[Withdrawn] FASP laboratory handbook
Updated 13 September 2019
1. Introduction
This handbook brings together in one publication the national guidelines, recommendations, standards and specifications for Down’s syndrome (T21), Edwards’ syndrome (T18) and Patau’s syndrome (T13) screening that relate to the work performed by screening laboratories.
Each national NHS screening programme has a defined set of standards that providers have to meet to make sure local services are safe and effective.
Quality assurance (QA) is the process of checking that these standards are met and encouraging continuous improvement.
All NHS fetal anomaly screening programme (FASP) screening laboratories must:
- be accredited by the UK Accreditation Service (UKAS) to ISO Medical laboratories – requirements for quality and competence (ISO 15189)
- participate in external quality assurance (EQA) schemes accredited to ISO Conformity assessment. General requirements for proficiency testing schemes (ISO 17043)
- meet the screening programme QA requirements mapped to ISO 15189
- use ISO 15189 accredited reference laboratories
UKAS looks at ISO 15189 and the screening requirements on behalf of PHE screening quality assurance services (SQAS) and the national screening programme.
1.1 Conventions
Throughout this handbook:
- Down’s syndrome is referred to as trisomy 21 (T21)
- Edwards’ syndrome is referred to as trisomy 18 (T18)
- Patau’s syndrome is referred to as trisomy 13 (T13)
1.2 Related documents
This handbook is part of a suite of 5 documents.
The ultrasound practitioners handbook sets out the requirements for ultrasound practitioners involved in the pathway for first trimester screening for T21, T18 and T13.
The programme handbook provides operational guidance and information for all practitioners involved in screening for T21, T18 and T13 and the 18+0 to 20+6 (18 weeks and 0 days to 20 weeks and 6 days ) week fetal anomaly scan.
Service specifications No. 16 (Down’s syndrome, Edwards’ syndrome and Patau’s syndrome screening) and No. 17 (18+0 to 20+6 week fetal anomaly scan) outline the service and quality indicators expected by NHS England and the recommendations and standards of the UK National Screening Committee (UK NSC).
The screening standards define a set of standards relating to screening for T21, T18 and T13 screening and the 18+0 to 20+6 week fetal anomaly scan.
2. The NHS Fetal Anomaly Screening Programme
NHS population screening explained sets out the general principles of screening.
2.1 Background
The national FASP team, part of the PHE Screening division, ensures national consistency and provides expertise. It supports and manages implementation of fetal anomaly screening and the technical and professional development of the programme. It also makes sure quality and safety standards are maintained and continuously improved.
The programme has evolved since being set up in 2001 when the majority of screening was performed using maternal biochemistry in the second trimester. First trimester screening is now the recommended method. It combines maternal age, biochemistry and ultrasound measurement of fetal nuchal translucency (NT) to provide a pregnant woman with her chance of having a baby with T21, and/or T18 and T13.
To enable commissioners and SQAS teams to assess the quality of services, NHS FASP has standardised:
- national standards, guidance and chance cut-off for T21, T18 and T13 screening in the light of emerging evidence
- a statistical service to make sure laboratories have access to the best advice in maintaining their population medians
- specifications for chance calculation software to make sure all laboratories calculate chance results the same way
2.2 Screening policy
NHS FASP requires there to be equal access to uniform and quality-assured screening across England. Services should provide women with high quality information so they can make an informed choice about their screening and pregnancy options. PHE Screening education and training resources are available for staff covering all stages of the process, from informing women of test availability, through to understanding and supporting their decisions.
NHS FASP offers screening to all eligible pregnant women in England to assess the chance of babies being born with T21, and/or T18 or T13 and a number of physical conditions (unexpected development in the fetus).
National policy is to offer screening to assess the chance of the baby being born with T21, T18 or T13. The test of choice for both singleton and twin pregnancies is first trimester combined screening. Women can choose:
- not to have screening
- to have screening for T21, T18 and T13
- to have screening for T21 only
- to have screening for T18 and T13 only
More information on the conditions screened for can be found in the programme handbook.
If a woman accepts the offer of screening, the first scan usually takes place between 11 and 14 weeks of pregnancy along with a blood sample taken to test for T21 and/or T18 and T13. A second scan for fetal conditions takes place between 18+0 and 20+6 weeks. The timing of the scans allows for further diagnostic tests if required and ensures women have time to consider decisions about continuing their pregnancy.
The second scan is designed to identify physical conditions which indicate that:
- treatment before or after birth would be of benefit
- the birth should be in an appropriate setting so that treatment is optimised after birth
- the baby may die shortly after birth
Some women may choose not to be screened at all. It is important that this choice is respected.
Diagnostic testing can include chorionic villus sampling (CVS), which involves removing a small piece of tissue from the placenta, or amniocentesis, which involves removing a small amount of amniotic fluid (the fluid surrounding the baby in the uterus).
3. Screening tests
3.1 First trimester combined test
The combined test calculates the chance of the pregnancy being affected by T21 or T18/T13 by using:
- maternal age
- the NT measurement
- 2 biochemical tests – free beta hCG and PAPP-A
- the gestational age calculated from the crown rump length (CRL) measurement
The combined test can be performed when the CRL is between 45.0mm and 84.0mm, which corresponds to 11+2 to 14+1 weeks of gestation.
