Nutrition and health claims: guidance to compliance with Regulation (EC) 1924/2006
Updated 10 November 2021
Nutrition law
Following the UK’s departure from the EU on 31 January 2020, the UK entered a time-limited transition period until 31 December 2020. Now the transition period has ended, regulation is an autonomous matter for both the UK and EU as 2 separate legal and regulatory systems. The government remains committed to promoting robust food standards nationally and internationally, to protect consumer interests, and to ensure that consumers can have confidence in the food they buy.
Section 1 of this guidance outlines for businesses those changes relating to nutrition and health claims from 1 January 2021.
Executive summary
Introduction
On 30 December 2006, a Regulation of the European Parliament and of the Council of the European Union on nutrition and health claims made on foods was published. On 1 January 2021 ‘The Nutrition (Amendment etc.) (EU Exit) Regulations 2019’ and ‘The Nutrition (Amendment etc.) (EU Exit) Regulations 2020’ transferred responsibilities from EU organisations involved in the risk assessment and risk management processes covered by nutrition legislation to bodies in Great Britain (GB); and fixed inoperability’s of retained Regulation (EC) 1924/2006 that would otherwise have arisen. This guidance is due to be updated to fully incorporate the exit bulletin and any previous legislative changes. Section 1 of this guidance reflects changes to processes from 1 January 2021.
Intended audience
This guidance is intended to help food business operators, in particular those of small and medium-sized enterprises (SMEs). It may also be of use to others with an interest in the legislation, such as food law enforcement officers.
Purpose of this guidance
This guidance is designed to help you comply with the regulations if you choose to make a nutrition or health claim on your food product. It also explains the requirements for authorisation of new claims. The guidance may be read from cover to cover, but you might find it useful to begin by reading the sections immediately relevant to your questions. Food business operators with specific questions may wish to seek advice from their local food law enforcement agency which will usually be the trading standards or environmental health department of their local authority.
Legal status of guidance
These guidance notes, which should be read together with the legislation, have been produced to provide advice on:
- the legal requirements in respect of nutrition and health claims made on foods
- best practice in this area
The guidance notes on legal requirements cannot cover every situation and you may need to consider the relevant legislation itself to see how it applies in your circumstances. If you do follow the guidance notes they will help you to comply with the law. You are not required by law to follow best practice advice. To distinguish between the 2 types of information, all advice on best practice is in shaded boxes, with a heading of Best Practice. A quick-start guide is available which is an entry point to this guidance.
Section 1: guidance from 1 January 2021
1.1 Using this guidance
This section is to be read in conjunction with the remainder of the guidance which remains relevant and useful, with exception to any references to the EU, in helping businesses comply.
The protocol on Ireland/Northern Ireland (NIP) means that EU legislation relating to nutrition, as detailed in Annex 2 to the NIP, continues to be directly applicable in Northern Ireland.
From 1 January, EU Regulations and tertiary legislation relating to nutrition have been retained under the powers contained within the European Union (Withdrawal) Act 2018 as UK law. That retained EU legislation is subsequently amended by the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020.
This guidance sets out the practical effect of the changes to the legislation for industry, and the processes and procedures which food business operators, and other interested parties, must comply.
The Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020 transferred responsibilities from EU organisations involved in the risk assessment and risk management processes covered by nutrition legislation to bodies in Great Britain (GB).
They also made practical changes that resulted from this transfer to:
- applications
- frameworks for the scientific evaluation of applications/dossiers/files
- the factors taken into consideration when a risk management decision is required
Businesses seeking to submit applications, scientific dossiers, or files in accordance with the legislation covered by this guidance for consideration in the GB market should prepare those applications and requests in line with the advice in this section and submit them to the Department of Health and Social Care (DHSC). DHSC ensures that all documents are shared with the appropriate authorities in Scotland, and Wales, and Northern Ireland and, once deemed valid, the applicable expert committees.
Information is shared with Northern Ireland as all nutrition issues continue to be considered on a 4-nation basis: and, importantly, officials and ministers in Northern Ireland continue to play a vital role in policy development under the arrangements agreed in the UK-wide common framework for nutrition-related labelling, composition and standards (NLCS). Northern Ireland’s full participation in risk assessment and risk management processes ensure that any decisions taken in GB (England, Scotland and Wales) account for the potential impacts across the UK.
1.2 Appropriate authorities
The Nutrition (Amendment etc.) (EU Exit) Regulations 2019 transferred functions and powers previously held by the European Commission to legislate to give effect to a decision, such as whether to authorise applications for new health claims, to the following appropriate authorities.
The appropriate authorities are in:
- England: the Secretary of State
- Scotland: the Scottish ministers
- Wales: the Welsh ministers
Each appropriate authority may, therefore: make legislation equivalent to that which the European Commission would have made previously. However, the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 also provide concurrent powers for the UK Secretary of State to legislate for the whole of GB where devolved administrations in Scotland and Wales agree.
Note: functions and powers held by the European Commission have not been transferred to Northern Ireland. However, officials and ministers in Northern Ireland continue to play a vital role in policy development under the arrangements agreed in the UK-wide common framework for NLCS. Therefore, references are included in this guidance to ‘appropriate UK authorities’ where Northern Ireland officials and ministers are involved in risk assessment or risk management.
The Food Standards Agency (FSA) remains the designated Competent Authority in Northern Ireland.
1.3 Common framework for NLCS
Officials from the UK government and the devolved administrations in Scotland, Wales, and Northern Ireland have jointly developed a UK-wide Common Framework for NLCS.
As a devolved policy area NLCS is one of several identified in the UK government Frameworks Analysis which required more detailed discussion to explore whether a common framework agreement was needed to manage potential divergence within the UK after EU Exit and the governance and decision-making processes required for effective joint working and implementation.
Officials across all 4 nations have worked together to develop the NLCS Common Framework, which was provisionally agreed at the Joint Ministerial Committee (EN) on 3 September 2020.
Risk assessment and risk management (policy development) mechanisms
NLCS risk assessments consider the impact on a UK-wide basis aiming to deliver a consistent approach and process for businesses and enforcement authorities across the UK (with capacity maintained for nation-specific assessments where appropriate).
Decisions based on both scientific opinion and those wider risk management considerations are made by the appropriate authority (namely the Secretary of State, Scottish, Welsh, ministers or as appropriate with consent from the devolved administrations) through the establishment of 4-nation working arrangements which build on existing consensus-based policymaking.
While ministers retain the right to take individual decisions for their nation on areas within the scope of the NLCS Framework, the opportunity for consistency of approach across administrations is always sought in the first instance and where agreed, common policy recommendations made.
The ability to diverge where appropriate and proportionate is retained, while taking account of the impact on consumer safety and confidence, and the functioning of the UK internal market in reaching a final decision.
Dispute prevention and dispute resolution
Every effort is made at working level to resolve any disagreements in difference of approach. It is anticipated that the need for dispute resolution in areas within scope of the Nutrition Framework is unlikely. However, should it be needed, the dispute resolution established by the NLCS Framework will come into play.
1.4 Lists and registers
Where retained EU legislation amended by the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 requires a list or register to be established, each appropriate authority must produce and maintain a list or register.
Decisions made by the appropriate authorities as set out above, will result in the GB lists and registers needing to be updated periodically.
For convenience and clarity GB lists and registers, which consolidate all lists produced and maintained by the appropriate authorities are available on GOV.UK for food business operators and other interested parties.
Businesses may submit applications or dossiers in support of these lists being amended for consideration for use on the GB market to DHSC mailboxes, unless stated otherwise in this guidance. DHSC will centrally coordinate applications.
1.5 Northern Ireland
The Northern Ireland Protocol was published in October 2019 as part of the Withdrawal Agreement to address the ‘unique circumstances on the island of Ireland’.
The UK government published a Command Paper on its approach to the NIP on 20 May 2020 and further information can be found there, in addition to business guidance on GOV.UK.
The NIP was designed as a practical solution to avoiding a hard border on the island of Ireland, whilst ensuring that the UK, including Northern Ireland, could leave the EU as a whole. It therefore included a number of special provisions which apply only in Northern Ireland, for as long as the NIP is in force.
The NIP means that EU legislation relating to nutrition, as detailed in Annex 2 to the NIP, continues to be directly applicable in Northern Ireland.
Section 2 of the nutrition legislation information sheet remains relevant and useful in complying with the compositional and labelling requirements set out in EU law when read alongside the updates in this document relevant in Northern Ireland, such as:
- Making a claim in the European Union or Northern Ireland
- Section 4: how to make a claim
- Section 5: how to a make a nutrition claim
- Section 6: health claims
- Section 7: future control of nutrition and health claims
- Section 8: when do I need to comply with the regulation?
- Section 9: enforcement and compliance
- Section 10: questions and answers
- the appendices of this document
With regards to trade going from Northern Ireland to the rest of the UK: this has not changed. Northern Ireland businesses continue to be able to place their goods on the market throughout the rest of the United Kingdom without new restrictions.
Businesses seeking to submit applications in respect of requests for authorisation for the Northern Ireland or EU27 markets should forward them to the European Commission in accordance with the section on Making a claim in European Union or Northern Ireland.
Northern Ireland’s full participation in risk assessment and risk management processes ensures that any decisions taken in GB (England, Scotland and Wales) account for the potential impacts across the UK.
1.6 Nutrition and health claims for food being placed on the market within the EU from 1 January 2021
Regulation (EC) No. 1924/2006 sets out the legal framework for businesses wanting to make nutrition and/or health claims on their products. This is to ensure that claims made about a product are accurate and consumers are not misled. Nutrition and health claims are required to be based on scientific evidence and may only be used in commercial communications if they have been authorised following scientific assessment of substantiating evidence.
The Community Register of nutrition and health claims made on foods, lists all authorised and rejected claims set out in EU legislation:
- permitted nutrition claims that may be made on foods as listed in the Annex to Regulation (EC) No. 1924/2006
- authorised health claims that may be made on foods, other than those referring to the reduction of disease risk and to children’s development and health as listed in the Annex to Commission Regulation (EU) No. 432/2012
- permitted reduction of disease risk claims and claims referring to children’s development and health as set out in various Commission Regulations
- rejected health claims as set out in various Commission Regulations
- an application to use a claim that is not authorised and listed in the Commission Register for use in the EU, may be submitted via a Member State to the European Commission (EC) for consideration. The European Food Safety Authority’s (EFSA) Panel on Nutrition, Novel Food, and Food Allergens (NDA) conducts the scientific assessment of applications for new claims. EFSA’s scientific opinion is taken into consideration by the EC when deciding whether to authorise or reject an application
1.7 Nutrition and health claims for food being placed on the market within Great Britain from 1 January 2021
In amending Regulation (EC) No. 1924/2006, the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020 make a number of practical changes to the regulatory framework. This guidance sets out how GB’s nutrition and health claim system operates when accounting for those changes.
Sections 2 (introduction and summary), 3 (scope), 7 (future control of nutrition and health claims), and 9 (enforcement and compliance) of this guidance remain relevant and useful, with exception to any references to the EU therein, in helping food businesses to comply with the retained Regulation (EC) No. 1924/2006.
The NIP means that EU legislation relating to nutrition and health claims continues to be directly applicable in Northern Ireland.
Given this, sections 2 to 9 and the appendices in this guidance remain applicable in helping food businesses to comply with EU nutrition and health claims regulations, when read alongside the updates in this document relevant to Northern Ireland, such as making a claim in the EU or Northern Ireland.
1.8 GB Nutrition and Health Claims Register
All nutrition and health claims that are listed in the Community Register, as of 1 January 2021, have been adopted and included in the Great Britain Nutrition and Health Claims Register (GB NHC Register).
