Vaccine update: issue 323, June 2021
Published 1 July 2021
England is a world leader in childhood vaccinations, with one of the most comprehensive programmes in the world
The programme is under constant surveillance to make sure that we are giving the right number of doses at the right time, to give the best possible protection against infectious diseases.
As new vaccines are available we introduce them to the programme and we now are able to protect against 16 diseases, giving protection throughout the life course.
Unfortunately, however, Public Health England (PHE) data declined across the age groups with a decline in both infant and childhood vaccines and vaccines given to older people such as the shingles vaccine. Adolescents are also at risk if they have missed their Meningococcal A, C, W and Y (MenACWY), measles, mumps and rubella (MMR), teenage booster or the human papillomavirus (HPV) vaccines. School teams have worked tirelessly to make vaccination appointments to catch up but there are still children and young people who may have missed out.
PHE data show that, although coverage remains high, children’s vaccine uptake has been slowly decreasing since 2012 to 2013. This decline has been impacted by the pandemic resulting in more infants and children who are under-vaccinated. This means that some children have missed out and may remain vulnerable to serious or even fatal infections that are vaccine-preventable.
As the population leaves lockdown and more people circulate, there is a greater risk of outbreaks of infectious diseases such as measles. We have seen a greater volume of families attending A&E centres due to winter-type viruses such as Respiratory Syncytial Virus (RSV) and bronchitis, which demonstrates that viruses other than COVID-19 are circulating again.
Despite media speculation that anti-vaccine groups and social media messaging may be damaging the immunisation programme, there is currently no evidence of a major impact on parental confidence in England.
Parental confidence in the national immunisation programme is at an all-time high and our work shows that parents trust the information they get on vaccination from their healthcare professionals over and above any other channel. Part of the reason for the decline in coverage is therefore related to how people can best access and use local services and their knowledge of the availability.
In 2019 the Royal Society of Public Health (RSPH) undertook a survey, included in a report, to identify barriers to vaccination across the life course. The report stated that accessibility and convenience of vaccination services can be important determinants of vaccine uptake and this may be particularly true for parents who are not explicitly anti-vaccination, but perhaps are more questioning, as reassurance from a healthcare professional (usually a nurse) is the most effective way of encouraging them to vaccinate.
Based on that survey, the most common barriers to getting vaccinated were:
- 49% reported timing of appointments
- 46% reported availability of appointments
- 29% reported childcare duties
Healthcare workers who work in GP surgeries also acknowledged that parents, especially those in work, struggle with busy schedules.
Top 10 tips for increasing vaccine uptake
There are various actions you can take and it is important to revisit these 10 points periodically and consider if there is anything your practice can adopt or change to maximise your uptake and get everyone up to date with their vaccinations.
1. Send invites and reminders
Ensure that everyone is invited for their vaccination in good time, and that those who do not attend when they are due get a reminder. This may mean ensuring that your IT system is configured correctly for call and recall or checking local arrangements with your Child Health Records Department.
Evidence shows that call and recall is the single most effective intervention to increase vaccine uptake. If you have a high rate of non-attendance for vaccine appointments, you could consider using text message reminders (e.g. iPlato), and including information about the cost of missed appointments, as this can significantly reduce the rate of non-attendance.
2. Check children, young people, pregnant women and older adults are up to date
When children and young adults (particularly those up to the age of 25 years), pregnant women and older adults visit the GP practice, receptionists, practice nurses and GPs can use this opportunity to check whether they’re up to date and offer them an appointment for any missing vaccinations. Sounds simple, but making sure everyone is doing their bit to check status can really make the difference in getting coverage rates high.
3. Check your IT system flags when people have missed out
Check with your IT supplier that your system is configured correctly to flag patients with outstanding vaccinations. For those using EMIS, videos on how to do this for shingles are available.
Similar advice for TPP/SystmOne practices will be available shortly.
4. Record vaccinations with the appropriate codes
Vaccinations that are not recorded using the appropriate Read 2, CTV 3 or SNOMED codes aren’t counted in uptake figures and may make your practice coverage seem lower than it actually is. This is particularly important when patients move to the UK from abroad.
PHE has released an online tool to support the coding of vaccines given abroad.