If the ultrasound measurement shows that the CRL is less than 45.0mm, the woman should be recalled for a further scan to measure the NT. If the CRL is greater than 84.0mm, the second trimester quadruple test should be offered.
The first trimester combined test allows earlier decision making for women. In practice, the following 2 models are available for performing the combined test.
Maternal blood specimen taken (from 10 weeks onwards) before the ultrasound scan
The biochemistry results can then be made available at the time of the NT scan and the combined test result can be calculated at the time of the appointment. Although the result may be calculated by the sonographer, the laboratory remains responsible for the software that calculates the screening result. Midwifery and/or ultrasound departments must have a process in place to share ultrasound measurements and final screening results with the laboratory to enable timely audit of all results.
Maternal blood specimen taken at the time of the ultrasound scan
The combined test result is then made available within a few days of the biochemistry results being authorised by the laboratory.
The gestational age, for the purpose of standardising marker values, should be calculated by use of CRL obtained by ultrasound measurement. Combined screening can be offered (including in vitro fertilisation (IVF) pregnancies) where the CRL measurement of the baby is between 45.0mm and 84.0mm. Where the CRL is above 84.0mm then gestational age should be calculated using the head circumference (HC).
Maternal age in IVF pregnancies using donor eggs should be derived from the date of birth or age of the donor at the time of egg collection as this is required for calculation of prior chance. See Down’s syndrome screening: risk calculation software requirements.
NHS FASP recommends that the T21 and T18 and T13 screening chance result generated from first trimester combined screening must not be recalculated up or down following the initial screening test, or at the 18+0 to 20+6 week fetal anomaly ultrasound scan, due to the presence or absence of a single ultrasound marker of less predictive power than increased nuchal fold.
3.2 Second trimester quadruple test
There will always be a need for a screening test in the second trimester for women who book too late for first trimester testing or when an NT measurement cannot be obtained in the first trimester.
The quadruple test uses maternal age and 4 biochemical markers measured from 14+2 weeks until 20+0 weeks – alpha fetoprotein (AFP), human chorionic gonadotropin (hCG) (total, intact or free beta subunit), unconjugated oestriol (uE3) and Inhibin-A. Although this combination of markers has a lower detection and a higher screen positive rate (SPR) than the combined test, it is the nationally recommended screening strategy for the second trimester.
An ultrasound scan will be required to date the pregnancy and a fetal HC is the recommended measurement for women presenting in the second trimester. If the HC is ≥ 101.0mm then the blood sample for the quadruple test may be taken.
If the CRL is > 84.0mm but the HC is < 101.0mm there are 2 options.
You can either assess the gestational age of the pregnancy using the HC and, from that, calculate the date the woman will be between 14+2 and 20+0 weeks of pregnancy and offer an appointment for blood sampling.
Or you can rebook the woman for a further scan at 15 weeks of pregnancy (calculated from the HC) where the HC can be re-measured and bloods taken.
For more information regarding the practicalities of a solution to combining dating and screening requirements at the first pregnancy scan see A practical solution to combining dating and screening for Down’s syndrome.
3.3 National standards
The national standards in table 1 below give the thresholds for the national programme. They are reported on each year by the Down’s syndrome screening Quality Assurance Support Service (DQASS) and the National Congenital Anomaly and Rare Diseases Registration Service (NCARDRS).
Table 1: thresholds for performance
| Screening strategy | Trisomy | Standardised DR | Standardised SPR (acceptable) | Standardised SPR (achievable) |
|---|---|---|---|---|
| Combined | T21 | 85% | 2.2 to 3% | 2.3 to 2.9% |
| Combined | T18/T13 | 80% | 0.1 to 0.2% | 0.13 to 0.17% |
| Combined | T21/T18/T13 | 80% | 2.2 to 3% | 2.3 to 2.9% |
| Quadruple | T21 | 80% | 3.2 to 4% | 3.3 to 3.9% |
DR = detection rate
SPR = screen positive rate
4. Markers used in screening tests
4.1 Maternal age
All women have a chance of having a baby with T21, T18 or T13. This chance increases with age. The older the mother, the greater the chance that she will have a baby with one of these conditions.