This means that where the European Commission had not taken a decision on an application related to a nutrition or health claim by 1 January 2021, a new application must be submitted to the appropriate GB authorities for assessment if the applicant wishes for the claim to be authorised for use in the GB market. Where a scientific opinion regarding the efficacy of a claim is available, from the European Food Safety Authority or other scientific advisory body, this should be included with an application in accordance with Commission Regulation 353/2008 (paragraph 5 of the Annex) and Part 1.7 of the application form. The appropriate UK authorities will determine whether any such opinion is sufficient to inform a risk management decision or if further risk assessment is required.
All authorised and rejected nutrition and health claims will be listed in the GB NHC Register, other than those health claims authorised on the basis of proprietary data which will be recorded in a separate Annex to the GB NHC Register.
The GB NHC Register, and the separate Annex, are available on GOV.UK.
Communication of changes
Any future amendments to the GB NHC Register will be communicated via regular bulletins published on GOV.UK.
1.9 Nutrition claims
Only nutrition claims listed in the GB NHC Register, may be used in GB from 1 January 2021. The only exceptions to this are:
- trademarks or brand names that are also nutrition claims (subject to the conditions of Article 1.3 and Article 28.2 of retained Regulation (EC) No. 1924/2006)
Products that make a nutrition claim must continue to:
-
meet the specific conditions of use as set out in the Annex to retained Regulation (EC) No. 1924/2006
-
present nutrition labelling as required by Article 7 of retained Regulation (EC) No. 1924/2006
-
comply with general conditions set out in Article 5 of retained Regulation (EC) No. 1924/2006 and any general requirements outlined in existing guidance such as those that relate to alcoholic beverages
New GB nutrition claims
The appropriate UK authorities may, after consulting an expert committee, amend the list of permitted nutrition claims contained within the Annex to Regulation (EC) No. 1924/2006 by making regulations. Authorised and rejected nutrition claims will be added to the GB NHC Register.
If you wish to apply for a claim to be authorised for use in the GB market please contact the appropriate GB authorities via the DHSC mailbox (which centrally coordinates applications for all GB nations).
If you wish to submit an application for a claim to be authorised for use in:
-
England only, please contact the competent authority via the DHSC mailbox
-
Scotland only, please contact the competent authority via the Food Standards Scotland mailbox
-
Wales only, please contact the competent authority via the Welsh Government mailbox
The appropriate authority will consult the United Kingdom Nutrition and Health Claims Committee (UKNHCC), and any other appropriate scientific advisory committee (SAC) when considering a new nutrition claim. If a claim is authorised by the appropriate UK authorities and added to the Annex, any specific conditions associated with that claim will apply.
Businesses wishing to make new nutrition claims in the EU or Northern Ireland market following the end of the transition period should refer to the section on making a claim in the EU or Northern Ireland.
1.10 Health claims
Only authorised health claims listed in the Great Britain Nutrition and Health Claims Register may be used in the GB market from 1 January 2021. The only exceptions to this are:
-
general, non-specific claims (subject to the conditions of Article 10.3 of retained Regulation (EC) No. 1924/2006)
-
trademarks or brand names that are also health claims (subject to the conditions of Article 1.3 and Article 28.2 of retained Regulation (EC) No. 1924/2006)
Products that make a health claim must continue to:
-
meet the specific conditions of use as set out in the GB NHC Register
-
present nutrition labelling (subject to the conditions of Article 7 of retained Regulation (EC) No. 1924/2006)
-
comply with any general requirements as set out in the remainder of this guidance document, such as those that relate to alcoholic beverages
Health claims authorised on the basis of proprietary data
Health claims that have been authorised on the basis of proprietary data are listed in a separate Annex to the GB NHC Register. Products that make a health claim authorised on the basis of proprietary data must continue to do all of the following:
-
meet the specific conditions of use as set out in the Annex to the GB Register
-
present nutrition labelling (subject to the conditions of Article 7 of Regulation (EC) No. 1924/2006)
-
comply with any general requirements as set out in the remainder of this guidance document, such as those that relate to alcoholic beverages
New GB health claims
From 1 January 2021 anyone wishing to make a new health claim on a product in GB that is not included in the GB NHC Register must submit an application for that claim to be assessed and authorised before it can be used.
See application forms, which contain supplementary information on completing an application for a claim.
An application may be made for:
- claims based on newly developed scientific evidence, or those which include a request for the protection of proprietary data
- reduction of disease risk claims and claims referring to children’s development and health
Applications seeking authorisation of a claim for use in the GB market should be submitted to the competent authorities via the DHSC mailbox (which centrally coordinates applications for all GB nations).
Applications seeking authorisation of a claim for use in:
-
England only, please contact the competent authority via the DHSC mailbox
-
Scotland only, please contact the competent authority via the Food Standards Scotland mailbox
-
Wales only, please contact the competent authority via the Welsh Government mailbox
We recommend that applicants complete a Medicines Borderline Advice Form to be submitted to the Medicines and Health Products Regulatory Agency (MHRA) prior to submitting a health claim for assessment, to confirm whether or not the claim they wish to make about a nutrient or substance would be considered medicinal. Medicinal claims may not be made on food, and an application for a claim that was considered medicinal would therefore not be permitted.
We recommend that applicants check with the Food Standards Agency whether their food would be considered a novel food in GB.
Food business operators wishing to make new health claims in the EU or Northern Ireland market following the end of the transition period should refer to the section on making a claim in the EU or Northern Ireland.
Applications for claims based on new or emerging science or proprietary data: Article 13(5)
Article 13(5) of retained Regulation (EC) No. 1924/2006 provides for the authorisation of health claims based on newly developed scientific evidence and/or which include a request for the protection of proprietary data to the GB NHC Register. The process to be used is set out in Article 18 of retained Regulation (EC) No. 1924/2006
Regulation (EC) No. 1924/2006, did not define ‘newly developed scientific evidence’. Our understanding therefore remains that, in this context, a claim based on newly-developed scientific evidence may be a claim that either:
- has never been made before
- is based on evidence that has become available since 31 January 2008
Therefore, the process contained in Article 18 of retained Regulation (EC) No. 1924/2006may be used to submit health claims other than those referring to disease risk reduction or to children’s development and health.
For example, a new application for a claim which received a negative opinion from EFSA after submission under Article 13(2), and for which relevant information has come to light since 31 January 2008, could be submitted via this route.
In the event of an application being submitted that has received a previous negative assessment by EFSA (or other assessment outside of the UK/GB), the applicant should state the reason or reasons for submitting the new application, and provide a description of the changes made to the application. Any new, relevant information that the applicant wishes the expert committee to consider, should be highlighted within the application and clearly outlined in the reason for the request.
Health claims based on new or emerging science, or health claims based on proprietary data, require authorisation prior to use. Retained Regulation (EC) No. 1924/2006 specifies the procedure for such authorisations. To have a claim authorised an application with supporting information (which is kept confidential) must be submitted to the relevant competent authority via the appropriate mailbox. See the new claims section.
Within 14 days of the date of receipt the competent authority, working with the applicant where necessary, conducts a validity check of the application (to ensure it comes within the scope of the regulation) and acknowledges receipt in writing (the acknowledgement shall state the date of receipt of the application). The competent authority then sends the application to the United Kingdom Nutrition and Health Claims Committee (UKNHCC) for scientific assessment and to the other relevant authorities for information who ensure that proprietary data remains confidential.
An application must contain all the following information about the claim:
- the name and address of the applicant
- a statement confirming whether the application is for authorisation of the claim for use in Great Britain, or either in one of England, Scotland, or Wales only
- the nutrient or other substance, or the food or the category of food, in respect of which the health claim is to be made and its particular characteristics
- a description of the claimed effect and whether or not it is based on the essentiality of the nutrient
- a copy of the studies, including, where available, independent, peer-reviewed studies, which have been carried out with regard to the health claim and any other material which is available to demonstrate that the health claim complies with the criteria provided for in retained Regulation (EC) No. 1924/2006
- where appropriate, an indication of the information which should be regarded as proprietary accompanied by verifiable justification
- a copy of other scientific studies which are relevant to that health claim
- a proposal for the wording of the health claim for which authorisation is sought including, as the case may be, specific conditions for use
- a summary of the application
From the date that the UKNHCC receives a valid application from a competent GB authority it has 5 months to provide its opinion to the relevant authorities. The UKNHCC has the option to request further information about the application if necessary. If the UKNHCC requests any further information, the overall time limit is extended by 1 month, with the applicant required to submit the requested information within 15 days (this is known as the ‘stop the clock’ process’). The UKNHCC forwards its opinion to the relevant authorities and the applicant as well as making it public. The applicant and members of the public have 30 days to make comments to the relevant competent GB authority via the appropriate mailbox (see the new claims section).
The appropriate UK authorities have 2 months from receipt of that UKNHCC scientific opinion to decide whether the claim should be authorised. The appropriate UK authorities take into account:
- the UKNHCC’s scientific opinion
- relevant provisions in law
- any enactments
- other factors relevant to the matter under consideration, and will consult one another in reaching a view
Authorised claims are added to the GB NHC Register together with any conditions of use. Similarly, if the claim is rejected it is added to the GB NHC Register together with the reasons for the rejection.
Once authorised and added to the GB NHC Register the claim is available for use on any product that meets with the requirements of the Regulation, and any conditions of use specified. If, however, any of the supporting scientific data or other information has been granted data protection, it cannot be used by any other applicant for 5 years in accordance with Article 21. This is reliant on all the following:
- the scientific data or other information being designated as proprietary by the applicant when the application is made
- the prior applicant having exclusive right of reference to the proprietary data at the time the prior application was made
- the health claim not being able to be authorised without the submission of the proprietary data by the applicant
This aims to protect proprietary data, but also, to a certain extent, protects particular claims as the Regulation requires manufacturers to be in a position to scientifically justify any claims they make. It does not stop the same claim being submitted with another scientific justification by another food business operator.
Applications for reduction of disease risk claims and claims referring to children’s development and health: Article 14
Retained Regulation (EC) No. 1924/2006 requires disease risk reduction claims and claims which refer to children’s development and health to be authorised prior to use, and specifies a procedure for such authorisations. Once authorised, a claim will be added to the GB NHC Register and can be used on any product that meets the conditions of the Regulation and the conditions of use specified. To have a claim authorised for use in the GB market an application with supporting information must be submitted to the relevant competent GB authority via appropriate mailbox (see the ‘new claims’ section).
Within 14 days of the date of receipt the competent authority, working with the applicant where necessary, conducts a validity check of the application (to ensure it comes within scope of the regulation) and acknowledge receipt in writing (the acknowledgement shall state the date of receipt of the application). The competent authority then forwards the application to the UKNHCC for its assessment and make the application and any supplementary information available to other relevant authorities. The UKNHCC makes the summary of the application available to the public.
An application must contain all the following information about the claim:
- the name and address of the applicant
- a statement confirming whether the application is for authorisation of the claim for use in Great Britain, or either in one of England, Scotland, or Wales only
- the nutrient or other substance, or the food or the category of food, in respect of which the health claim is to be made and its particular characteristics
- whether it is a reduction of disease risk claim or a claim referring to children’s development and health, if the former what the proposed risk factor for the disease to which the claim refers is
- a copy of the studies, including, where available, independent, peer-reviewed studies, which have been carried out with regard to the health claim and any other material which is available to demonstrate that the health claim complies with the criteria provided for in retained Regulation (EC) No. 1924/2006
- where appropriate, an indication of the information which should be regarded as proprietary accompanied by verifiable justification
- a copy of other scientific studies which are relevant to that health claim
- a proposal for the wording of the health claim for which authorisation is sought including, as the case may be, specific conditions for use
- a summary of the application (which the UKNHCC makes public)
From the date that the UKNHCC receives a valid application from a competent GB authority it has 5 months to provide its opinion to the relevant UK authorities. The UKNHCC, or the competent GB authority through the UKNHCC, may request further information about the application if necessary. If any further information is requested the overall time limit is extended by up to 2 months following the date of receipt of the requested information submitted by the applicant (this is known as the ‘stop the clock’ process’). To facilitate a timely response and approval, it is important to submit a well-prepared dossier that includes all relevant information.