5. Plan sufficient appointments
Assess your practice register to make sure you have sufficient appointments slots to vaccinate everyone. If you are aware of insufficient capacity or you have waiting lists for vaccination at your practice, seek support from your local clinical commissioning group. Evidence suggests that practices with more clinic slots have higher coverage.
6. Speak confidently about vaccines
Keep up to date with the latest information about vaccines and vaccination so you and your staff feel confident when answering questions from patients. This can be done by subscribing to Vaccine update and by regularly checking the PHE immunisation collection on GOV.UK.
7. Provide leaflets, stickers and posters
Evidence suggests that NHS and PHE leaflets and posters are among the most trusted sources of information among parents of young children. These can be ordered free of charge for your waiting room so that your patients have a clear visual reminder about the need for routine and selective vaccination.
It is really important for families and individuals to understand what is offered, why it is important and to be encouraged to be fully vaccinated at every stage of the life course. Both paper and digital copies (that can be used on your practice website or waiting room TV screen) are available.
Some of the material is also available in multiple languages. It is important to provide accessible leaflets for people who are blind or partially sighted such as braille. We have large print available for older adults, simple text versions for people with low literacy, and easy read versions for people with a learning disability.
We also have translated versions available to download and print and as paper copies for the COVID-19 and flu programme. We hope to expand on this range this year to include the infant and childhood programme resources. Currently the HPV and MMR leaflets are available in a range of translations.
There are posters, leaflets, stickers and a range of accessible publications to support the routine and selective programmes. We also have video versions in British Sign Language (BSL) available to download and show on screens or on your websites.
Visit our Health Publications website and register your practice and see what is available.
8. Create a call-back system
Create a system where a nurse or doctor can phone back and discuss vaccination with parents or patients who are unsure. A strong recommendation from a healthcare worker is effective at encouraging people to get vaccinated. Consider offering your reception staff training so they understand the importance of vaccination and can encourage people to attend too.
9. Signpost people to online resources
There are several online resources to signpost people to if they want to read more about vaccination, including the NHS.UK vaccination page and the Oxford Vaccine Knowledge project. You can also make these sites available to people on your practice website.
10. Offer additional and more flexible appointments
Check that you are offering more convenient clinic times for people, such as evenings and weekends, for vaccination appointments. The RSPH report found that those GPs who offered more flexible appointments were effective in overcoming the barrier for most people.
As the most trusted source of advice on immunisations, healthcare professionals are on the frontline in reaching those not yet vaccinated, but we all have a role to play. We must all speak confidently about the value of vaccines and leave the public in no doubt that they are safe and save lives.
By working together with the whole practice team you can help to engage people in your area, including those in under-served and high risk groups, and ensure that they are protected against potentially serious diseases.
Throughout the pandemic it has been clear that working with trusted local stakeholders, charities, religious leaders including food-banks providers, St Johns Ambulance and Community Champions has contributed to higher uptake of the COVID-19 vaccinations through trusted messaging.
Working with the relevant organisations and teams in your area may also help to increase uptake of the routine and selective immunisations on our national immunisation schedule.
Top 10 tips were adapted from the Health Matters blog.
Impact of COVID-19 on childhood vaccination
Impact of COVID-19 on childhood vaccination data to April 2021
Early vaccine coverage data uploaded on ImmForm is extracted at 6 months of age to assess vaccine coverage for Hexavalent vaccine doses 1 to 3, and at 18 months to assess vaccine coverage for MMR1.
Vaccine coverage data extracted from ImmForm up to and including April 2021 indicates that:
87.9% of infants completed the 3-dose course of Hexavalent vaccine by 6 months of age. This is 0.9 percentage points lower compared to April 2019 and 3.8 percentage points higher compared to April 2020.
For children scheduled to receive MMR1 vaccine from March 2020 onwards, vaccine coverage measured at 18 months of age remains approximately 86.0%.
In April 2021, 86.7% of infants were vaccinated with MMR1 by 18 months of age, 1.1 and 0.9 percentage points lower than April 2019 and April 2020, respectively.
All children who have missed out on their routine vaccinations during the COVID-19 pandemic remain eligible for their vaccines. As physical distancing and lockdown measures change throughout the course of the pandemic, it is possible that there may be further impact on primary immunisations.
It is therefore important for GPs and local teams to continue offering routine immunisations, check that any infants or children impacted during the pandemic are rescheduled for their immunisation and, where required, consider implementing catch-up or recovery plans.