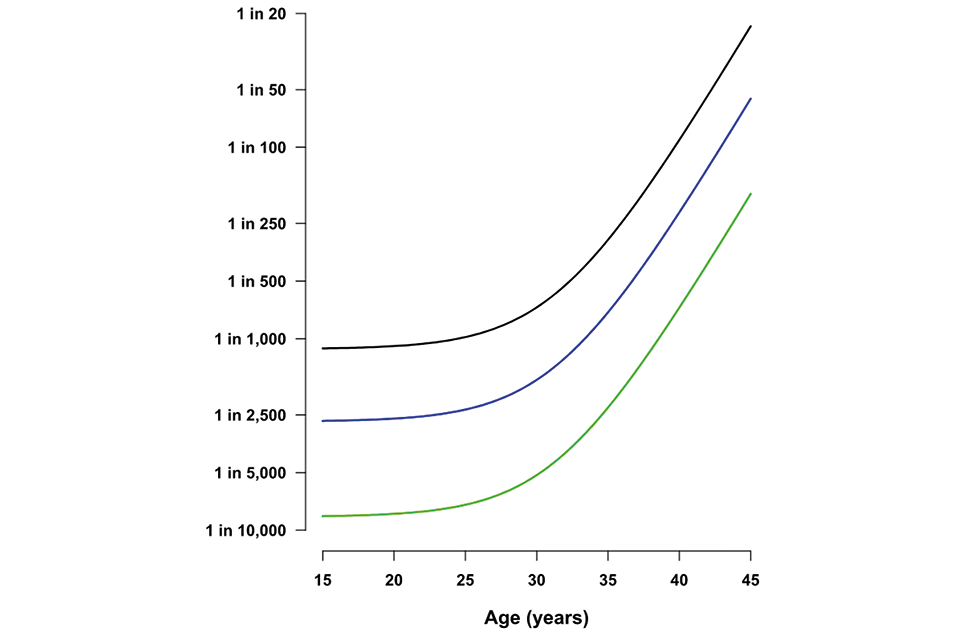
Graph illustrating the chance of a pregnancy being affected by Down’s syndrome, Edwards' syndrome or Patau’s syndrome (y axis) according to maternal age (y axis). The graph shows the risk of all 3 conditions rising steeply after the age of 30
Table 2: chance of having a pregnancy affected by T21 according to maternal age
| Maternal age | Chances of having a pregnancy affected by Down’s syndrome | Probability |
|---|---|---|
| 20 years | 1 in 1,500 | 0.07% |
| 30 years | 1 in 900 | 0.1% |
| 40 years | 1 in 100 | 1% |
4.2 Biochemical markers
There are 5 analytes (commonly referred to as markers) measured by the laboratory that are used to calculate the chance of a pregnancy being affected by T21, T18 or T13 – or 6 if human chorionic gonadotropin (hCG) and its free beta subunit are considered as 2 separate analytes.
Screening laboratories must use the recommended combination of biochemical markers for both the first and second trimester screening tests.
Alpha fetoprotein
AFP is a glycoprotein of 591 amino acids produced by the yolk sac and the fetal liver. Its level in fetal serum increases until the end of the first trimester and then gradually decreases. Concentrations are much lower in maternal serum but continue to rise until about week 32 of pregnancy. Decreased levels in the second trimester are associated with Down’s syndrome.
Human chorionic gonadotropin
hCG is a glycoprotein of 244 amino acids produced by the developing embryo and later by the placenta. It is a dimeric molecule composed of an alpha and a beta subunit. The alpha subunit is common to several other hormones (luteinising hormone (LH), follicle stimulating hormone (FSH) and thyroid stimulating hormone (TSH)).
The beta subunit is unique to hCG. Concentrations of hCG rise exponentially after conception, reaching a peak at about 9 to 12 weeks, then falling to reach a plateau at about 20 weeks. Some of the beta subunit (less than 1% of the intact dimeric hCG) is free in the blood and this molecule can be measured by the laboratory as a distinct entity. A raised level of hCG and/or the free beta subunit in the first and second trimester is associated with an increased chance of a pregnancy affected by T21. A decreased level is associated with both T18 and T13.
Unconjugated oestriol
Oestriol is one of the 3 main steroid hormones produced by the feto-placental unit during pregnancy. It is made in the placenta from the 16-hydroxydehydroepiandrosterone produced by the fetal liver. Once in the maternal circulation, most of the oestriol undergoes conjugation with glucuronides or sulphate but about 10% remains as the unconjugated form. Low levels of uE3 in the maternal circulation in the second trimester are associated with T21.
Inhibin-A
Inhibin-A is a dimeric molecule comprised of an alpha and a beta polypeptide chain linked by a disulphide bridge (a similar molecule called Inhibin-B has the same alpha chain but a different beta chain). It is produced by the corpus luteum and the placenta during pregnancy with levels increasing during the first trimester, then declining to reach a plateau in the second trimester before increasing again in the third trimester. Elevated levels in the second trimester are associated with pregnancies affected by T21.
Pregnancy associated plasma protein – A (PAPP-A)
PAPP-A is a large zinc glycoprotein produced by the placenta where it is thought to regulate the activity of factors responsible for the growth of the placenta. Low levels in maternal blood in the first trimester are associated with pregnancies affected by T21, T18 and T13.
4.3 Effect of vaginal bleeding on biochemical markers used in screening for T21, T18 and T13
There are concerns that a history of significant maternal vaginal bleeding might change the levels of biochemical markers used in the combined and quadruple tests. The NHS FASP recommends women should be offered the screening tests in the normal way (calculating the chance based on maternal age, NT, free beta hCG and PAPP-A levels or the chance based on AFP, free beta hCG, Inhibin and uE3). This is because current evidence suggests that the biochemical marker levels are not significantly different in women with this history.
4.4 Effect of ‘vanished twin’ on biochemical markers used in screening for T21, T18 and T13
Combined screening
When ultrasound shows an empty second pregnancy sac, the biochemical markers appear no different to those in a singleton pregnancy and the combined test of NT, PAPP-A and free beta hCG can be used to calculate the chance result.
When ultrasound shows a second sac containing a non-viable fetus (sometimes called ‘vanished’ twin), it is possible there could be a contribution to the maternal biochemical markers for many weeks.