If the UKNHCC gives a positive opinion on the claim, its opinion contains:
- details of the applicant, the claim, and the nutrient or other substance referred to
- a proposal for the wording of the claim
- where necessary, any conditions or restrictions on use, including compulsory warnings
The opinion, whether negative or positive, together with details about the reasoning for that opinion, is sent to the appropriate UK authorities and is made available to the public on GOV.UK. The applicant and members of the public have 30 days to make comments to the relevant competent GB authority via appropriate mailbox (see the ‘new claims’ section).
Claims authorised by the appropriate UK authorities for use in the GB market are added to the GB NHC Register together with any conditions of use. Rejected claims are also added to the GB NHC Register together with the reasons for their rejection.
1.11 Modification, suspension and revocation of authorisations
In accordance with Article 19 of retained Regulation (EC) No. 1924/2006 an applicant and/or user of a claim, authorised for the purposes of Article 13 or Article 14, may apply for a modification of that health claim to be authorised: following the procedures set out in Articles 15 to 18.
The appropriate GB authorities may also, on their own initiative, request that a claim be reconsidered.
Following a request from an appropriate GB authority, the UKNHCC shall issue a scientific opinion on whether a health claim authorised for the purposes of Article 13 or 14 still meets the conditions laid down in retained Regulation (EC) No. 1924/2006.
The UKNHCC shall make available its opinion to the appropriate UK authorities, the original applicant of the claim in question, and the public. The applicant, user, or member of the public have 30 days, the publication of the opinion, to make comments to the appropriate UK authorities via the DHSC mailbox (which centrally coordinates comments for all GB nations).
The appropriate UK authorities, taking into consideration the opinion of the UKNHCC and any comments received, may by regulations modify or revoke the claim in question.
In cases of urgency, the appropriate UK authorities may exercise the power to make regulations to modify or revoke a claim without allowing for the 30-day comment period.
1.12 ‘On hold’ health claims
As set out in a Department of Health Bulletin (2014) intended for interested parties entitled Article 13(1) ‘on hold’ Health Claims Spreadsheet, (2014 Bulletin) on hold claims are those which may be used while they are still under consideration, subject to the transition measures in Article (28)(5) of the Nutrition & Health Claims Regulation (EC) 1924/2006. See the full list of ‘on hold’ claims referenced by the 2014 Bulletin.
‘On hold’ claims are still under consideration in the EU. However, from 1 January 2021 GB has its own system for authorising claims separate from the EU authorisation system.
The UK government and devolved administrations in Scotland, Wales, and Northern Ireland will launch a call to evidence, seeking information from stakeholders so that the full scale of the ‘on hold’ claims issue may be understood. Following the call for evidence, a decision will be made on the approach to ‘on hold’ claims for use in the GB market.
As it is the intention of the UK government and devolved administrations in Scotland, Wales to minimise disruption to business following the end of the transition period, we are providing business reasonable time to plan accordingly. ‘On hold’ claims may continue to be used in accordance with the 2014 Bulletin until a decision is made following the call for evidence.
1.13 Generic descriptors
Retained Regulation (EC) No. 1924/2006 continues to allow for appropriate authorities to make regulations granting derogations from Article 1.3 following the receipt of an application for the GB market by business.
The Nutrition (Amendment etc) (EU Exit) Regulations 2019 revoked Commission Regulation (EU) No. 907/2013 that set out the application procedure for generic descriptors, this was because the provisions did not work for UK only applications.
The procedure and requirements for applications made by businesses for generic descriptors is set out below. Applications for the GB market should be completed in line with the requirements set out in the GB generic descriptor application process section of this guidance. Applications should be submitted for GB consideration to the DHSC mailbox (which centrally co-ordinates applications for all GB nations).
Retained Commission Regulation (EU) No. 2019/343 which provides for derogations from Article 1(3) of Retained Regulation (EC) No 1924/2006 is applicable. It has been subsequently amended by the Nutrition (Amendment etc.) (EU Exit) Regulations 2020. The Annex to retained Regulation (EU) No. 2019/343 contains all the generic descriptors permitted for use in GB following the end of the transition period.
Applications for generic descriptors other than those addressed by Commission Regulation (EU) No. 2019/343 currently being considered by the EU have not been authorised before the end of the transition period. We therefore recommend that any applications currently under consideration in the EU are submitted for consideration by the UK appropriate authorities for use the GB market to the DHSC mailbox (which centrally coordinates applications for all GB nations).
GB generic descriptor application process
An application may be made for the use of a generic descriptor in Great Britain or either in one of England, Scotland, or Wales only.
Applications seeking authorisation of a claim for use in the Great Britain should be submitted to the competent authorities via the DHSC mailbox (which centrally coordinates applications for all GB nations).
Applications seeking authorisation of a claim for use in:
-
England only, please contact the competent authority via the DHSC mailbox
-
Scotland only, please contact the competent authority via the Food Standards Scotland mailbox
-
Wales only, please contact the competent authority via the Welsh Government Mailbox
The application shall be submitted electronically using the format set out below. Appropriate GB authorities may request a paper copy if they require it. For the data referred to in supporting data and additional information, a list of references alone is not sufficient.
On receipt of an application the appropriate GB authority shall:
-
acknowledge receipt of the application in writing within 14 days of its receipt. The acknowledgement shall state the date of receipt of the application
-
forward the full application to the other appropriate UK authorities for which the application concerning the use of the generic descriptor is made
The appropriate authority shall verify, without delay, and taking into account information provided by the other appropriate authorities, whether the application contains all required information as listed in the section generic descriptors: mandatory information. Where the application does not contain all the elements required under the ‘generic descriptors: mandatory information’ section, the appropriate authority shall request the necessary additional information from the applicant and inform the applicant of the period within which that information shall be provided.
An application shall be considered as not valid in cases where an applicant does not provide further information as requested by the appropriate authority. In such a case the appropriate authority shall inform the applicant, and other appropriate authorities, indicating the reasons why the application is considered not valid.
The appropriate authority shall forward the valid application to the other appropriate authorities, without delay and inform the applicant thereof.
The appropriate authorities concerned shall provide their opinion to one another within 6 weeks from the date of transmission of the valid application. The opinion shall state whether the generic descriptor fulfils the conditions for obtaining an exemption pursuant to Article 1(4) of Retained Regulation (EC) No 1924/2006, and whether it is supported by the elements referred to in the section generic descriptors: mandatory information (under ‘The generic descriptor subject to the application’, ‘the class of foods or beverages which the generic descriptor covers’ and ‘supporting data in relation to the use of the generic descriptor’) and shall give the reasons justifying that opinion. The opinions shall be submitted in writing.
After receiving the valid application from appropriate authority, and the opinion(s) referred to in this process, the appropriate UK authorities may, within a reasonable time, initiate the procedure of approval of the generic descriptor seeking the opinions of an expert committee as appropriate.
1.14 Generic descriptors: mandatory information
The application shall consist of the following information.
A summary of the application:
- the name and the address of the applicant
- the generic descriptor subject to the application
- a brief description of the particularity of the class of foods or beverages which the generic descriptor covers
- the nation for which the application concerning the use of the generic descriptor is made by the applicant
Applicant details:
- name, address and contact details of the food business operator submitting an application and/or of the person authorised to communicate with the appropriate authority on behalf of the applicant
Applications for the authorisation of a generic descriptor may also be submitted by trade associations, acting on behalf of their members and shall include the name, address and contact details of the trade association submitting an application and/or of the person authorised to communicate with the appropriate authority on behalf of the trade association. Information about the support of the application by the members of the trade association would be desirable
The generic descriptor subject to the application:
- the generic descriptor as used in the language(s) where it is traditionally used
- a description of the generic descriptor in English, where appropriate
- the nation where the generic descriptor is used
The class of foods or beverages which the generic descriptor covers:
- an indication of the class of foods or beverages marketed under the generic descriptor for which the application is made
- a detailed description, highlighting the particularity and the elements that distinguish the class of foods or beverages marketed under the generic descriptor, for which the application is made, from other products falling within the same class of foods or beverages
Supporting data in relation to the use of the generic descriptor:
- relevant bibliographical or otherwise verifiable evidence demonstrating the presence on the market of the class of foods or beverages with the generic descriptor, over at least a 20-year period, in the nation (s), prior to the date of entry into force of this Regulation
Additional information
Additional information in relation to the use of generic descriptors that must be provided if requested on the appropriate authority’s initiative: supporting data in relation to the understanding/perception of the consumer.
Recipient appropriate authority and the appropriate authorities concerned may require the additional data by the applicant on the following types of information, prior to the submission of the application to the appropriate authority, where they consider it necessary for the assessment of the application:
-
relevant evidence or information related to consumer understanding and perception of the effects that could be implied by the generic descriptor. Such data shall cover the nations where the generic descriptor is used
-
relevant evidence or information demonstrating that the consumer links the generic descriptor with the specific class of foods or beverages mentioned under ‘the class of foods or beverages which the generic descriptor covers’
1.15 Risk assessment
Risk assessment functions related to nutrition and health claims have been assumed by the United Kingdom Nutrition and Health Claims Committee (UKNHCC), namely conducting the scientific assessment of applications for new claims and providing opinions to the appropriate UK authorities on nutrition and health claims post exit.
1.16 Risk management
Risk management functions related to nutrition and health claims have been assumed by the appropriate UK authorities in respect of:
- making regulations
- publishing guidelines
- authorising applications for the GB market
- maintaining the GB Nutrition and Health Claims Register
Wording of claims
Decisions regarding the final wording of a claim have been taken by the appropriate UK authorities when considering whether to authorise a claim. Comments submitted to the appropriate GB authorities by applicants following the publication of the UKNHCC’s scientific opinion, consumer understanding, and the opinion of the UKNHCC is all taken into consideration when establishing the final wording of a claim.
Panel and secretariat
The UKNHCC is a new expert committee established under the remit of Public Health England, an executive agency sponsored by the Department of Health and Social Care. The UKNHCC is administered and resourced by civil servants from within PHE.
Appointments to the committee are made on merit and in accordance with the principles of the Government Office for Science Code of Practice for Scientific Advisory Committees and the Governance Code on Public Appointments issued by the Minister for the Cabinet Office. The Chair and members are appointed as individuals, on a personal basis, to fulfil the role of the committee, not as representatives of their particular profession, employer or interest group, and have a duty to act in the public interest.
Applications and scientific dossiers
See updated nutrition and health claim application forms for use in GB (containing further information on completing an application). This is similar the EFSA application form, only minor amendments have been made, and there are no changes in respect of the scientific dossiers that applicants are required to submit in support of a nutrition or health claim.
Scientific dossiers may therefore be submitted to the appropriate GB authorities for consideration using the same format as those submitted to the EU until further notice.
Applications are being considered in turn. To facilitate timely evaluation, the appropriate UK authorities recommend that applications are not submitted shortly before any scheduled meeting of the UKNHCC.
If the UKNHCC receives an application that is not suitably completed or provides insufficient evidence to complete an assessment, it may request further information from the applicant in accordance with previous sections of this guidance:
- applications for Claims Based on New or Emerging Science or Proprietary Data: Article 13(5)
- applications for Reduction of Disease Risk Claims and Claims Referring to Children’s Development and Health: Article 14
Under the stop the clock process, the evaluation of an application is suspended until the applicant provides the requested information. Applicants must provide requested information in line with any timings set out in this guidance.
