MAVIS Hub Edition 119
Published 2 August 2021
1. Product News
Prid Delta 1.55 g Vaginal Delivery System for Cattle - Product defect recall alert
Salmovac 440 Product defect recall alert
Availability of Lactating Cow Intramammary Antibiotics
2. Enforcement
Animal medicine seizure notice: Mr Hinds, Cinderford, Gloucestershire
Animal medicine seizure notice: Parcel addressed to Belfast, County Antrim
Animal medicine seizure notice - Parcel addressed to Craigavon, County Armagh
Animal medicine improvement notice: Swinfen Veterinary Centre
Animal medicine improvement notice: Corner Veterinary Centre
Animal medicine seizure notice - Parcel addressed to Ballymena, County Antrim
Animal medicine seizure notice: Dr Grant, West Wickham, Kent
3. AMR (Antimicrobial Resistance)
3.1 Communications
The VMD is supporting RCVS Knowledge’s Farm Vet Champions initiative on AMR, which has recently been launched. The project promotes the appointment of Farm Vet Champions (FVCs) within practices throughout the UK to unite and empower UK farm animal veterinary practitioners as they establish good antimicrobial stewardship in practices and on farms. The VMD is also supporting the Farm Vet Champions promotional campaign to raise awareness of the project.
3.2 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met virtually on 8 June 2021 to discuss the recent findings in antibiotic resistance (AMR) in bacteria of importance to animal and public health. The meeting included updates from the VMD on the delivery of the National Action Plan. Guest speakers from Scotland, Wales and Northern Ireland also provided updates on farm level usage in their respective areas and an update was provided on the Arwain Vet Cymru and Farm Vet Champions schemes. Additionally, guest speaker Alison Mather from the Quadram Institute provided a presentation on the genomic epidemiology and mobility of AMR across species. Minutes from previous meetings are available on GOV.UK.
3.3 Policy
The UK’s hosting of the G7 Presidency has seen commitments on AMR made in the health ministers’ declaration, the climate and environment ministers’ declaration and the finance ministers’ declaration These include commitments on AMR in the environment stemming from antimicrobial drug manufacturing, consideration of options for strengthening supply chains, and exploration of antimicrobial valuation principles and market incentives. The VMD will contribute to cross-government efforts to implement these commitments, where relevant to our work area, over the coming months.
4. Inspections
During the period from 01/04/21 to 30/06/21 the Distribution and Supply Chain Inspection Section carried out a total of 215 inspections of retail and feed business premises to assess their compliance with the Veterinary Medicines Regulations 2013. Due to the current pandemic, most of these inspections were conducted as remote assessments. However, on-site routine inspections have now re-started.
4.1 Retail Premises
We inspected 161 retail premises (veterinary practice premises and SQP retailers) and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 74 | 46.0% |
| 4 | 71 | 44.1% |
| 3 | 15 | 9.3% |
| 2 | - | 0% |
| 1 | 1 | 0.6% |
The most common deficiencies that required corrective action were:
- Controlled Drug (CD) Registers not maintained as required
- Broach dates exceeded or not recorded
- Cascade labels either missing or missing required information
The requirements for retailers are published on Gov.UK
4.2 Feed Business Operator premises
We inspected 54 Feed Business Operator (FeBO) premises and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 20 | 37.0% |
| 4 | 25 | 46.3% |
| 3 | 8 | 14.8% |
| 2 | 0 | - |
| 1 | 1 | 1.9% |
The most common deficiencies that required corrective action were:
- Supply against incomplete Medicated Feedingstuffs Prescriptions /invalid prescriptions
- HACCP plan not documented/implemented/maintained as required
- Feed stability issues
The requirements for Feed Business Operator (FeBO) premises are published on GOV.UK
5. Guidance Updates
Important Information for applicants of marketing authorisations: New Applications
UK-Canada Trade Continuity Agreement (TCA)
Veterinary medicine wholesale dealer’s authorisation (WDA)
Reminder: CAP Conversion Conditions
Fees applied to animal medicine authorisation applications
Report a product defect: veterinary medicine
Controlled drugs: recording, using, storing and disposal
Submission of an application for an animal medicine authorisation
Resistant bacteria from animals of possible risk: Contingency plan
Parallel submission of generic applications
Application and authorisation information hub explainer
6. Statistics
VMD Published Standards 2021 to 2022: Monitoring performance
Residues of veterinary medicines in food:2021
7. Stakeholder engagement
Vets: help us to improve the Special Import Service
8. Corporate News
Job Advert for 2 Senior Scientific Officers
Job Advert Senior Scientific Officer
Vacancy for Head of Policy and Communications in the Antimicrobial Resistance Team
Vacancy for an Antimicrobial Use, Stewardship and Stakeholder Engagement Manager
Veterinary Medicines Directorate annual report and accounts 2020 to 2021
Vacancy for Higher Scientific Officer in the Biologicals Team
9. Organogram as at 1 August 2021
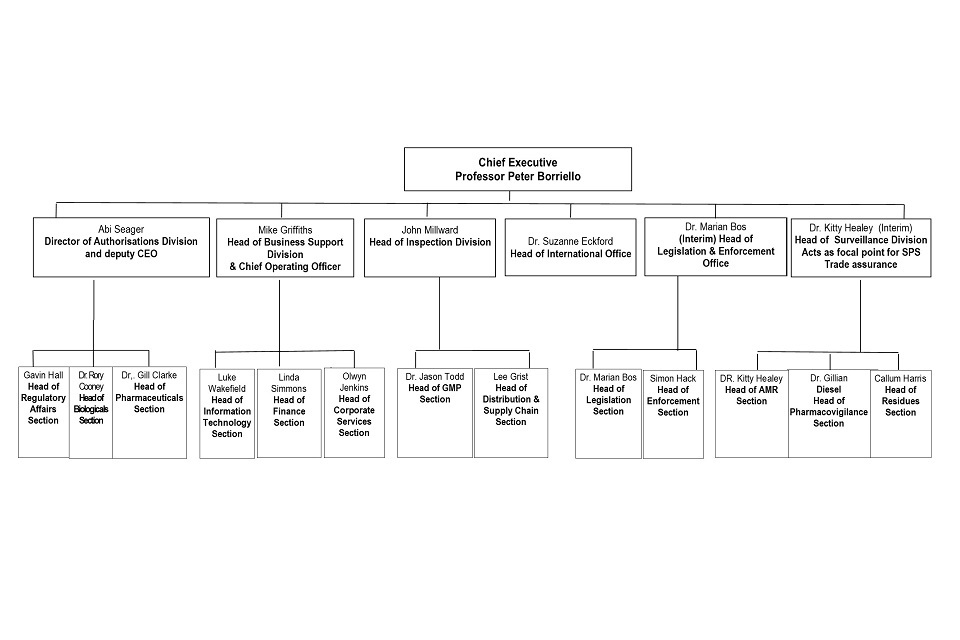
VMD Organogram as at 31 July 2021