In this event, where a woman has accepted screening, it is recommended the CRL and NT measurements of the viable fetus should be undertaken. There should also be a local policy in place to provide referral to a health professional, with the relevant skills and knowledge, to discuss the options available for testing that fall outside the remit of NHS FASP. For example, use of NT measurement and maternal age alone.
Quadruple screening
In women eligible for quadruple screening (women who present too late for the offer of a combined screening test or when the NT cannot be measured) where the ultrasound shows an empty second pregnancy sac or a second sac containing a non-viable fetus, levels of inhibin may be higher than the usual range and AFP may also be increased.
Given that AFP is decreased in Down’s syndrome and inhibin is increased, these effects tend to cancel each other out.
For this reason, it is suggested (Huang and others, 2015) that the detection rate of the quadruple test is unlikely to be reduced in vanished twins. Therefore, for those women presenting too late for the offer of combined screening or those in which NT cannot be measured with an empty second pregnancy sac or second non-viable fetus, the quadruple test can be offered.
4.5 Ultrasound markers
The offer of antenatal screening for Down’s syndrome, Edwards’ syndrome and Patau’s syndrome depends on very specific criteria. Reporting ultrasound measurement to one decimal place, for example 45.0mm rather than 45mm, is required because the measurements correlate to specific gestational ages.
Nuchal translucency
NT is the ultrasound appearance of a collection of fluid under the skin behind the neck of the fetus in the first trimester of pregnancy. The thickness of the NT is measured by the sonographer and used in calculating the chance of the pregnancy being affected by T21, or T18 and T13. An increased NT measurement is associated with an increased chance of these autosomal trisomies as well as other fetal conditions such as cardiac conditions. But, as with all screening tests, a pregnancy with an increased NT may also have a normal outcome.
Where screening in the first trimester using the combined screening strategy is accepted, the biochemical component of the test must be completed. Therefore, where an NT measurement of ≥3.5 mm is recorded, a blood sample must be taken but referral should not be delayed to await the biochemistry marker levels. As soon as the results are available, they should be forwarded to the clinician to support discussion of further investigative options with the woman.
Crown rump length
The gestational age of the fetus is calculated from the ultrasound measurement between the top of the head (crown) to the bottom of the buttocks (rump). This is the CRL. The gestational age in days can be calculated by the use of tables or an equation from the CRL measurement.
The first trimester screening test is primarily described within a gestational age timeframe (10+0 weeks to 14+1 weeks). However, entry into the screening programme within the laboratory should be based on CRL measurements of 45.0mm to 84.0mm rather than gestational age of weeks and days. It is important the CRL is measured accurately because the concentration of the biochemistry markers depends on the gestational age of the fetus.
Head circumference
If the CRL is greater than 84.0mm, it is recommended that the gestational age of the fetus is calculated using the fetal HC. Ultrasound measurements of the biparietal diameter (BPD) using ‘outer to outer’ calliper placement and the occipital-frontal diameter (OFD) using ‘outer to outer’ calliper placement are used to calculate the head circumference which can then be used to date the pregnancy.
Information about NHS training for competency in the measurement of NT and CRL and the recommended measurements charts can be found in the ultrasound practitioner’s handbook.
5. Singleton pregnancies
5.1 Combined test chance results
For women screened using the combined test, dependent of their screening choice, the following chance results will be reported:
- a term chance of T21 and a term chance of T18/T13
- a term chance of T21 only
- a term chance of T18/T13 only
5.2 Quadruple test chance results
Quadruple screening can be offered between 14+2 and 20+0 weeks.
For women screened using the quadruple test a single term chance of T21 will be reported.
6. Twin pregnancies
Having more than one baby in a pregnancy complicates screening. Each feto-placental unit will contribute to the concentrations of the biochemical markers used in the chance calculation. In the first trimester there is an NT measurement for each fetus.
6.1 Combined screening in twin pregnancies
The test of choice for twin pregnancies is first trimester combined screening. Every opportunity must be made to maximise the offer of first trimester combined screening. Chance results to be reported are:
- a term chance of T21 and a term chance of T18/T13
- a term chance of T21 only
- a term chance of T18/T13 only
For women screened using the combined test, where a dichorionic twin pregnancy is identified, the chances will be reported for each fetus. In a monochorionic twin pregnancy, both fetuses are either affected or unaffected so the chance will be the same and a single ‘pregnancy’ chance will be reported.
6.2 Quadruple screening in twin pregnancies
For the small number of women who have a twin pregnancy and miss the opportunity of having first trimester screening, we can offer the choice of a second trimester quadruple test for T21 only.
This means the decision-making process is more difficult for women as this test is less sensitive than first trimester combined screening and any subsequent decisions about invasive diagnostic testing and selective reduction will have to be made later in the pregnancy.
The policy takes into account the fact that:
- chorionicity is likely to be difficult to ascertain in the second trimester and therefore unknown
- the chance of a T21 birth from a dizygotic twin pregnancy is higher than that from a singleton pregnancy
This is as an interim policy to provide equity of choice for women while further evidence and other strategies become available.
Quadruple screening in twin pregnancies can be offered to women:
- who present for the first time in the second trimester or
- where the NT could not be measured in the first trimester
For this reason, women considering screening should have a discussion with a healthcare professional with an interest in multiple pregnancies to help facilitate informed choice.