The UKNHCC resumes the evaluation of the application once it receives the requested information, producing its opinion within the time remaining of the original 5-month evaluation period on the date of suspension plus any additional time (1 month for Article 13.5 claims and 2 months for Article 14 claims).
An application may be withdrawn by the applicant up to the moment the UKNHCC adopts its opinion. A request for withdrawal of an application must be submitted to the appropriate authority to which the application was submitted.
Scientific opinion
In conducting a scientific assessment, the UKNHCC uses a framework similar to the Scientific Advisory Committee on Nutrition (SACN) Framework for the Evaluation of Evidence.
Opinions are produced with either a favourable or unfavourable conclusion, that is either positive or negative.
In the event the UKNHCC provides a negative opinion, the opinion will set out reasons for that.
In the event the UKNHCC provides a positive opinion, the opinion shall include the following information:
- the names and address of the applicant
- the nutrient or other substance, or the food or the category of food, in respect of which a claim is to be made and its particular characteristics
- a summary of the evidence submitted in support of the claims and an assessment of its validity
- a proposal for the wording of the health claim, including, as the case may be, the specific conditions of use
- where applicable, conditions or restriction of use of the food and/or an additional statement or warning that should accompany the health claim on the label and in advertising
1.17 Domestic enforcement provisions
Legislation which provided for enforcement of Regulation (EC) No. 1924/2006 in each part of the United Kingdom’s prior to its withdrawal from the European Union still applies.
1.18 Making a claim in the EU or Northern Ireland
From 1 January 2021, there is a separate system for authorising nutrition and health claims for the GB market.
Businesses wishing to make new nutrition and health claims in the EU or Northern Ireland market following the end of the transition period must continue to comply with the requirements of Regulation (EC) No. 1924/2006.
We recommend that food business operators wishing to submit applications for new claims in the EU or Northern Ireland from 1 January 2021 refer to the extensive guidance on making nutrition and health claims in the European Union published by EFSA.
Section 2: introduction and summary
2.1 Introduction
When making a voluntary nutrition or health claim you must comply with the requirements of European Regulation (EC) No 1924/2006, on nutrition and health claims made on food. This section provides background information about Regulation 1924/2006 and a brief summary of the key controls it introduced.
This was the first piece of specific legislation to deal with nutrition and health claims and seeks to protect consumers from misleading or false claims. It harmonised legislation across the European Community making it easier to trade and aids food business operators in complying with the law. The Regulation makes it easier to identify nutrition and health claims that can justifiably be used on a specific product.
If you make, or plan to make, a nutrition or health claim, as well as using the rest of these guidance notes you are advised to consult your Primary Authority/Home Authority (see section 9.2) where you have one to ensure you meet with the requirements of the Regulation. You should start by reading sections 3 and 4 of the guidance, which outline the scope of the Regulation and give general information about making claims. By answering the questions at the end of section 4 you can identify the additional sections of this guidance that are relevant to you. In addition, you may find it helpful to read section 10, which includes the answers to specific questions about the Regulation.
2.2 Background
On 30 December 2006 a Regulation of the European Parliament and of the Council of the European Union on nutrition and health claims made on foods was published as Regulation (EC) 1924/2006. A corrigendum with the legal text of the Regulation was published on 18 January 2007. This amends the text that was originally published and reflects the agreed Regulation. In January 2008 2 amendments to the Regulation were agreed. Regulation (EC) 107/2008 amends the comitology procedures2 laid down by the Regulation and Regulation (EC) 109/2008 introduces a transition period for claims referring to children's development and health. A consolidated version of the Regulation, which takes into account these amendments was published on 4 March 2008. A copy of this Regulation can be found on the website of the European Commission.
2.3 What is a nutrition claim and what is a health claim?
Article 2 defines a nutrition claim as any claim which states, suggests or implies that a food has particular beneficial nutritional properties due to the presence, absence, increased or reduced levels of energy or of a particular nutrient or other substance, and includes claims such as "source of calcium", "low fat", "high fibre" and "reduced salt".
Article 2 defines a health claim as any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health. This would include claims such as "calcium helps maintain normal bones". More general claims such as "good for you" may also be health claims, and the Regulation takes these into account. Further advice about what is and is not a nutrition or health claim and what does and does not have to comply with the Regulation can be found in section 3.
2.4 Key requirements of the Regulation
Although the key requirements of the Regulation are outlined below, there may be more specific requirements not mentioned here. You are therefore recommended to consult the rest of the guidance to ensure you comply with the Regulation.
- Claims must comply with the general requirements of the Regulation as specified in Article 3, which include not being false, ambiguous or misleading, not encouraging or condoning excess consumption of a food and not implying that a balanced diet cannot provide necessary nutrients.
- If a claim is made Article 7 makes it obligatory to provide nutrition labelling in most cases. See sections 5.2 and 6.1 for more information about providing nutrition labelling.
- Article 8 means that only nutrition claims listed in the Annex to the Regulation can be made on food and only if the product meets with the specific conditions of use for that claim. For example, "low fat" can only be made on products containing no more than 3g of fat per 100g for solids.
- Claims must not be made on alcoholic beverages containing more than 1.2% by volume of alcohol, with limited exceptions for reduced energy or reduced alcohol and low alcohol content claims (Article 4).
- Health claims which suggest that health could be affected by not consuming the food cannot be made on food (Article 12).
- Health claims which make reference to the rate or amount of weight loss cannot be made on food (Article 12).
- Health claims which make reference to recommendations from individual doctors, health professionals or associations other than national associations of medical, nutrition or dietetic professionals and health-related charities cannot be made on food (Article 12).
- As specified by Article 10, health claims must be authorised and included in the list of authorised health claims in the EU Register to be used on food; the EU Register will be built up gradually as claims are authorised. Products will also have to meet the specific conditions of use stated.
Article 4 of the Regulation puts in place provisions for regulations to be adopted that may restrict the use of claims on certain foods or categories of foods based on their nutritional composition (nutrient profile). Nutrient profiles should have been adopted by 19 January 2009 but this deadline has not been met. According to Article 28 of the Regulation, food business operators will have 2 years to comply with these controls once the profiles are adopted in Europe.
2.5 Key dates (Article 28)
Although the Regulation applied from 1 July 2007, Article 28 puts in place transitional measures that mean you may have until January 2022 to comply with different specific aspects of the Regulation. For full details of the transitional periods, see section 8. In some instances, the Regulation does not provide exact end dates for transition periods but instead allows for periods following certain decisions. For example, there is a 2-year transitional period for products to comply with the controls relating to nutrient profiles, following their adoption (Article 28).
Section 3: scope
3.1 Introduction
Whether a claim has to comply with the Regulation (is within scope) or not will depend on the nature of the claim, where the claim is made and why the claim is made. This section offers guidance on the scope of Regulation 1924/2006 and when claims will need to comply with its requirements.
For certain food types or products, there is specific legislation which may control the use of labelling, and which is not therefore a voluntary nutrition or health claim. This section includes guidance on when and how claims in these cases are controlled by the Regulation.
Appendix 4 includes a flow diagram to help you work out whether or not you need to comply with the requirements of the Regulation. This flow chart should be used in conjunction with the rest of the information in this section.
3.2 Nature of the claim: nutrition claim
Article 2 of the Regulation defines a nutrition claim as any claim, which states, suggests or implies that a food has particular beneficial nutritional properties due to the presence, absence, increased or reduced levels of energy or of a particular nutrient or other substance. Nutrition claims provide factual information about the nutritional composition of the food. Some examples of nutrition claims are "reduced energy", "contains calcium", "low fat", "high fibre" and "contains lycopene".
As specified in the Annex, rather than control the exact wording of nutrition claims, the Regulation will control anything that has the same meaning to the consumer as one of the claims listed in the Annex. For example, it is our view that "contains no fat" would be subject to the conditions for "fat free". As well as controlling different wordings the Regulation also controls the use of pictorial or symbolic representations that have the same meaning to the consumer (Article 2).
It is a requirement of the Regulation (Article 5) that consumer understanding is taken into account when deciding whether a claim is controlled by the Regulation. Where a claim states, suggests or implies a nutritional benefit, and consumers are likely to view it as such, it will need to comply with the controls of the Regulation.
The Regulation only controls nutrition claims that refer to beneficial nutritional properties (Article 2) and does not control claims that refer to non-beneficial nutritional properties such as "high in fat" or "high in salt". In some cases the context of the claim will need to be considered to decide if the nutritional property would be beneficial to the consumer. For example, in our view "high in calories" on a ready meal is unlikely to be a beneficial nutritional property; however, the claim "high energy" on a drink aimed at athletes could be a positive attribute of the product.
In some cases the claim will be neither beneficial nor non-beneficial and will refer to a statement of fact, such as "contains 10g of fat". Such statements must not be misleading and are controlled by the Consumer Protection from Unfair Trading Regulations 2008, the Food Safety Act 1990[footnote 1] and European Regulation 178/2002, which make it an offence to mislead consumers and give out false information. If, however, the information is presented in a way that implies it is beneficial to consumers, such as "contains only 10g of fat" or "contains less than 10g of fat", our opinion is that it would need to comply with Regulation 1924/2006 on nutrition and health claims made on foods. The presentation of nutrition information is controlled by Directive 90/496/EEC on nutrition labelling for foodstuffs (as amended) and has been transposed into UK law by the Food Labelling Regulations, 1996 (as amended). This Directive sets out the content and format of nutrition declarations whether or not they are given voluntarily.[footnote 2]
Statements which do no more than highlight the presence or absence of an ingredient (as defined by the Food Labelling Regulations 1996 (as amended)), that has not been added, enhanced or removed for the purpose of highlighting a health or nutrition benefit, developing a health or nutrition function in the food, or improving the nutritional profile of the final food may be regarded as being outside the scope of the Regulation, unless presented in a way that suggests or implies to consumers that the product has beneficial nutritional properties as defined in the Regulation. Examples here might be "contains no additives". Any claim included in the Annex has already been considered and deemed to be a nutrition claim. Use of a claim included in the Annex, such as "no added sugar", must comply with the requirements of the Regulation. 5. You should also read the answers to frequently asked questions which can be found in sections 10.1, 10.2 and 10.3.
3.3 Nature of the claim: health claim
Article 2 of the Regulation defines a health claim as any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health. Health claims are different from nutrition claims as they refer to, or imply, a function in the body. For example "contains calcium" only refers to the composition of the food and is a nutrition claim. In contrast "calcium is needed for the maintenance of normal bones" refers to the function of calcium in the body and would be considered a health claim. Other examples of health claims include "probiotic" and "helps you lose weight".
Articles 10, 13 and 14 of the Regulation divide health claims into different types, which are then controlled in different ways. If you intend to make one of the following types of claims you will need to read the rest of this guidance, in particular sections 4 and 6, to ensure you comply with the requirements of the Regulation.
- Claims referring to the role of a nutrient or other substance in growth, development and functions of the body. For example "calcium is needed for the maintenance of normal bones".
- Claims referring to psychological and behavioural functions. For example "helps improve concentration".
- Claims referring to slimming or weight control or a reduction in the sense of hunger or an increase in the sense of satiety or to the reduction of the available energy from the diet. For example "keeps you feeling fuller for longer".
- Claims about general, non-specific benefits of the nutrient or food for overall good health or health-related well-being. For example "good for you" or "healthy".
- Claims referring to children's development and health. This includes health claims solely referring to the development and health of children (aged up to 18 years), and where the scientific substantiation only relates to children. For more information about what might distinguish a claim referring to children's development and health from other claims please see the European Commission's guidance on implementation of the Regulation.