Impact of COVID-19 on childhood vaccination counts up to week 19, 2021
This report presents vaccination counts data up to week 19 of 2021 (compared with the same period in 2020 and 2019) in the charts below, and should be read in conjunction with the[last published full report: Impact of COVID-19 on childhood vaccination counts to week 17, and vaccine coverage to March 2021 in England, Health Protection Report 15(8).
The charts below show that:
-
the number of dose 1 Hexavalent vaccine doses delivered in England in week 19 (Figure 1) were lower than during the same week in 2019 but higher than during the same week in 2020. The overall difference in vaccination counts delivered in 2021 was lower than in 2019, though slightly higher than in 2020 (Figures 1 and 3)
-
the number of dose 1 MMR vaccine doses delivered in England in week 19 (Figure 2) were lower than during the same week in 2019 and 2020. The overall difference in vaccination counts delivered in 2021 was lower than in 2019 but higher than in 2020 (Figures 2 and 4)
-
in 2021, vaccination counts for Hexavalent and MMR vaccine were 6.5% and 18.1% lower in week 19 in 2021, compared to week 19 in 2019, respectively
It should be noted that a drop in birth rates associated with the pandemic and the public health measures enacted is expected, with the greatest drop likely to occur in women delivering babies in December 2020 (infants first eligible in February 2021). Any drop in births compared to previous years may result in a drop in vaccinated infants.
In figures 1 and 2 it should be noted that direct comparisons for the weekly data between 2019, 2020 and 2021 should be made with caution since the days do not map the same weeks (week 1 data for 2021 only accounts for 3 days).
Also, that school holidays (often coinciding with family holidays) are for the 2020 calendar year. These holidays may vary slightly by year and by local area. School holidays for the 2019 to 2020 academic year were in weeks 43, 52, 53, 1, 8, 15, 16, 19, 22, 30 to 36. School holidays for the 2020 to 2021 academic year are in weeks 44, 52, 53, 7, 13, 14, 18, 22, 29 to 35.
Hexavalent vaccination counts to week 19
Figure 1 below shows weekly dose 1 Hexavalent vaccination counts in infants younger than 6 months in The Phoenix Partnership (TPP) practices open in both 2019 and 2020, or both 2019 and 2021, in England: 2019, 2020 and 2021. School holidays for the 2020 calendar year are shown in grey (see Health Protection Report 15(8) report for full notes on this graph).
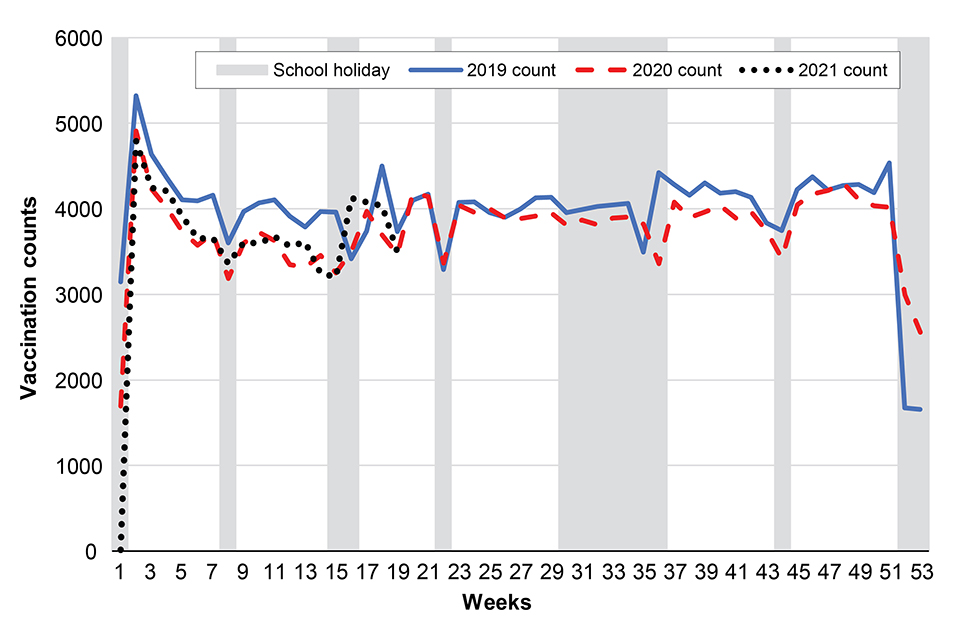
Figure 1
MMR vaccination counts to week 19
Figure 2 below shows MMR 1 vaccination counts in infants aged 12 to 18 months in TPP practices open in both 2019 and 2020, or both 2019 and 2021, in England: 2019, 2020 and 2021. School holidays for the 2020 calendar year are shown in grey (see (see Health Protection Report 15(8) report for full notes on this graph).