There are likely to be between 500 and 1,600 women with twin pregnancies in the eligible population each year who fall outside the combined testing programme and may be offered second trimester quadruple testing. Pregnancies in this group are likely to be of uncertain chorionicity compared to the general population of twin pregnancies. Among these, fewer than 4 affected pregnancies would be expected.
Monochorionic twins
The chance of a T21 birth from a monochorionic pregnancy is lower than that from a singleton pregnancy due to a higher fetal loss rate amongst affected pregnancies.
The performance of screening in monochorionic twins is comparable to that of singletons: a detection rate is 80% for a standardised SPR of 3%.
Dichorionic twins
The chance of a T21 birth of at least one baby from a dichorionic twin pregnancy is higher than that from a singleton pregnancy.
In dichorionic twins, where one is affected and the other unaffected, the performance is poorer due to the markers being less discriminatory but is better than using maternal age only, where the detection rate is only 30% for a 5% SPR.
In dichorionic twins the detection rate is 40% to 50% for a standardised SPR of 3%.
It should be noted that the approach used in calculating a quadruple twin pregnancy chance results is referred to as ‘pseudo’ risk/chance in the literature. This is the established methodology currently available and simply means that the chance would be accurate in predicting a false-positive rate (which relates only to the marker distributions in unaffected twin pregnancies). The chance is a pregnancy related chance that is not fetal specific.
The term chance cut-off of 1 in 150 is applied to the pseudo risk/chance.
Because the calculation of chance results in twin pregnancies relies on limited evidence and assumptions, the chance estimate should be interpreted by suitably experienced practitioners.
The risks of miscarriage and other procedure related complications are higher in twin pregnancies, usually quoted as 1 in 50. If one fetus is affected, selective reduction may be an option.
Please refer to NICE guidance on screening for T21, T18 and T13 in higher multiple pregnancies (triplets and more).
7. Chance cut-off
NHS FASP defines the national cut-off and this is currently set at 1 in 150 at term for both first and second trimester screening tests. A woman with a chance of 1 in 150 or greater (1 in 2 to 1 in 150) of having a pregnancy affected by T21 or T18 and T13 in the first trimester or T21 only in the second trimester is considered to be in the ‘higher chance’ group.
Women in this group are offered diagnostic test such as CVS or amniocentesis to directly investigate the fetal chromosomes.
For women having screening using the combined test, dependant of their screening choice, up to 2 chance results are reported:
- a chance for T21 and a chance for T18/T13
- a chance for T21 only or T18/T13 only
The cut-off is based on a chance at term rather than a chance at the time of the screening test. The main reason for this is that the original studies on the likelihood of having a Down’s syndrome affected pregnancy is based on the birth prevalence of the condition before screening was implemented.
There is a significant fetal loss rate between the time of screening and birth but the loss rate is not exactly known. A chance at the time of screening would need to make assumptions about the fetal loss rate during the various stages of pregnancy. This will be kept under review.
7.1 Chance calculation software
The software used to calculate the T21, and T18 or T13, chance result from the biochemical and ultrasound markers is complex and best provided and supported by commercial suppliers. Laboratories must use chance calculation software that is CE marked and complies with EU directives, and, where planned, utilise upgrades of this software.
NHS FASP developed a specification for the chance result calculation software for laboratories in England. Software in use in screening laboratories must meet the requirements of this specification.
8. Request form and data fields
Laboratory request form and data fields includes the data fields that need to be included when designing a laboratory screening request form for T21, T18 and T13. Supplier software and data entry methods may vary. To maximise the potential for supporting screening laboratories effectively and driving quality improvement in the programme’s performance, all data items specified in Appendix 1 would be helpful to the DQASS service, and NHS FASP recommends, as request forms and electronic data fields are developed, these are included.
Fields highlighted in bold text in Laboratory request form and data fields must be provided to DQASS.
9. Report formats
The chance result report may contain a number of information fields, for example, biochemistry marker values, the NT measurement, the T21 chance, the T18 and T13 combined chance, as well as the necessary demographics of the mother, date of the specimen and destination of the report.
Achieving the right balance is important to make sure there is enough information to understand the chance assessment report but not so much that it has the potential to cause confusion. Only results and comments relating to the conditions screened for as part of the NHS screening pathway for T21, T18 and T13 in this pregnancy should be included on the laboratory report.
In addition to the data given on the screening request form, the report must include:
- analytical results for each biochemistry marker and the NT measurement for the first trimester combined test, with units of measurement and corrected multiple of the median (MoM) value
- all relevant information used for calculating the screening result, such as date of birth, maternal weight, smoking history, ethnicity, scan measurement data and, where appropriate, IVF, diabetes, previously affected pregnancy and twins
- the factors for which the markers were corrected, such as weight, smoking, ethnicity and, where appropriate, IVF, diabetes, previously affected pregnancy and twins
- the T21 prior chance and adjusted chance
- the T18 and T13 combined prior chance and adjusted chance
- the classification of the chance into the higher and lower chance category with an indication of the chance cut-off used
The chances will be reported for each fetus for women screened using the combined test, where a dichorionic twin pregnancy is identified. In a monochorionic twin pregnancy, both fetuses are either affected or unaffected so the chance will be the same and a single chance will be reported. For the quadruple test a pregnancy chance will be reported.