- Claims that state, suggest or imply that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease.
- Prohibited claims (see section 4.2).
The Food Labelling Regulations 1996 (as amended) state that medicinal claims, which claim that a food has the property of preventing, treating or curing a human disease, are not permitted to be used on or about food and this will continue to be the case. As a result it will NOT be possible to make statements such as "eating long chain omega-3 may prevent or improve symptoms of heart disease". Products that make claims such as this may be subject to medicines legislation. For further information on medicine controls please see the website of the Medicines and Healthcare products Regulatory Agency (MHRA)
Statements referring to government health messages are not covered by the Regulation unless they make an explicit nutrition or health claim. For example, making a statement such as "in line with government salt targets" or "the government recommends eating 2 portions of fish a week, one of which should be oily" are not, in our view, controlled by the Regulation. Such statements would need to comply with the Consumer Protection from Unfair Trading Regulations 2008, the Food Safety Act 1990 and European Regulation 178/2002 which make it an offence to mislead consumers and give out false information. If, however, a nutrition or health claim is also made, such as ‘the government recommends eating 2 portions of fish a week, one of which should be oily, because it’s good for your heart’, the additional health claim would need to comply with the requirements of the Regulation. You should also read the answers to frequently asked questions which can be found in section 10.2.
3.4 Where the claim is made: commercial communication (Article 1)
Article 1 means that the Regulation only applies to claims made in a commercial context. To decide whether or not you need to comply with the requirements of the Regulation you should consider why the claim is being made and in what context. If a health claim is made in a commercial context it will have to comply with the requirements of the Regulation. These are outlined in this guidance document and include the requirement for health claims to be authorised and listed in the EU Register. However, if the same claim is made in a non-commercial context it is outside the scope of the Regulation, does not have to comply with the conditions laid down therein and is not a claim as defined in the Regulation, rather an independent statement.
In some cases it is easy to identify what is and what is not a commercial communication. Recital 4 gives some examples of non-commercial communications – dietary guidelines or advice issued by public health authorities and bodies, and information in the press and in scientific publications. In our view, any form of product labelling or packaging to be delivered as such to the final consumer (see below) would be commercial. As would product specific advertising in any form, including in print, broadcast, internet or direct mail, promotional features in print media, in-store promotion and catalogues or product directories, whether printed or on-line (this is not an exhaustive list). In other cases it may not be so easy to define what is, and what is not a commercial communication. As specific circumstances will vary, it is likely that decisions about whether a claim is being made in a commercial context would need to be made on a case-by-case basis. Although you should always consult your Local Authority for their view, there is some general guidance below on what is a commercial communication. To help you decide if a claim is being made in a commercial context you can apply the following tests. There will be many cases where no one test is determinative, but the combination of results may give an indication as to whether or not a claim is being made in a commercial context.
- What is the primary object of the claim and its context? If it is in the form of information to get people to change behaviour, such as decrease or increase consumption of certain substances for their own benefit, and is not directly used to advertise or promote a product or specific ingredient within a product, then this may be characterised as non-commercial. If the primary object is to induce the final consumer to eat a specific product, to the benefit of the manufacturer or retailer (either directly financial or indirectly, e.g. reputation), it is commercial.
- If a third party makes the claim, have they been paid to make the claim (commercial) or are they doing it to provide "information" (not commercial)?
- If the third party has not been paid, is the claim within the information they give likely to lead to gain in kind, or some intangible future benefit (indirectly commercial, in the sense that the inducement may call into question the independence of the information)?
As a general rule, a newspaper article will not be covered by the Regulation. However, it is our view that there might possibly be circumstances when a newspaper "article" could be deemed to be a "commercial communication" and therefore, subject to the Regulation. For example, where it is aimed at the final consumer of a product (as a consumer of that product), written as part of a promotional campaign by a food business representative and for commercial rather than scientific or informative purposes. Promotional activity of this kind will probably be deemed to be commercial even if it is presented to the final consumer as "journalism".
You should also read the answers to frequently asked questions which can be found in section 10.4.
3.5 Where the claim is made (Article 1)
The Regulation applies to claims made in commercial communications about foods to be delivered as such to the final consumer (Article 1). Food labels, advertising and other forms of presentation such as leaflets and menus may be commercial communications within the scope of the Regulation depending upon the circumstances.
While the Regulation applies to claims made in commercial communications about foods it is our opinion that it will not control claims made in communications within trade (business to business), to doctors or other health professionals, or to their organisations, whether the claim is in the labelling, advertising or other presentation of the food. This is provided that the recipients are acting within the scope of their professional activities and that they are not being addressed as final consumers of the foods. It therefore follows that if the information were, at any time, conveyed to final consumers within a commercial context, any claims made would need to comply with the requirements of the Regulation.
The Regulation does not generally apply to claims made for foods in transactions between one business and another. However, as an exception to this, it does apply in relation to foods intended for supply to restaurants, hospitals, schools, canteens and similar mass caterers (Article 1(2), paragraph 3).
Since the Regulation applies to leaflets and menus where these are commercial communications, caterers making nutrition claims such as "low fat" or health claims such as "superfood" or "healthy option", or using symbols to imply these things, need to comply with the Regulation. Section 3.7 provides advice for caterers and others involved with healthy eating schemes. Section 3.8 provides advice about diet codes on menus for hospital in-patients. Section 3.9 mentions catering in schools.
3.6 Where the claim is made: trademarks and brand names
If a trademark or brand name appearing on a food or in the presentation or advertising of a food implies a nutrition or health claim, it will come within the scope of the Regulation. Article 1 of the Regulation exempts such brand names or trademarks from having to be authorised or be present in the Annex of nutrition claims or the EU Register of health claims. However, such brand names or trademarks must be accompanied by a prominent, related, authorised and listed claim.
You should also read the answers to frequently asked questions which can be found in section 10.5.
3.7 Where the claim is made: healthy catering
Healthy catering award schemes such as those set up by local authorities or by individual caterers or chains do not necessarily constitute claims. However, depending upon where and how an award scheme 'badge' is used it may fall within the scope of the Regulation.
General marketing materials, e.g. a sticker or poster on the door or in the window of a catering establishment indicating that the business has achieved a healthy catering award, would generally be permitted without having to comply with the Regulation as long as they do not make any link between particular foods or dishes and health. However, if reference to an award scheme appears on a menu, menu board or shelf ticket, e.g. as a logo or symbol alongside certain dishes, then this would imply that a health claim or a nutrition claim is being made about these dishes. In these cases the Regulation would apply, in the same way as it applies to any other claim or reference to a healthy option on a menu. In most cases references to award schemes that are linked to specific dishes are likely to fall into the category of claiming a general, non-specific benefit from eating the food as referred to in Article 10(3) of the Regulation. To be used, these must be accompanied by a permitted specific health claim which explains to consumers what makes the product healthy e.g. "maintains normal cholesterol levels for a healthy heart". The EU list of permitted health claims can be found in the EU Register of health claims.
So, what are the options for highlighting healthier choices? Most health claims refer to specific nutrients or substances that may be present in only some foods e.g. "calcium helps build strong bones". There are unlikely to be many authorised health claims that could be applied to a whole range of dishes. So, when thinking about establishing a healthy catering award scheme, careful consideration should be given to any aspect of the scheme which requires caterers to highlight healthier options.
Caterers may find it easier to make nutrition claims about dishes, such as "low fat" or "reduced calorie", and this could have the advantage of making the claimed benefit clearer to consumers than a general reference to a product being "healthy". However, this would require caterers to know enough about the nutrition content of a dish to be sure that it met the conditions for making the claim. If you, as the caterer, don't know the amount of certain nutrients in your dishes, there are other ways in which you could tell consumers about the steps you've taken to make products healthier. Talking about what steps you are taking to improve the foods you offer in a factual way, without referring to any associated health benefits, is not likely to fall within the scope of the claims Regulation. For example, "we are using extra vegetables and less cheese on our pizzas" would not be a claim whereas "we are using extra vegetables and less cheese on our pizzas to help you lose weight and keep your heart healthy" includes a health claim. 6. You may also find you can make claims about the ingredients you are using rather than the finished products, in which case you would only need to know the nutritional content of the ingredient, which your supplier may be able to give you. For example, instead of saying "lots of our puddings are low in fat" you could say "we use skimmed milk when we make our delicious custard because it's low in saturated fat". You must make clear to customers exactly what it is that a particular claim is being made about as implying that the overall product is low in saturated fat when it is not would be misleading.
Nutrition claims such as "low fat", "source of fibre" and "reduced calorie" can be used as long as they are included in the EU list of authorised nutrition claims and products using the claims comply with the set criteria. For example, to claim that a product is low fat it must contain no more than 3g of fat per 100g or, for liquids, 1.5g of fat per 100ml.
Nutrition claims that have the same meaning to customers as those on the permitted list can also be used as long as products meet the criteria to use the listed claim. For example, we consider that a claim "less than 5% fat" is likely to mean the same to a consumer as "low fat" so can only be used on products that contain no more than 3g of fat per 100g. Statements of fact that do not imply the food is beneficial, such as "contains 5g of fat" or "250 calories", are not nutrition claims so do not have to comply with the Regulation. However, they must not be misleading in order to meet the requirements of general consumer protection legislation. See sections 3 and 5 of this guidance for more information about nutrition claims.
To make a comparative claim, such as "reduced calorie" or "lower fat", the comparison should be with 2 or more foods of the same category whose composition does not allow them to make a claim. The claim must also state how much the food/dish is reduced when compared to the same quantity of the other food(s). For example, it would be misleading to state that a sandwich is reduced calorie if it only contains 30% fewer calories than the most calorific sandwiches available and other sandwiches available have a similar or lower calorie content to the one claiming to be "reduced".
If you wish to use a symbol (such as a tick) as a key to denote menu items that are, for example, 'low fat', you may do this as long as the associated claim you use with it is authorised and the product/dish meets the criteria to use the claim. Your dish must contain no more than 3g of fat per 100g if you wish to use the "low fat" claim. You should take care when choosing what symbol to use as, if it implies a health claim such as "helps you lose weight" (e.g. a tape measure around a silhouette of a waist), it can only be used on products that meet the criteria to use a relevant authorised health claim.
Health claims could be used to designate beneficial properties of certain dishes and the same rules apply as for health claims used in other contexts – see section 6 of this guidance. Once the EU list of permitted health claims has been adopted, claims about general, non-specific benefits of a food for overall good health or health-related well-being, such as "healthy" or "superfood", will only be allowed if they are accompanied by a specific permitted health claim. The product(s) would have to meet the conditions of use for the accompanying claim. This is because customers can understand "healthy" to mean very different things, so it is important that it is made clear to them exactly what it is that makes the product beneficial to health. For example, if you are calling something "healthy" because it maintains normal cholesterol levels then in future you would only be able to describe it as "healthy" if you also use a permitted claim about maintenance of normal cholesterol and your dish would need to meet the conditions to use that permitted claim. This applies not only to specific products, but also to product ranges labelled as "healthy" or if reference is made to all or some of the products on offer at a catering establishment being "healthy".
These general references to overall good health must be accompanied by a health claim and it is not sufficient to use a nutrition claim such as "low fat". If you are using the term "healthy" or other such general reference to overall good health to indicate products that have beneficial nutritional properties, like reduced fat and salt content, then you should use the appropriate nutrition claim instead of using the word "healthy" . For example, if you are calling something "healthy" because it is low in fat then you should use a "low fat" claim instead of the word "healthy" and your dish must contain no more than 3g of fat per 100g.