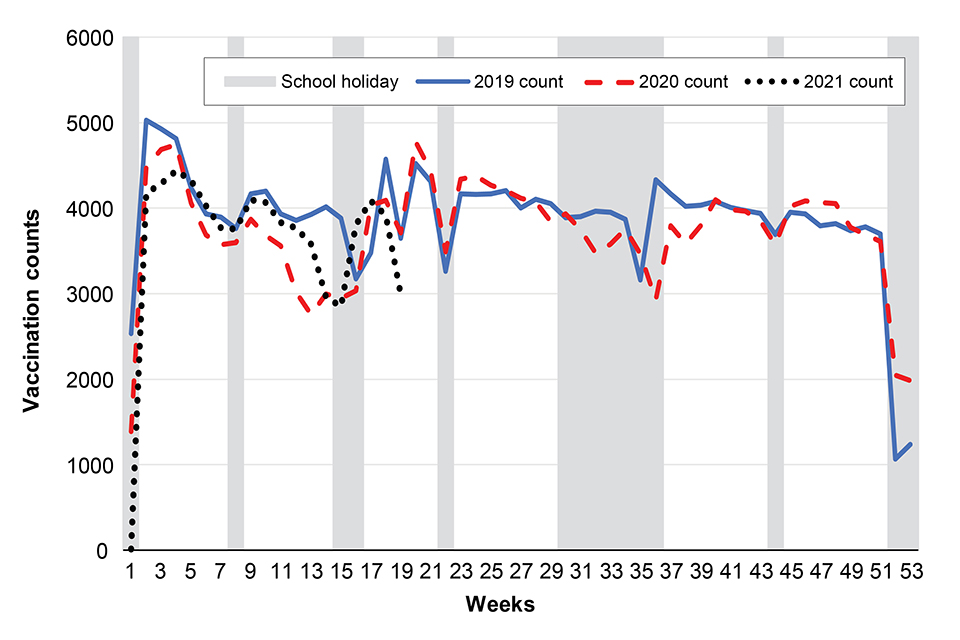
Figure 2
Percentage change in vaccination counts in 2021 and 2020 compared with 2019, to week 19
Figure 3 below shows percentage change in dose 1 Hexavalent vaccination counts (in infants under 6 months) in 2021 and 2020 compared to 2019, by week, in TPP practices in England. (Direct comparisons for the weekly data between 2019, 2020 and 2021 should be made with caution since the days do not map the same weeks: week 1 data for 2021 only accounts for 3 days.)
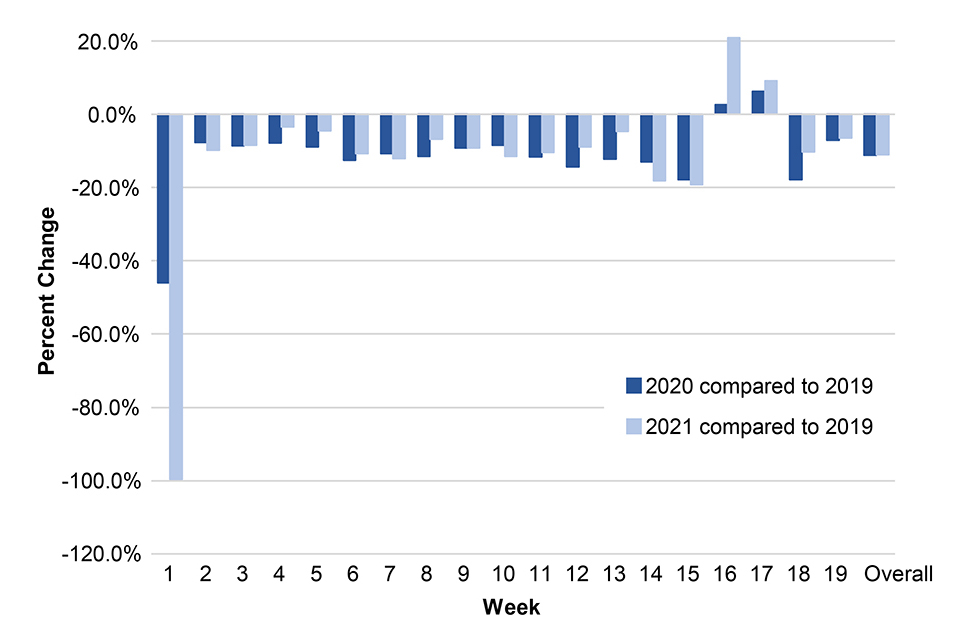
Figure 3
Figure 4 below shows MMR 1 vaccination counts (in infants aged 12 to 18 months) in 2021 and 2020 compared to 2019, by week, in TPP practices in England. (Direct comparisons for the weekly data between 2019, 2020 and 2021 should be made with caution since the days do not map the same weeks: week 1 data for 2021 only accounts for 3 days.)