See Lower chance result letter templates for examples of letters for informing women of their screening chance results.
10. Specimen transport and storage
Blood taken for T21, T18 and T13 screening should be collected into a plain or gel serum separating tube (SST). It is important that the correct order of draw is followed. A screening sample should always be taken first if a full blood count sample is being taken at the same time.
Tubes containing ethylene-diamine-tetra-acetic acid (EDTA) must be avoided due to significant interference of EDTA in the immunoassays used to measure the serum markers for screening.
Blood samples taken for T21, T18 and T13 screening should be processed as soon as possible. This is because free beta hCG is likely to increase in concentration over time due to the dissociation of the intact hCG molecule.
This effect is temperature dependent. The rate of deterioration of the sample increases with increasing temperature. The guidance below is for samples transported and stored at room temperature as this is most likely to be the conditions in clinics and during transport to laboratories. However, during periods of increased temperatures, providers should consider both storing and transporting samples in cool bags to reduce the effects on the markers, particularly free beta hCG. Whole blood samples should not be frozen or transported on dry ice as this makes the sample unsuitable for analysis.
If a gel separator tube is not used, it is good practice for the sample to be centrifuged and separated from the clot within 24 hours of collection. If this is not possible, samples may remain as whole blood at room temperature for up to 48 hours. Samples received as whole blood after 48 hours from sample collection should be rejected.
Table 3: screening sample stability acceptance criteria
| Sample Type | Room temperature (20°C) | 4°C |
|---|---|---|
| Whole blood (unseparated) | 48 hours | 5 days |
| Serum (separated) | 72 hours | 14 days |
Serum samples are stable up to 72 hours at room temperature.
All samples that have been transported or stored at room temperature for longer than 72 hours should be rejected.
The stability of the samples is significantly improved by refrigeration at 4°C, with whole blood samples and serum stable for several days. High temperatures (over 30°C) should be avoided as deterioration of free beta hCG occurs within 4 hours following sample collection.
11. Analysers and kits
There are several suppliers in the UK providing assays and analysers that are Conformité Européene (CE) marked for use in T21, T18 and T13 screening.
NHS FASP does not have a preferred supplier and laboratories must decide which analyser best suits their purpose.
Some assays are available on random access analysers which overcome the need to batch specimens. All assays should be verified and used according to the manufacturer’s instructions. Any deviations must be fully validated in compliance with Medical laboratories – Requirements for quality and competence (ISO 15189:2012).
Analysers must be maintained according to the manufacturer’s instructions and a comprehensive maintenance record log compiled, preferably in an electronic format.
Standard operating procedures that would pass a UK Accreditation Service (UKAS) assessment must be in place in the laboratory for each assay and all procedures relating to the screening work of the laboratory.
12. Laboratory throughput
NHS FASP specifies the following throughput criteria for laboratories (or laboratory network):
-
A standalone screening laboratory must have a workload of at least 8,000 specimens per annum for each of the first and/or second trimester screening tests that are offered.
-
All laboratories with a workload of less than 8,000 specimens per annum for a specific screening test must be part of a network screening service for that test.
-
Each laboratory in a network screening service must have a minimum throughput of 2,000 specimens per screening test per annum.
-
In a network screening service the total aggregated specimens must be no less than 8,000 specimens per annum.
A laboratory network screening service must meet the following criteria:
-
The network screening service must be directed by a person who has executive accountability and the competence as assessed by UKAS to assume governance responsibility for the service. A clear governance structure that integrates with trust governance and quality structures must be outlined.
-
There must be an agreed contractual arrangement between all laboratories in the network screening service and the commissioners of the service.
-
The network screening service lead will receive one 6-monthly combined DQASS report.
-
Each laboratory in the network screening service must have identical screening policies.
-
Each laboratory in the network screening service must have identical analytical procedures, such as analysers and kit lots.
-
Each laboratory in the network screening service must have chance calculation software with an identical chance calculation algorithm and population parameters.
The above criteria should improve the quality of the screening programme.
Achieving precision in the DQASS assessment of laboratory performance
An annual throughput of 8,000 is needed to achieve effectiveness and statistical precision in the DQASS assessment of laboratory performance and to make accurate estimates of standardised SPRs.
In cases where laboratory throughput is small, DQASS cannot measure performance reliably. For example, in a DQASS report based on a sample size of less than 700 records over a 6-month period, the feedback to the laboratory was as follows:
There is insufficient data to assess whether or not there are substantial deviations from target. Overall the estimated median MoM shows a small positive bias, but is within 5% of target. Given the small sample size, any flag assigned to this marker should be interpreted with caution. The flag allocated may not be a reliable measure of performance due to low throughput.
An annual throughput of 8,000 enables the monthly mean log10 MoM within each cycle to be estimated to within ±0.1 standard deviations with 95% confidence.
Achieving sufficiently precise estimates of standardised SPRs
An annual throughput of 8,000 enables the SPR for each cycle to be estimated within ± 0.5% with 95% confidence.
Enabling proportionate quality improvements while sustaining and developing expertise
T21/T18/T13 screening is a specialist, complex and continually evolving area of work. A minimum laboratory throughput will enable this expertise to be concentrated and developed.