If the trade mark or brand name of a product or range of products implies a nutrition or health claim, for example "healthy choice™", then it will also be controlled by the Regulation (see section 3.6 of this guidance). These trademarks or brand names do not have to be in the EU Register of permitted nutrition or health claims but they must be accompanied by a prominent, related, authorised and listed claim; the claim must be relevant to the trade mark or brand name. However, trademarks or brand names that existed before 1 January 2005 do not have to comply with this requirement until January 2022.
Note that, in future, dishes for which claims are made will also have to comply with nutrient profiles (see section 7.2).
You should also read the answers to frequently asked questions which can be found in section 10.6.
3.8 Where the claim is made: menus for hospital in-patients
Diet codes such as: 'healthier eating' or a symbol to indicate this; a heart symbol to indicate dishes suitable for patients with diabetes or heart problems; 'higher energy'; 'contains \< or = 25g fat'; 'lower sodium: contains \< or = 26mmol sodium' are used on hospital in-patient menus for guidance on the suitability of dishes for use in therapeutic diets. The use of these codes is based on guidance produced by the Food Counts Specialist Group of the British Dietetic Association ('Delivering nutritional care through food and beverage services').
If used in commercial communications (e.g. in the labelling of foods sold at retail or in advertising to consumers) diet codes would be considered nutrition or health claims and would have to be used in compliance with the Regulation. There may be some cases where a menu is not a commercial communication, in which case any nutrition or health claims applied to individual foods or dishes on the menu would not be subject to the rules in the Regulation (see section 3.4 of this guidance for advice on how to decide if claims are being presented in a commercial context).
Whether or not a menu provided to hospital in-patients is a commercial communication will depend on a number of circumstances or factors and we have not produced an exhaustive list. In our view, a menu provided to hospital in-patients may not be a commercial communication if:
- its primary purpose is to provide information to shape patients' behaviour for the patients' benefit e.g. by enabling patients to follow dietary advice relevant to their medical conditions;
- it is not used to advertise or promote a product to the benefit of the caterer or another food manufacturer or retailer;
- in-patients are not paying for the food they receive.
- where a diet code is used it is applied to all items on the menu that fulfil the criteria for its use.
However, a menu provided to hospital in-patients would be seen as a commercial communication if:
- patients were paying for their meals;
- well-known brand names (e.g. those of products sold at retail) appeared on menus in conjunction with foods/dishes and diet codes;
- caterers used diet codes to encourage patients to choose dishes with the largest profit margins for the caterers;
- the use of diet codes on menus which also include the caterer's name could benefit the caterer in another way either directly or indirectly.
If a menu fulfilled any of these criteria then any diet code used on that menu would need to comply with the requirements of the nutrition and health claims Regulation.
Best practice
We understand that meals in hospitals may be provided for patients by in-house caterers or by private caterers. We would recommend that caterers, procurement professionals and dietitians should adhere to the advice above when developing specifications and evaluating food provision tenders for providing meals for hospital in-patients.
We would also recommend that catering company names are not put on menus.
3.9 Where the claim is made: menus in schools
In our view, the advice in section 3.8 could also apply to menus in schools or in similar settings.
3.10 Where the claim is made: use of recommendations of, or endorsements by, national associations of medical, nutrition or dietetic professionals and health-related charities
The Regulation does not put in place specific rules concerning recommendations of, or endorsements by, national associations of medical, nutrition or dietetic professionals and health-related charities, but instead states that national rules can apply (Article 11). There are no such rules in place in the UK. However, if the recommendation or endorsement implies a nutrition or health claim it must comply with the general provisions of the Regulation.
If the recommendation or endorsement implies a nutrition or health claim then that claim must be authorised and included in the Annex of nutrition claims or the EU Register of health claims. If the recommendation or endorsement implies a reference to general, non-specific benefits of a food for overall good health then, in accordance with Article 10(3), it would need to be accompanied by a relevant, authorised health claim. Please see section 6.1 for further details. In some cases, the recommendation or endorsement may be a trademark, for example a charity logo. If so, and the trademark could be construed as a nutrition or health claim, it must comply with Article 1(3) and be accompanied by a relevant, authorised nutrition or health claim. Please see section 3.6 for further details.
Best practice
Sometimes national associations of medical, nutrition or dietetic professionals and health-related charities establish fundraising partnerships with food businesses and may allow their logo or details of the association or charity to appear in the labelling, presentation or advertising of food. You must be clear if such a partnership is purely for fundraising purposes as consumer research shows that consumers often infer health benefits from products associated with the name of a health-related national association or charity.
In addition to complying with the Regulation, professional codes of practice, such as the Health Professions Council's Standards of Conduct, Performance and Ethics, or the British Dietetic Association's code should also be adhered to.
3.11 Why the claim is made
Article 2 clarifies that the Regulation controls only voluntary nutrition and health claims made on foods and does not apply to statements or descriptions that are required to be present by other EU or UK food legislation. Regulation (EU) No 1308/2013 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products, requires that certain products with a fat content of 60-62% must be labelled "three-quarter-fat", or may be described as "reduced fat". In these cases use of the term "reduced fat" would not need to comply with the requirements of the nutrition and health claims Regulation.
The Food Labelling Regulations 1996 (as amended) require foods to be marked or labelled with the name of the food. Where there is no legal or customary name, "the name used for the food shall be sufficiently precise to inform a purchaser of the true nature of the food and to enable the food to be distinguished from products with which it could be confused and, if necessary, shall include a description of its use". It may be that the only way of complying with this requirement is to include a statement 18 that is tantamount to a claim. For example if zinc is added to an orange juice drink consumers would need to be made aware of this and reference would need to be made in the name of the product, such as "orange juice drink with added zinc". To the extent that wording is necessary to comply with mandatory labelling requirements, it is our view that this would not be a claim subject to the nutrition and health claims Regulation. However, the name of the product is only required to appear once and so in our example any additional reference to zinc would go beyond this mandatory requirement and would have to fully comply with the controls in the nutrition and health claims Regulation.
Please note, that when labelling with the name of the product, it is necessary to comply with the Consumer Protection from Unfair Trading Regulations 2008, the Food Safety Act 1990 and European Regulation 178/2002, which make it an offence to sell a food with a label that falsely describes a food or is likely to mislead consumers as to its nature or substance or quality.
Best practice
To try to ensure that reference to a vitamin or mineral in the name of a product is not likely to mislead consumers – for example "orange juice with added zinc" - we recommend that the zinc should be present in a significant amount as required by the nutrition claim "SOURCE OF [NAME OF VITAMIN/S] AND/OR [NAME OF MINERAL/S].
3.12 Additional legislation controlling claims
Foods for particular nutritional uses (PARNUTS), natural mineral water, spring water and bottled water intended for human consumption are controlled by specific legislation. As specified by Article 1(5), both the Regulation on nutrition and health claims and the specific legislation will apply to these products. Where the specific legislation controls claims it will take precedence. In all other cases the Regulation on nutrition and health claims will apply. Further guidance on application of the claims Regulation to PARNUTS is contained in the European Commission's guidance to interpretation. However, please note that in June 2011 the European Commission adopted a proposal that would amend the rules on foods for particular nutritional uses therefore, such foods may be subject to the Regulation in the future.
Regulation 258/97 concerning novel foods and novel food ingredients provides for the possibility of mandatory labelling, sometimes as a condition of approval. To the extent that statements are required to be made by the novel food approval, they will not have to comply with the requirements of the Regulation on nutrition and health claims. In the vast majority of cases the labelling requirements for novel foods are not in a form that constitutes a claim.
3.13 Do I need to comply with the Regulation?
Please see appendix 4 for a flowchart, which will help you decide whether you need to comply with the controls in Regulation 1924/2006.
Section 4: how to make a claim
4.1 Introduction
As well as complying with any specific controls applicable to nutrition or health claims, as outlined in sections 5 and 6, you must also ensure you comply with the general requirements of the Regulation. This section gives further information on these requirements.
It is worth noting that Article 28 puts in place various transitional periods that give you time to comply with the requirements of the Regulation. During these periods certain claims or products, which do not comply with the Regulation, may remain on the market. Full details of the transitional periods can be found in section 8.
4.2 Prohibited claims (Article 4 and article 12)
Article 4 of the Regulation contains specific controls on the use of claims on alcoholic beverages. Article 12 of the Regulation does not allow the following health claims to be made on food:
- claims which suggest that health could be affected by not consuming the food
- claims which make reference to rate or amount of weight loss
- claims which make reference to recommendations of individual doctors or health professionals
4.3 Claims on alcoholic beverages (Article 4)
Article 4(3) prohibits beverages that contain more than 1.2% by volume of alcohol from making health claims and nutrition claims other than "low alcohol", "reduced alcohol", and "reduced energy" claims, which should be used in accordance with national rules.
The use of a "low alcohol" claim in the UK continues to be controlled by the Food Information Regulations 2014.This places strict conditions on the use of the description "low alcohol" or "any other word or description" which implies the same. Such products must be no more than 1.2% alcohol by volume and labelled as such.
"Reduced alcohol" claims are expressly permitted by the Regulation, subject to national rules. Although there are, at present, no national rules controlling such claims, a "reduced alcohol" claim must be made with care, as it must not contravene the Food Information Regulations 2014 (see paragraph 73 above) i.e. it must not imply the "reduced alcohol" product is a "low alcohol" one i.e. 1.2% alcohol by volume or less. Whether a consumer is misled will depend on how the product is labelled and presented.
In our view, the term "light" could also be used (as an alternative to "reduced alcohol"), if used with sufficient care. Again, in order to comply with the Food Information Regulations 2014, you would need to avoid giving the impression that the product was a "low alcohol" product.
As a matter of best practice we recommend that such claims are only made when the alcohol value is reduced by at least 30% as this is consistent with the "reduced [name of the nutrient]" claim in the Annex of the Regulation.
If either of these claims is made, when exporting products to other countries you will need to check the individual requirements of the importing country.
If you use the term "light" in relation to reduced energy content only, and not alcohol content, you need to make sure that this is clear both in the way the claim is presented, for example in the context of the product labelling, and in the way the product is named.
As there is no specific UK legislation controlling "reduced energy" claims on alcohol, our view is that the criteria for the claim "energy-reduced" in the Annex of Regulation (EC) 1924/2006 should apply, except the requirement to indicate the characteristic(s) which make(s) the food reduced in energy as this is likely to constitute a prohibited nutrition claim on alcohol. Therefore, if an "energy-reduced" claim or a "light" claim referring to energy content were made on an alcoholic beverage, it could simply state "reduced energy – X% less calories" and not "reduced energy – X% less calories – low in sugar" as "low in sugar" is a nutrition claim that cannot be made on alcohol.
In the case of reduced energy and reduced alcohol claims the requirements of Article 9 of the Regulation, which deals with "comparative claims", should be met when making the claim.
Beverages that normally contain alcohol but have had these levels reduced to 1.2% or less by volume of alcohol will be treated as any other non-alcoholic beverage and can make any of the nutrition claims in the Annex as long as the product meets the conditions of use of the claim.
You should also read section 10.7.
4.4 Claims which make reference to rate or amount of weight loss
These are claims that state, suggest or imply a loss of a measurable amount of weight over a period of time, or loss of a measurable amount of weight. Previously, our view was that reference to periods of time alone, particularly in general terms such as "rapid" or "fast", might not be subject to this prohibition provided that such claims did not mislead consumers. However, at the meeting of the European Commission's working group on nutrition and health claims on 15 January 2010, the Commission and Member States concluded that any claims about fast or rapid weight loss are prohibited by Article 12(b). Similarly, testimonials or 'before and after' photographs which state or imply a rate or amount of weight loss are prohibited.
