre
The UK’s FAO Reference Centre for AMR hosted a workshop in Nigeria to bring together multiple (Nigerian) government agencies to successfully draft their first national action plan for residues monitoring. Due to the Coronavirus pandemic, several overseas visits by the Centre have been postponed.
4. Inspections
All VMD inspections postponed due to coronavirus (COVID-19)
During the period from 01/12/19 to 29/02/20 the Inspections and Investigations Team carried out a total of 259 inspection visits of retail and feed business premises.
- Retail Premises
There were 199 retail premise inspection visits (vet practice premises and SQP retailers). The compliance ratings for these inspection visits are as follows (5 being the highest, 1 being the lowest):
| Rating | Number of premises | Percentage | ||
|---|---|---|---|---|
| 5 | 73 | 36.68% | ||
| 4 | 90 | 45.23% | ||
| 3 | 25 | 12.56% | ||
| 2 | 4 | 2% | ||
| 1 | 4 | 2% | ||
| NICO (No inspection carried out) | 3 | 1.51% |
(discrepancy of 0.2% in total due to rounding)
The most common issues that required corrective action were:
- Insufficient temperature records for ambient and/or cold chain products
- Broach dates not recorded or exceeded
- Controlled Drug (CD) Registers not maintained as required
The requirements for retailers are published on Gov.UK Retail of veterinary medicines
- Feed Business Operator premises
There were 60 Feed Business Operator (FeBO) premise inspection visits. The compliance ratings for these inspection visits are as follows (5 being the highest, 1 being the lowest):
| Rating | Number of premises | Percentage | ||
|---|---|---|---|---|
| 5 | 22 | 36.67% | ||
| 4 | 25 | 41.67% | ||
| 3 | 9 | 15% | ||
| 2 | 3 | 5% | ||
| 1 | 1 | 1.67% |
(discrepancy of 0.1% in total due to rounding)
The most common issues that required corrective action were:
- HACCP plan not documented/implemented/maintained as required
- Homogenity test issues
- Cleanliness and/or cleaning records not of the required standard
The requirements for Feed Business Operator (FeBO) premises are published on Gov.UK Manufacturing and supplying veterinary medicines for animal feed
5. Guidance Updates
Post-Transition period action deadlines will go beyond 2020
VMD Export Certificates being issued electronically
Veterinary medicine wholesale dealer’s authorisation (WDA)
SQPs temporarily allowed to prescribe and authorise supply of veterinary medicines remotely
Veterinary practice premises: temporary change of premises
Veterinary surgeons: temporary changes to the retail supply of veterinary medicines
SQP retailer premises: temporary change of premises
Extemporaneous products: temporary change of supply
Approval of premises for retail supply of veterinary medicines by suitably qualified persons (SQP)
Authorisations to manufacture veterinary medicines
Coronavirus (COVID-19) VMD News and Guidance
6. Surveillance
Veterinary pharmacovigilance in the UK annual review 2018
7. Research and Statistics
Top ten imported veterinary medicines - Quarterly report 1 January 2020 - 31 March 2020
8. Stakeholder Engagement
Launch of a new charity to improve veterinary medicines regulation worldwide
8.1 Feedback results - company meeting questionnaires 2019/2020
During 2019/2020 a total of 31 face to face company meetings were arranged to discuss on going applications or potential applications. A further nine teleconferences were also held.
Based on previous years only a relatively small number of questionnaires were returned, which is perhaps not surprising given the increased activity around preparations for UK Exit from the EU.
We do, however, encourage companies to complete the questionnaires as this not only supports the range of qualitative measures we have in place, but provides valuable information that we use to help us to improve our services.
We achieved our objective with the overall median score from feedback surveys for individual VMD company meetings to be at least good for 90% or more. All companies who submitted a return rated the VMD as at least good. Out of a score of 5 the usefulness of the VMD advice was rated on average at 4.4. The rating for VMD staff being well prepared was 4.6, and the scores for individual teams (efficacy, safety, quality and administrative) were rated between 4.5 and 5. It was not possible to provide a score for the biologicals team on this occasion.
The VMD is happy to meet with you. Should you wish to have a face to face meeting to provide regulatory advice on applications, please contact Chris Abbott here at the VMD c.abbott@vmd.gov.uk who can make the necessary arrangements. He can also arrange a teleconference if that is better suited to your needs.
When considering the need for a meeting please give careful consideration to the areas you would like to discuss. This will help us to identify the relevant VMD attendees. Please also provide an agenda and any supporting presentations at least five working days in advance of the meeting so that we can prepare and be ready to provide the required advice.
9. Corporate News
The VMD’s old GSI email address will no longer work from 1 April
VMD web services: update 4 March 8am
VMD switchboard service unavailable: Update - now working
The VMD remains open for business
VMD: Civil Service People Survey 2019
The VMD switchboard service: update 8 April
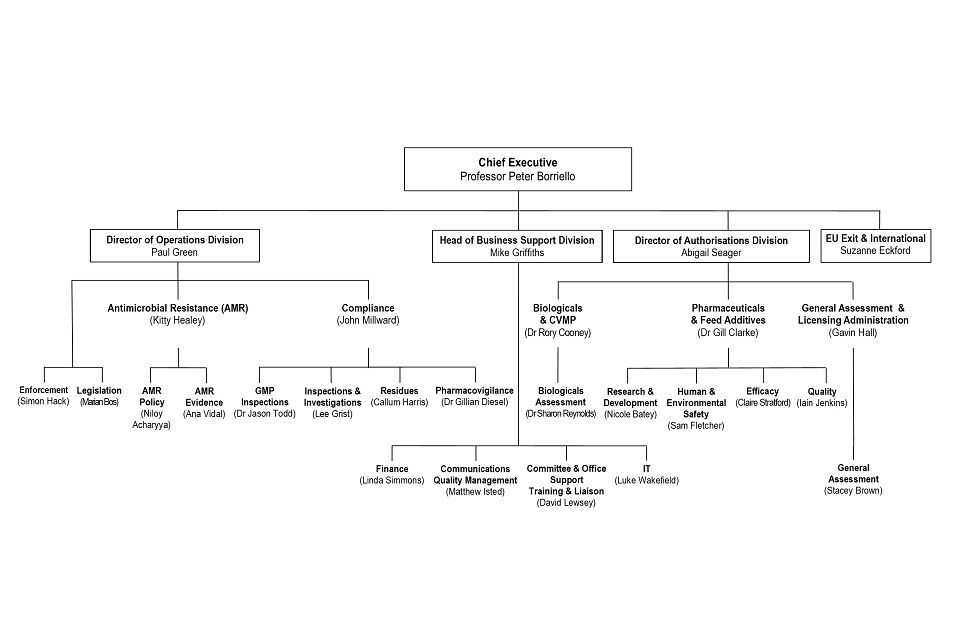
VMD Organogram as at 30 April 2020