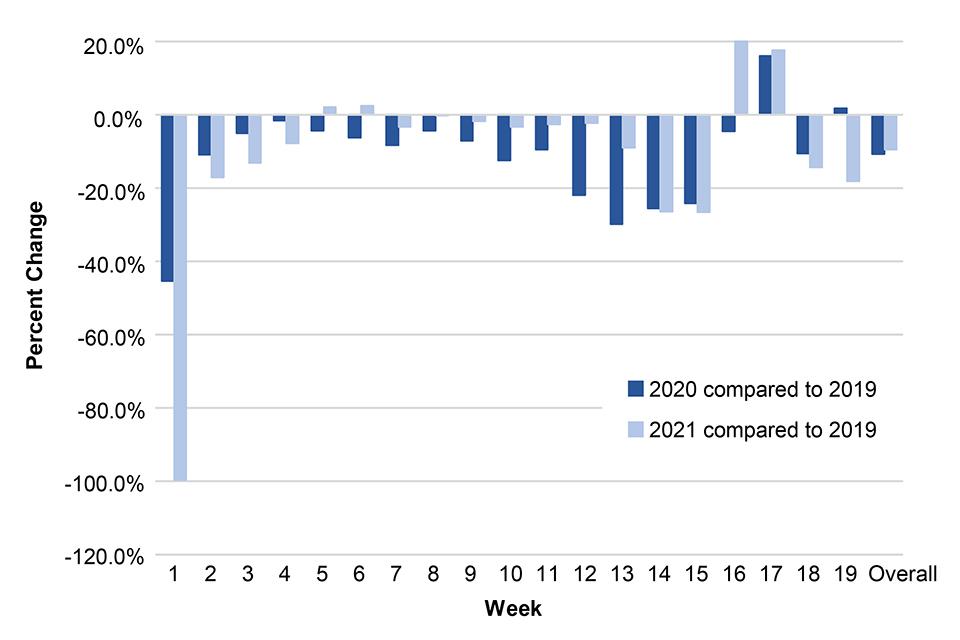
Figure 4
Methods
Aggregated weekly vaccination counts from 2019, 2020 and 2021 for dose 1 Hexavalent (diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b (Hib) and Hepatitis B) delivered to infants 6 months and younger, and MMR1 (first dose of measles, mumps and rubella vaccine) to children between the ages of 12 and 18 months, were provided by the GP information technology supplier, TPP. Weekly trends in vaccination counts were compared between 2019, 2020 and 2021.
TPP represents approximately 38% of data for all GP practices in England and some regions are less represented than others.
Meningitis B guidance and slide set updated
The meningitis B information document for healthcare professionals and the meningitis B slide set have been updated. The information document now includes an updated section on catching up on missed doses of Bexsero vaccine, hyperlinks to relevant resources and an updated reference list.
The meningitis B training slide set includes information on meningitis B and the vaccination programme.
Rotavirus vaccine Patient Group Direction (PGD)
Version 05.00 of the Rotavirus vaccine (Rotarix®) PGD template is available on GOV.UK. This replaces version 04.00, which expires at the end of this month.
This PGD template requires organisational authorisation in line with the Human Medicines Regulations 2012 before they are a legally valid PGD. It is advised that PHE PGD templates are organisationally authorised in accordance with local procedures before sharing with providers.