Does the prohibition in Article 12(b) also apply to claims in commercial communications about the rate or amount of weight loss that an individual has experienced or could experience on a weight-loss programme (WLP)? It is not the intention of this guidance to prevent a weight-loss programme providing consumers with the information they need to make an informed decision about whether the programme is suitable for them. False or misleading claims about a weight-loss programme would fall within the scope of the Consumer Protection from Unfair Trading Regulations. Many WLPs include, as well as a food component, other elements e.g. about exercise; learning new eating habits; support aimed at providing practical tools to help individuals follow the programme and maintain a healthy weight afterwards. The food component of WLPs may involve consuming 'normal' foods and drinks; specially formulated meal or snack replacement products (e.g. shakes and bars); food supplements; or combinations of these things. So, is a health claim for a weight-loss programme within the scope of the Regulation, and Article 12(b)?
At the time of writing, claims such as 'drop a dress size in a month', 'I lost a stone in 6 weeks', 'I lost 2" off my hips', 'I used to be a size 24 now I'm a size 18', 'lose weight fast' are made about weight-loss programmes, for example in leaflets and on websites seen by members of the general public; in some cases they are presented in testimonials. We consider such leaflets and websites to be commercial communications.
Overall, our view is that when considering whether a claim about a rate or amount of weight loss associated with a WLP would be prohibited by the Regulation, you need to determine whether there is sufficient linkage between the claim and the food element of the programme to lead to a breach of Article 12(b). It follows then, that a claim about a rate or amount of weight loss associated with a WLP consisting of only a food element is within the scope of the Regulation and would be prohibited by Article 12(b).
In the case of a weight-loss programme that includes other elements as well as food, when assessing whether the claim is linked totally or partly to the food element, you should consider the context in which the claim is made and how it is presented, as well as the wording of the claim. We offer some advice on circumstances in which claims and products might be considered linked in sections 3.4 and 10.4 of this guidance. To illustrate with some specific examples (this is not an exhaustive list), we would consider a claim about a rate or amount of weight loss to be linked to the food element of a WLP if:
- it could be seen at the same time as pictures of food or text about the food to be consumed on the programme (e.g. on a webpage or in a leaflet) thereby establishing a link, in a consumer's mind, between the food and the claim. This may be particularly important in those cases where WLP-branded food products are marketed, even if the products are not an essential element of the programme. The Divisional Court's judgement in the case of Cheshire County Council v Mornflake Oats Ltd (1993) is relevant here
- it were made in the context of information only or largely about the food element of the programme. An Advertising Standards Authority (ASA) adjudication of 27 April 2011 illustrates this point
We would also advise you to take into account our view that, in the case of a programme, where the food element consists of advice about portion control or general, healthy eating, a claim about rate or amount of weight loss may not be a claim as defined in Article 2(2)(1) of the Regulation, since it does not relate to 'particular characteristics' of the food, but rather to the amount consumed.
Furthermore, it is important to note that the Committee on Advertising Practice and Broadcast Committee on Advertising Practice advertising codes include rules that apply to marketing communications for weight control and slimming foodstuffs and diets (see section 9.1 of this guidance for more information about these codes). The rules recognise the Article 12(b) prohibition on health claims that refer to a rate or amount of weight loss attributed to food products; they also state that marketing communications must not make claims about specific weight loss. When determining whether communications are advertisements for the purposes of the codes, especially in digital media, the ASA makes its assessments on a case-by-case basis.
4.5 Claims which make reference to recommendations of individual doctors or health professionals
Article 12(c) prohibits, in commercial communications, health claims, which make reference to recommendations of individual doctors or health professionals or associations other than national associations of medical, nutrition or dietetic professionals and health-related charities. Our understanding is that this prohibition was put in place due to concern that, in commercial communications, the added weight of perceived professional expertise might unduly influence consumers, and the objective of the Regulation is that consumers should not be misled in any way.
Article 12(c) of the Regulation prohibits a very specific type of health claim e.g. Dr X recommends Brand Y calcium food supplement because calcium is needed for healthy bones' in an advertisement for that supplement.
When writing in commercial communications, whether these be product labels, in-store leaflets, advertising copy or some other form, care would need to be taken to use health claims from the EU register of authorised claims, once these are available, when describing the relationship that exists between a food category, a food or one of its constituents and health. In straightforward terms, health claims which make reference to recommendations of individual doctors or health professionals and other associations not referred to in Article 11 are prohibited and therefore will not be authorised in the EU. However, if an authorised health claim were used in a context such as the example given above, this would, in our view, be prohibited.
Some people have expressed concern that Article 12 (c) might be interpreted to mean that individual doctors or other health professionals would not be able to make legitimate health claims in any communication, whereas anyone else may do so. We consider that experts in their field should be able to express their professional expertise and the Regulation does not seek to hinder this; it makes a distinction between commercial and non-commercial communication (please see section 3.4 for guidance on how to distinguish between commercial and non-commercial communications). In the following paragraphs we use questions and examples of various scenarios in order to illustrate how we interpret Article 12(c) of the Regulation.
Best practice in commercial communications
Could a health professional recommend a branded product that is also making a health claim? In our view this could be permitted however any recommendation would have to be presented in a way that could not be construed as an unauthorised health claim, or as misleading to the consumer. If a health professional were to recommend a product bearing an authorised health claim, the 2 components would need to be separated in presentation to the consumer to avoid the 2 being read together as an unauthorised health claim of the kind set out above. So, in our view, the following health claims would be prohibited in commercial communications:
-
'Dr X recommends Brand Y calcium food supplement' in an advertisement, that includes health claims, for that supplement
-
'Dr X says calcium is needed for strong bones' in an advertisement including the product name of that food supplement
The claims are prohibited because the claim and the doctor's recommendation are linked by virtue of being in the same advertisement and therefore are seen as an authorised claim.
Similarly, in our view, it would not be permissible to include a statement such as 'Developed with Professor XX' on a food supplement pack or in a food supplement advertisement bearing a health claim.
In contrast, the claim: 'Dr X recommends Brand Y calcium food supplement' in an advertisement that does not include any health claims would not be prohibited by Article 12(c) neither would a statement such as 'This product has been developed by a leading dietician' since no health claim is being made.
If authorised health claims about calcium are used e.g. in a product label or product leaflet about a calcium-containing food supplement product and the information is attributed to a [named] health professional would this be prohibited by Article 12(c)? In our view it is difficult to see how this would not be perceived as a prohibited claim. However, context and overall presentation would be very important. Similarly, it might be difficult for a health professional to write about the relationship that exists between a food category, a food or one of its constituents and health without contravening the prohibition in Article 12(c).
In non-commercial communication
However, remember that the prohibition in Article 12(c) does not cover non-commercial communications such as general advice about the contribution of a food category, a food or one of its constituents to health, which may be written by a health professional.
If a health professional (e.g. a dietitian) gives expert opinion or advice that includes a health claim, is this excluded from the scope of Article 12(c)? In our view, it would be excluded if it were given in a non-commercial communication. You should also read the answers to frequently asked questions which can be found in section 10.8.
4.6 Medicinal claims
Claims that state, suggest or imply that a food has the property of preventing, treating or curing a human disease will continue to be prohibited by the Food Labelling Regulations 1996 (as amended). Products that make claims such as this may be are subject to medicines legislation. For further information on medicine controls please visit the website of the Medicines and Healthcare products Regulatory Agency .
4.7 'X% fat-free'
In addition to these prohibitions in Articles 4 and 12 of the Regulation, use of the nutrition claim 'X% fat-free' is prohibited by virtue of the conditions applying to the nutrition claim 'fat-free' (see the EU Register of nutrition claims).
4.8 General requirements that all claims must fulfil (Article 3, 5 and 6)
All claims that are made on food must comply with general criteria laid out in the Regulation. Articles 3, 5, and 6 require that:
a) claims are not false, ambiguous or misleading; authorisation of claims goes some way to guaranteeing this, but care is still needed over context and presentation
b) claims do not give rise to doubt about the safety and/or nutritional adequacy of other foods (Article 9 specifically addresses comparative nutrition claims)
c) claims do not encourage or condone excess consumption of a food
d) claims do not state, suggest or imply that a balanced and varied diet cannot provide appropriate quantities of nutrients in general. Although there are provisions in place to adopt exemptions to this requirement, none currently exist
e) claims do not refer to changes in bodily functions which could give rise to, or exploit fear in the consumer, either textually or through pictorial, graphic or symbolic representations
f) the presence, absence or reduced content in a food or category of food of a nutrient or other substance in respect of which the claim is made has been shown to have a beneficial nutritional or physiological effect, as established by generally accepted scientific evidence
g) the nutrient or other substance for which the claim is made is either:
-
contained in the final product in a significant quantity as defined in Community legislation or, where such rules do not exist, in a quantity that will produce the nutritional or physiological effect claimed as established by generally accepted scientific evidence. In the case of vitamins and minerals, Directive 90/496/EEC (as amended by Directive 2008/100/EC) refers to 15% of the RDA when considering what constitutes a significant amount. In all other cases there should be evidence to show it has a benefit
-
not present, or is present in a reduced quantity so as to produce the nutritional or physiological effect claimed, as established by generally accepted scientific evidence. This will apply to claims such as "low fat" and "reduced fat".
h) where applicable, the nutrient or other substance for which the claim is made must be in a form that is available to be used by the body. It is not altogether clear how to interpret 'where applicable' in Article 5(1)(c). Reference to existing lists of compounds generally agreed to be bioavailable may be a simple, effective way to demonstrate bioavailability without recourse to complicated testing that may be difficult to interpret. Lists of vitamins and minerals and of their sources that may be included in food supplements or in fortified foods are included in the European legislation (Directive 2002/46/EC and Regulation 1925/2006 respectively) and recital 11 of 2002/46 and recital 10 of 1925/2006 state that the vitamin and mineral sources listed in the legislation have been assessed on the basis of safety and availability. So, in the case of a claim for a vitamin or mineral evidence of availability might be taken as read providing a substance listed in the legislation is used. However, there may be particular circumstances where this wording is relevant and helpful: certain dietary components exert their beneficial health effect by reducing the absorption of another component and are not themselves absorbed (e.g. plant sterols reduce blood cholesterol levels by reducing the absorption of dietary cholesterol from the gut) therefore they are not 'in a form that is available to be used by the body' in the same way that vitamins / minerals are
i) compliance with the specific conditions set for nutrition and health claims (section 5 and 6 deal with this respectively) as the case may be;
j) if called upon to do so by the enforcement authorities a food business operator must be able to justify the claim. In some cases this may be by reference to the EU Register and use of references to generally accepted scientific evidence, unless the scientific substantiation is subject to data protection. Food business operators will also have to show that the nutrient or other substance to which the claim relates is present in a significant amount and is available to be used by the body
k) nutrition and health claims shall only be permitted if the average consumer can be expected to understand the beneficial effects as expressed in the claim
The Regulation applies to the food ready for consumption in accordance with manufacturers' instructions (Article 5). In our opinion this should only apply where the food could not or should not be consumed otherwise. For example, the requirements of the Regulation would apply to a dehydrated product, only after water has been added in accordance with the instructions. Where the product can be consumed without following the manufacturer's instructions, the requirements would apply to the food as sold. For example breakfast cereal does not have to be eaten with milk and therefore the claim should apply to the cereal as sold and not rely on milk being added.
You should also read the answers to frequently asked questions, which can be found in section 10.9.
4.9 Specific information about making nutrition and health claims
If your claim complies with the general conditions of use outlined in this section you should now ensure you comply with the specific conditions of use applicable to nutrition and health claims. If you are unclear whether you are making a nutrition claim or a health claim you should consult sections 3.2 and 3.3 for guidance. If you want to make a nutrition claim please go to section 5. If you want to make a health claim please go to section 6.