It will also enable DQASS to give additional focused support where required.
The principle of a network screening service with a minimum of 2,000 samples per test per year enables laboratories with a smaller throughput to maintain the ability to monitor performance while working in a consistent way to a set of common standards. It also allows capacity and capability building to develop proficiency and technical expertise in a safe and efficient way.
Promoting efficiency
A high throughput, particularly where technology in use requires batching of samples, means that laboratories will be able to meet recommended turnaround times for results. Poor turnaround times could delay diagnosis and ongoing management options.
The workload required to effectively and efficiently run a small throughput laboratory is about the same as, if not more than, a laboratory with a larger throughout. It could be argued that the effort and resources needed for internal and external QA are greater and therefore less cost effective.
Enabling laboratories internal quality control (IQC) to detect biases in MoM values in a timely way
Although DQASS provides a highly beneficial external QA service, it should be remembered that this is a retrospective analysis on the whole. In addition to the retrospective audit feedback that DQASS provides, laboratories must also have IQC processes that are performed frequently enough to enable timely changes where required.
This enables laboratories to be responsive to changes in real time. A high throughput is required to detect changes in biases in a timely manner.
13. Internal quality control
Because the chance calculation involves the results of several markers, it is vital that each measurement is as accurate as possible to minimise the inaccuracy of the final chance reported.
The quality control procedures employed by the laboratory must be rigorous enough to detect problems with the assays, not only on a day-to-day basis but also to check problems like a gradual drift of results with time and changes that may occur when a new batch of reagents is brought into use.
Manufacturer’s recommendations for IQC must be strictly adhered to, but regarded as the minimum requirements. Additional controls should be included, especially if an assay is particularly difficult.
It is good practice to include validated IQC material from another supplier rather than rely solely on the controls provided by the manufacturer of the assay kit. Senior staff should examine long-term trends in IQC data to detect assay drift. Particular attention should be paid to comparing patient and IQC results when a new batch of reagents is introduced.
Significant shifts in population medians have been observed on occasions with the introduction of a new reagent batch, which can then impact on the risks reported if it is not anticipated and allowed for. Similar effects have been seen after analyser maintenance and vigilance is required at all times.
14. Quality assurance
14.1 Quality assurance of screening programmes
Each NHS screening programme has a defined set of standards that providers have to meet to ensure local services are safe and effective.
All commissioners and service providers must refer to the public health functions agreement (Section 7A) NHS FASP service specification (No 16), and supporting standards and the laboratory handbook to make sure a service is set up correctly and is meeting the standards and guidance set by NHS FASP.
QA is the process of checking these standards are met and encouraging continuous improvement. It includes:
- advice on the development of national quality standards
- monitoring how services meet (or fail to meet) standards
- providing expert screening advice for incident management
- facilitating quality review of services, including peer advice
- supporting, on a day-to-day basis, those involved in commissioning or providing screening services
QA covers the entire screening pathway, from identifying who is eligible to be invited to screening, through to referral and treatment where required/appropriate.
The aim of QA is to maintain minimum standards and drive continuous improvement in the performance of all aspects of screening and to make sure all women and their babies have access to high quality screening wherever they live. QA is essential to minimise harm and maximise the benefits of screening.
Formal QA visits to local screening programmes provide the forum for a peer review of the whole multidisciplinary screening pathway (an assessment of the effectiveness of team working within the local screening programme and associated referral sites).
Regional QA teams:
- advise providers and commissioners about reducing risks in local screening programmes
- assess the robustness of local arrangements through audit, as part of peer review and in the investigation of any incidents as they occur
- act as a conduit for information and dialogue at national, regional and local levels, and sharing learning
- participate in a formal process of QA, which is the responsibility of each local screening programme
- monitor the performance of the local programmes in a variety of ways such as review of statistics, regional meetings or informal visits – all of which offer a valuable insight into the activity of a local programme
Regional QA teams will review reports of assessments and surveillance of screening laboratories by UKAS.
14.2 Quality assurance of NHS FASP laboratories
Laboratories undertaking T21, T18 and T13 screening must:
- be accredited by the UKAS to ISO Medical laboratories – Requirements for quality and competence (ISO 15189) or be CPA accredited and actively transitioning towards ISO 15189
- participate in EQA schemes accredited to ISO Conformity assessment. General requirements for proficiency testing schemes (ISO 17043) (for example, National External Quality Assessment Service (NEQAS) scheme for peptide hormones)
- submit data to the DQASS as specified in sections 8 and 15 of this handbook
- meet the screening programme QA requirements mapped to ISO 15189
- use ISO 15189 accredited reference laboratories
UKAS will look at both ISO 15189 and the screening requirements on behalf of the SQAS and NHS FASP.