Please note that PHE PGD templates now include an extended list of practitioners who may be authorised to operate under the PGD (see section 3). These have been included to reflect the expanded roles of allied health practitioners and to allow greater flexibility to commissioners of immunisation services. Authorising organisations may choose to limit the practitioners that are authorised to work to the PGD such as to reflect local commissioning arrangements. This is optional and can be detailed in the limitations to authorisation (see section 2).
Authorising organisations must not alter, amend or add to the clinical content of this document (sections 4, 5 and 6); such action will invalidate the clinical sign-off with which it is provided. In addition authorising organisations must not alter section 3 ‘Characteristics of staff’. Only sections 2 and 7 can be completed within the editable fields.
Revised MenACWY vaccine (Menveo® or Nimenrix®) PGD template
This PGD template supports the administration of MenACWY conjugate vaccine to individuals eligible for national routine MenACWY vaccination programme and university freshers (catch-up) programme. It also covers administration of MenACWY vaccine for outbreak control and contacts of confirmed cases, in accordance with the guidance for the public health management of meningococcal disease in the UK.
The MenACWY PGD V04.00 is valid from 1 July 2021 to 31 July 2023.
Practitioners must not use this PGD template until it has been authorised in Section 2. This is a legal requirement in line with the Human Medicines Regulations 2012. Practitioners should follow local policy/procedures to access authorised PGD documents.
This PGD template should be used with reference to current national guidance, the Green Book, and summary of product characteristics.
Social media cards for university students
There are 4 designs of the social media cards suitable for digital displays and social media or websites available to download and share.
Green Book of Immunisation – chapter 2 consent
The consent chapter of the Green Book has been updated.
Revisions include:
- changed the introduction to include reference to the General Medical Council (GMC) maintained factsheet on relevant legislation and case law
- new section on the principles of consent which includes guidance on how information should be shared and what information to include
- updated section on ‘Who can give consent’, to include updated information on the Mental Capacity Act (MCA) with supporting references, updated supporting references for 16 and 17 year olds and updated supporting references on Gillick competency
- previous section on ‘other issues’ has been divided into new sections on consent at the time of immunisation and disagreement between parents
- edits to the section recording consent
- edits to the guidance and resources section to include updated supporting information
- updates to the sections on consent for all 3 Devolved Administrations
Learning disability week
This year’s theme was art and creativity and it is clear that the role of the creative arts has been significant in helping people with a learning disability and their families to stay connected, support social interaction, provide welcome opportunities to engage with others and stay positive during lockdowns and the pandemic.
It is an opportunity to reflect on the resources we provide for children and adults with a learning disability to have their routine vaccinations. We have a full suite of resources for the COVID-19 vaccination and annual flu programmes.
We hope to progress further resources for the infant and preschool core vaccinations over the coming months.
Reasonable adjustments
We have developed 2 resources for use by clinicians and non-clinicians supporting the COVID-19 vaccination programme. They provide top tips on communicating with people with a learning disability and autistic people and highlight the range of reasonable adjustments that should be considered to ensure that a vaccination appointment goes well.
The Misfits Theatre company are a theatre and social group led by people with learning difficulties. They are recognised for their role as providers of unique training solutions, working with both private businesses and the public sector. Their aim is to enable people with learning difficulties to be creative, have fun and reach their potential through participation, performance, volunteering and employment.
They offer creative workshops for people with learning difficulties.
The Misfits Theatre Company have been telling us how reasonable adjustments can help people with a learning disability and autistic people live longer and healthier lives.
Reasonable adjustments are a legal requirement to make sure health services are accessible to all disabled people. Please watch the film below to find out how a simple reasonable adjustment can make a big difference to a person’s experience of quality and access to care.
Information video about the coronavirus vaccine for people with a learning disability and autistic people
This is a short film (weblink 24) which talks about coronavirus and the coronavirus vaccine. It describes how important it is to have the vaccine and what you should do after you’ve had the vaccine. Everyone on the GPs learning disability register will be invited to have their Coronavirus vaccination if they haven’t already had it.
Vaccine supply for routine vaccination programmes
PPV23 vaccine for the pneumococcal programme is now supplied by PHE
Since 1 June 2021, pneumococcal polysaccharide vaccine (PPV23; Pneumovax®23) for the routine immunisation programme and immunisation of those with underlying medical conditions has been supplied by PHE via ImmForm. This replaces local PPV23 procurement from wholesalers. Pneumovax®23 supplied by PHE is presented as single units of solution for injection in a pre-filled syringe (PFS).
Clinical prioritisation for PPV vaccination remains in place. Refer to the Vaccine update: issue 318, March 2021 for details of this prioritisation. Refer to chapter 25 of the Green Book for further details of the PPV23 vaccination programme.
Ordering controls are initially in place for PPV23 to enable PHE to balance incoming supplies and outgoing orders. We will ease or remove these ordering controls as soon as demand stabilises. A maximum ordering allocation has been assigned to each ImmForm account. These maximum ordering allocations will be renewed by 1 August 2021 once further supplies are received.
Please see ImmForm for the most up to date information on ordering controls.
Additional orders and out-of-schedule deliveries
Requests for extra vaccine will only be considered on a case by case basis. Requests should be emailed to the ImmForm Helpdesk at helpdesk@immform.org.uk. Allow sufficient time before your order cut-off. Out of schedule deliveries will be by exception only.
Viper antivenom has changed
The viper antivenom product supplied via ImmForm has recently changed from ViperaTAb® to Viperfav®. The products have different active ingredients, formulations and presentations:
| Product | ViperaTAb® | Viperfav® |
|---|---|---|
| Source of immune sera | Ovine | Equine |
| Licensed status | Unlicensed in the UK | Unlicensed in the UK |
| Storage | Store in a refrigerator between 2°C and 8°C | Store in a refrigerator between 2°C and 8°C |
| Presentation | Each pack includes 2 x 4ml vials, containing 100mg F(ab’) 2 fragments each | Each pack includes 1 x 4ml vial containing F(ab’) 2 fragments |
| Initial treatment recommendation | The initial dose of ViperaTAb® is the contents of 2 x 4ml vials (i.e. 1 pack per patient) | The initial dose of Viperfav® is the contents of 1 x 4ml vial (i.e. 1 pack per patient) |
Recommendations for the treatment of common adder bites and the administration of Viperfav® can be found on TOXBASE.
To minimise wastage, please use all locally held stocks of in date ViperaTAb® to treat eligible patients, before switching to Viperfav®.
Change to dTaP/IPV vaccine for both the preschool booster and maternal pertussis dTaP/IPV programmes
Boostrix-IPV® is currently supplied for both the preschool booster and maternal pertussis dTaP/IPV programmes. This has recently changed from Repevax®.
The 2 vaccines are equivalent. To minimise wastage, please use all your locally held stocks of Repevax® to vaccinate eligible individuals, before switching to Boostrix-IPV®. There is no other change to the preschool booster or maternal pertussis immunisation programme.
Further details about this programme can be found in chapter 24 of the Green Book
Update to Bexsero Patient Information Leaflet
Every pack of Bexsero (Meningitis B vaccine; 10 doses) is supplied with a pad of 10 Patient Information Leaflets (PILs), as well as there being a single PIL inside each Bexsero pack. Since September 2020, an updated version of the PIL pad has been distributed with Bexsero orders. Please dispose of the single PIL from inside the pack, as it will be out-of-date.
We will advise further when the PIL supplied in the pack is in line with the PIL pad.
MMR vaccine ordering
To rebalance central supplies of both MMR vaccines please consider ordering M-M-RvaxPRO® as your first choice, which is available without restriction. Customers in England and Wales who require Priorix®, for example because you serve communities that do not accept vaccines containing porcine gelatine, may order up to 6 packs of Priorix® per ImmForm account per week.
For assistance please contact the ImmForm Helpdesk at helpdesk@immform.org.uk. Customers in Scotland should refer to their local ordering restrictions.
The EU Falsified Medicines Directive (FMD) and Delegated Regulation as applicable to PHE supplied vaccines for the national immunisation programme
From 11pm on 31 December 2020, when the UK’s EU exit transition period ended, the ‘safety features’ Delegated Regulation (2016/161) under the EU Falsified Medicines Directive (FMD; 2011/62/EU) no longer applied in Great Britain.
This means that in Great Britain, end users of the majority of prescription-only medicines, including the FMD-compliant products supplied by PHE via ImmForm, are no longer required to verify or decommission the unique identifiers on serialised packs. Serialised packs can nonetheless continue to be dispensed for as long as they are still in date.