The laboratory must be able to demonstrate strong oversight and leadership for screening, including:
- senior leadership for screening in the laboratory
- contingency plans for screening
- participation in cross-organisational and multidisciplinary arrangements
- management review and annual screening report
The laboratory must be able to demonstrate good communication and having worked together with other services in the screening pathway, including:
- documented procedures for communication with other services
- formal agreements with referral laboratories
- incorporation of screening into the laboratory quality management system
- standard operating procedures to track samples from point of receipt to authorisation and reporting of screen positive results to clinical services
- inclusion of users in assessment and review
The laboratory must have effective procedures to assess and manage safety and performance, including:
- documented risk management policy for the laboratory as part of the overall risk management arrangements for the screening pathway
- audit schedule in line with screening risks and performance requirements
- procedures to audit and ensure screening is completed – from point of receipt of test to communicating screen positive results to clinicians
- compliance with PHE Screening guidance for managing safety incidents
- provision of standards and key performance indicators (KPIs) and other data and screening activity data to NHS Screening programmes
15. Education and training
15.1 e-learning resources
Providers are responsible for funding minimum training requirements to maintain an effective screening workforce including continuing professional development (CPD). NHS FASP and UK NSC provide online educational resources to support CPD.
Those specific to laboratory based staff include:
- screening for Down’s syndrome, Edwards’ syndrome and Patau’s syndrome e-learning resource
- antenatal and newborn screening resource
It is the responsibility of providers to make sure training is completed satisfactorily and recorded and that a system is in place to assess ongoing competency.
The provider should also ensure that there are adequate numbers of appropriately trained staff in place to deliver the screening programme in line with best practice guidelines.
NHS FASP holds annual meetings for biochemistry staff involved in the screening pathway to discuss programme developments and offer support to the laboratories in driving quality improvement. DQASS also holds regular workshops and information updates. Attendance by a representative of all laboratories is recommended and encouraged.
16. Sign up to the PHE Screening blog
The PHE Screening blog is the best way to keep in touch with news, views and updates relating to all the work of PHE screening and NHS FASP. Subscribe to the blog to sign up for email alerts.
Follow @PHE_Screening on Tiwtter.
17. Information for the public
Screening tests for you and your baby booklet
Screening tests for you and your baby is the recommended information booklet covering both antenatal and newborn screening.
Each screening programme is described in a standard format. This makes it easier for the public to compare the various tests and, crucially, to understand that some decisions are more complex than others.
18. Screening standards
Screening standards provide a way of measuring how well screening programmes are performing in important areas. KPIs are a subset of the screening standards for the NHS screening programmes.
These contribute to the quality assurance of screening programmes but are not, in themselves, sufficient to quality assure or performance manage screening services. They help local screening providers identify potential problems so they can be put right and have led to changes in practice and implementation of measures to prevent errors occurring in the screening pathway.
NHS FASP screening standards that are relevant for the T21, T18 and T13 biochemical screening laboratories are as follows:
Standard 1: coverage and identifying population (T21/T18/T13 screening). Laboratory responsibility: data source.
Standard 3a: test performance – screen positive rate (SPR) (T21/T18/T13). Laboratory responsibility: data source.
Standard 3b: test performance – detection rate (DR) (T21/T18/T13 screening). Laboratory responsibility: data source and responsible for submission of monthly report of higher chance results to NCARDRS.
Standard 5: test turnaround time (T21/T18/T13 screening). Laboratory responsibility: data source and responsible for submission to NHS FASP programme as part of annual standards data reporting process.
KPI FA1 is: ‘The proportion of laboratory request forms providing complete data prior to screening analysis and submitted to the laboratory within the recommended timeframe of 10+0 to 20+0 weeks’ gestation’.
Although this KPI is not a reflection of the performance of the laboratory, the laboratory provides the data for completion of the statistics.
NHS FASP screening standards that are relevant for the T21, T18 and T13 diagnostic (genomic) laboratories are as follows:
Standard 9a,b,c,d: Diagnose (T21/T18/T13 screening and 18+0 to 20+6 week fetal anomaly ultrasound). Laboratory responsibility: data source (submitted annually via Association for Clinical Genomic Science).
18.1 Reports to NCARDRS
All biochemical screening laboratories must provide a monthly report of all higher chance results from the NHS FASP T21, T18 and T13 screening pathway using either the combined or quadruple test. This report must use the dataset requirements provided by the NCARDRS team for antenatal screening.
19. Screening safety incidents
A screening safety incident is any unintended or unexpected incident(s) that could have or did lead to harm to one or more persons who are eligible for NHS screening – or to staff working in the screening programme.
A screening safety incident can affect populations as well as individuals. It is an actual or possible failure in the screening pathway and at the interface between screening and the next stage of care.
Although the level of risk to an individual in an incident may be low, because of the large numbers of people offered screening, this may equate to a high corporate risk. It is important to ensure that there is a proportionate response based on an accurate investigation and assessment of the risk of harm. Due to the public interest in screening, the likelihood of adverse media coverage with resulting public concern is high even if no harm occurs.
A screening laboratory must have an incident management policy in line with managing safety incidents in NHS screening programmes. This guidance outlines how screening incidents should be reported and investigated. The escalation procedure within the host trust should be described and an up-to-date list of incidents and their associated investigation reports maintained.
Local risk management policies require staff to assess the risk associated with laboratory processes and how they can be improved within the laboratory. One way is to prepare a risk assessment of the screening pathway in the laboratory to identify risks and review how they could be mitigated.
Lessons to be learnt from screening incidents are shared via the PHE Screening blog.
Laboratories are also expected to inform the Medicines and Healthcare Products Regulatory Agency (MHRA) of any adverse incidents that causes, or has the potential to cause, unexpected or unwanted effects involving the safety of patients or other persons.