Registering for a new or updating your existing ImmForm vaccine ordering account
When you register for or update an existing ImmForm account, Public Health England as a wholesaler of vaccines need to verify the requesting customer.
Please ensure you have your professional regulatory body registration number or Wholesaler Dealer Licence and an organisation code which can be verified when requesting updates or requesting a new vaccine ordering account. For more information please see the ImmForm how to register helpsheet.
Movianto UK drivers delivering ImmForm products are not able to phone delivery points
Please note that Movianto UK drivers delivering ImmForm products are not able to phone a delivery point upon arrival at the delivery location. Customers are expected to make arrangements ahead of the scheduled delivery day to receive their deliveries.
ImmForm customers should report long-term changes to opening hours for deliveries
Customers should report long-term changes to the days and times when they can accept deliveries, such as routine training days and closures, by contacting Movianto UK Customer Care at MoviantoUK.NHSCC@movianto.com.
Customers are reminded to be prepared for any break in deliveries due to absences or holidays and to order accordingly. Please make sure you have sufficient room in your fridge for any additional vaccine you wish to stock. Deferred orders can also be placed in advance. Out of schedule deliveries cannot be arranged for failure to place orders in good time.
Vaccine supply for the non-routine programme
Hepatitis A vaccine
Adult
- GSK: Havrix adult PFS singles and packs of 10 are available
- Sanofi Pasteur: Avaxim PFS singles and packs of 10 are available
- MSD: VAQTA adult is available
Paediatric
- GSK: Havrix paediatric PFS singles and packs of 10 are available
- MSD: VAQTA paediatric is available
Hepatitis B vaccine
Adult
- GSK: Engerix B PFS singles and packs of 10 are available
- GSK: Engerix B vials singles and packs of 10 are discontinued
- GSK: Fendrix is available
- MSD: HBVAXPRO 10 µg is available
- MSD: HBVAXPRO 40 µg is available
Paediatric:
- GSK: Engerix B paediatric singles are available
- MSD: HBVAXPRO 5µg is available
Combined hepatitis A and B vaccine
- GSK: Twinrix adult singles and packs of 10 are available
- GSK: Twinrix paediatric is available
- GSK: Ambirix is available
Combined hepatitis A and typhoid vaccine
- Sanofi Pasteur: Viatim is available
Typhoid vaccine
- Sanofi Pasteur: Typhim singles and packs of 10 are available
- Emergent: Vivotif is available (expiry 30 September 2021) to order until 30 June 2021. After this an OOS is expected until middle of 2022
Rabies vaccine
- Valneva: Rabipur is currently available
- Sanofi Pasteur: Rabies BP is currently available
Pneumococcal polysaccharide vaccine (PPV)
- MSD: Supply of Pneumovax 23 (PPV23) PFS is currently limited (Please refer to ImmForm for National Immunisation Programme (NIP) supply status)
Pneumococcal polysaccharide conjugate vaccine (PCV)
- Pfizer: Prevenar 13 is currently available
Varicella Zoster vaccine
- GSK: VARILRIX is currently limited
- MSD: VARIVAX is available
- MSD: ZOSTAVAX is currently available
Diphtheria, tetanus and poliomyelitis (inactivated) vaccine
- Sanofi Pasteur: Revaxis is available
Diphtheria, tetanus, pertussis (acellular) and poliomyelitis (inactivated) vaccine
- GSK: Boostrix-IPV is currently limited
- Sanofi Pasteur: Repevax is available
MMR vaccine
- MSD: MMR Vaxpro is currently available
- GSK: Priorix is currently available
Meningitis ACWY vaccine
- GSK: Menveo is available
- Pfizer: Nimenrix is currently available
Yellow fever vaccine
- Sanofi Pasteur: Stamaril is available
Human papillomavirus vaccine (HPV)
- MSD: GARDASIL has been discontinued. Please note this applies only to non-routine supply. GARDASIL is still available to order via ImmForm for the routine HPV vaccination programme.
- MSD: Gardasil 9 is currently available
- GSK: Cervarix has been discontinued
Cholera vaccine
- Valneva: Dukoral is available
Japanese encephalytis vaccine
- Valneva: Ixiaro is available