Section 5: how to make a nutrition claim
5.1 Introduction (Article 8)
Subject to transitional periods, Article 8 requires that only nutrition claims in the Annex of the Regulation be used on food (see the EU Register of nutrition claims).
- If the claim you wish to make is in the Annex please go to section 5.2 – Claims on the list in the Annex (Article 7 and 8).
- If you are interested in a claim that is not in the Annex please go to section 5.3 – Claims not on the list (Article 28).
- If you want to make a reduced or increased claim please see the specific controls in section 5.4 – Reduced and increased claims (Article 9).
5.2 Claims on the list in the Annex (Article 7 and 8)
To use the claim you will need to ensure that your product meets the following requirements:
- Article 8(1) requires the product to meet the specific conditions of use of the claim as outlined in the Annex. For example in order to claim that a food is "fat free" the product must contain no more than 0.5g of fat per 100g or 100ml
- compliance with additional requirements (such as prohibition if an alcoholic beverage) as outlined in section 4
- Article 7 of the Regulation requires nutrition labelling to be presented, as required by Directive 90/406/EEC, except in the case of non-prepacked foodstuffs put up for sale to the final consumer or to mass caterers, a foodstuff packed at point of sale at the request of the purchaser or pre-packed with a view to immediate sale (Article 1 of the Regulation). However, Article 1 of the Regulation goes on to say that national provisions may apply in the absence of Community measures; in England, Scotland and Wales, national provisions are set out in Schedule 7, Part II, para 2 of the Food Labelling Regulations 1996 (as amended); similar provisions apply in Northern Ireland through the Food Labelling Regulations (Northern Ireland) 1996 (SR 383). For food supplements, the nutritional information must be produced in line with food supplements legislation (Directive 2002/46/EC).
- For further advice and guidance on the provision of nutrition labelling please see the guidance notes on nutrition labelling and on the food supplements legislation
You should also read the answers to frequently asked questions, which can be found in section 10.10.
5.3 Claims not on the list (Article 28)
Article 28(3) of the Regulation put in place a 3-year transitional period to January 2010 for nutrition claims that were not in the Annex when the Regulation was published. This only applied to claims in use before 1 January 2006 and allowed them to continue to be used until 19 January 2010. Since 19 January 2010, only those nutrition claims in the Annex may be used on foods.
If the claim was not in use before 1 January 2006, Article 8(1) means it cannot be used, unless it is added to the Annex. The general transitional period in Article 28(1) applied to actual products placed on the market or labelled before 1 July 2007. This transitional period allowed existing stock to be sold until its expiry date or 31 July 2009, whichever was sooner.
Article 8(2) allows the list of permitted nutrition claims to be updated to add new claims or amend existing ones. Additions to the list will be made via the Commission's Standing Committee procedure. If you wish to propose that a claim is added to the list please contact the European Commission. Once a claim is added to the Annex the specific conditions associated with that claim will apply.
You should also read the answers to frequently asked questions, which can be found in section 10.11.
5.4 Reduced and increased claims (Article 9)
First, Article 8(1) requires products to meet the conditions given in the Annex to make a reduced or increased claim. So, for example, to say "reduced fat" there must be at least a 30% reduction compared to similar products. In addition, reduced and increased claims must comply with the following conditions laid out in Article 9:
- the comparison must be between foods of the same category. Although the Regulation does not provide a definition of food category, the European Commission has produced guidance on how this should be applied. It states that products being compared should be foods belonging to a group of foods that are similar in terms of nutritional content. For example, a "dairy products" category would be too large and would allow a comparison to be made between the fat content of cheese and the fat content of milk. Instead, narrower food categories such as "milks", "fresh cheeses", or "yoghurts" could be considered. The notion of food category should also take account of the occasion of consumption and/or the purpose of consumption and in particular alternatives of consumption, for example butter and margarine. The key requirement is that the comparison helps consumers make informed choices
- the comparison must be between the product bearing the claim and a range of other products from the same category, which do not have a composition, which allows them to bear a claim, including foods of other brands. This is to avoid a situation where a comparison with a single product may not be representative of the market and may mislead the consumer. It is our reading of the Commission guidance that in some cases it may be possible to compare with only one product if that one product is representative of other products on the market. For example to make a reduced sugar claim on lemonade the comparison could be with the full sugar version of the product range, provided the full sugar version has similar sugar levels to comparable lemonades on the market
- the claim must state the difference in the quantity of the nutrient and/or energy (calorific value) between the same quantities of the 2 foods. This difference can be expressed as an average and as either a percentage or an absolute value
- when the claim "light" is used, the Annex requires the characteristic(s) which make(s) the food "light" to be indicated. Similarly, if the claim "reduced energy" is made the Annex requires the characteristics that make the food reduced in its total energy value to be indicated. In our view, a single indication can fulfil the requirements of both Article 9 and the conditions for using the "light" or "reduced energy" claim. For example, a label stating "light – X% less sugar". If the nutrient has been removed a statement indicating its absence can be made. For example "light – no sugar ". In the context of this statement, the % reduction need not meet the conditions in the Annex. See section 4.3 for information about using the claims "energy-reduced" and "light" on alcoholic beverages
- the claim 'extra light', or claims likely to have the same meaning to the consumer, can no longer be used since they are not in the Annex. This is also the view of the European Commission, see the European Commission's guidance
Within Commission guidance it is specified that "as much as" claims e.g. "as much calcium as a glass of milk", are not subject to Article 9, which specifies that a comparative claim should indicate the difference in the quantity of a nutrient or energy value. Although this type of claim does not have to comply with the specific requirements in Article 9 it is still considered to be a nutrition claim and would need to comply with the requirements of the Regulation, such as being included in the Annex of permitted claims. Since this type of claim is not included in the Annex it may no longer be used.
You should also read the answers to frequently asked questions, which can be found in section 10.12.
5.5 Future controls
Several of the Regulation's requirements do not apply now, but will apply to the use of nutrition and health claims in future. Details of these requirements can be found in section 7.
5.6 Checklist for making nutrition claims
You can only make a nutrition claim on your product if all the following are true:
-
the claim is a nutrition claim as defined (see section 3.2)
-
you’ve consulted section 3.4 and 3.5 and the claim is within the scope of the Regulation. If it is not in scope, there is no need to continue with the checklist as you do not have to comply with the Regulation. You should contact your local authority to ensure you comply with other legislation applicable to food
-
you’ve consulted section 3.11 and the claim in question is not mandatory according to other legislation. If it is mandatory there is no need to continue with the checklist as you do not have to comply with the Regulation. You should contact your local authority to ensure you comply with other legislation applicable to food
-
use of the claim also complies with any relevant specific legislation (see section 3.12 for details)
-
it’s not a prohibited claim (see section 4.2 for details)
-
the claim and the product comply with the general requirements outlined in section 4.8
-
the product complies with the specific conditions of use in the Annex
-
if it is a reduced or increased claim it complies with the conditions of Article 9 (see section 5.4)
-
nutrition labelling has been provided (see section 5.2 for details)
Section 6: health claims
6.1 Using a health claim (Article 7 and 10)
Article 10(1) only allows health claims that are on the list of authorised health claims in the EU Register to be used on food. The only exceptions to this are general, non-specific claims (Article 10(3)) and trademarks or brand names that are also health claims (see section 3.6).
Article 10(3) of the Regulation puts in place special requirements for claims about general, non-specific benefits of the nutrient or food for overall good health or health-related well-being, such as "good for you" or "healthy". These claims do not have to be added to the EU Register of authorised health claims to be made on food. Instead the claim may only be used if it is accompanied by an authorised claim that is included in the EU Register. The product would have to meet the criteria of use of the accompanying claim. In our view this only becomes a requirement once the EU Register of authorised claims is adopted.
The list of authorised health claims is, however, still being prepared. Until the list of claims is adopted the transitional periods in section 8 will apply and health claims will need to comply with existing national legislation. They will also need to comply with the following aspects of the Regulation.
To use any health claim the Regulation requires:
- compliance with the general requirements of the Regulation (such as prohibitions if an alcoholic beverage) as outlined in section 4
- full nutrition labelling as required by Article 7; the information should be that of group 2, as defined in Article 4(1) of Directive 90/496/EEC – implemented by Schedule 7 of the Food Labelling Regulations 1996. If the claim relates to a specific nutrient that does not appear in group 2, that nutrient and the amount present must be stated in the same field of vision as the nutrition labelling. However, nutrition labelling does not have to be provided in the case of a nonprepacked foodstuff put up for sale to the final consumer or to mass caterers; a foodstuff packed at point of sale at the request of the purchaser or pre-packed with a view to immediate sale. For food supplements, the nutritional information must be in line with food supplements legislation (Directive 2002/46/EC). For further advice and guidance on the provision of nutrition labelling please see the guidance notes on nutrition labelling and on the food supplements legislation
- for a product making a disease risk reduction claim, a statement that the disease has multiple risk factors and altering one of these factors may or may not have a beneficial effect. These claims must be authorised before they can be used on food
In future Article 10 of the Regulation will require other conditions to be met for health claims to be made, however these will not apply until the list of claims has been adopted in 2010 health claim has been authorised for use in the EU. If you would like more information about these requirements, please see section 7.
You should also read the answers to frequently asked questions, which can be found in section 10.13.
6.2 How to get a claim on the EU Register of authorised health claims
The list of health claims is still being prepared and the Regulation has 3 mechanisms for the addition of claims to the list. Which of the 3 mechanisms will be the appropriate route to gain an authorisation will depend on the nature of the claim and the evidence it is based upon. For claims which do not fall into one of the categories below and are based on generally accepted scientific evidence, please go to section 6.3. The Regulation puts in place specific requirements associated with disease risk reduction claims and claims referring to children's development and health that apply to both claims based on new science and claims based on generally accepted science. If you wish to make one of these claims please see section 6.4. If the claim is based on new or emerging science, or there is a request for data protection, please go to section 6.5.
6.3 Health claims other than those referring to the reduction of disease risk and to children's development and health (Article 13)
The Regulation provides for the establishment of a Community list of permitted health claims. For health claims that are based on generally accepted scientific evidence and are well understood by the average consumer the procedure for establishing, changing and adding to the list of permitted claims is set out in Articles 13(2), (3), (4) and (5). To establish the list, the Regulation allowed the UK and other Member States to put together lists of candidate health claims and submit these by 31 January 2008. The European Commission consolidated Member States' lists, comprising over 44,000 claims, into around 4,000 unique entries. The resulting list was sent to EFSA for scientific opinions before decisions are made about which claims to include in the Community list. Further information about the list of health claims undergoing assessment can be found in EFSA's register of questions.
It is a requirement of the Regulation that this list of claims be adopted before 31 January 2010 however, due to the large number of claims received and the time it is taking to process them, adoption of the list has been delayed. EFSA is publishing its opinions on Article 13 health claims in batches; the first 6 batches were published in October 2009, February and October 2010, April and July 2011, completing the assessments relating to food constituents other than botanicals. The European Commission plans to establish the list of authorised claims in 2 stages: the first part of the list, comprising health claims for non-botanical ingredients, will be established some time after June 2011 and then health claims for botanical ingredients will be considered after that. National legislation will continue to apply in areas not covered by relevant EC law provisions until the Community list of authorised claims is established.
For certain claims, EFSA was initially unable to provide a conclusive assessment of scientific substantiation therefore, the European Commission made a 'further assessment' process available so that new data could be submitted between 1 June and 30 September 2011. The 2 categories of claims involved are: those on micro-organisms which EFSA considered to be insufficiently characterised; and those for which EFSA concluded that the evidence provided was insufficient to establish a cause and effect relationship.
Article 13(4) provides the mechanism for any changes to the list of permitted claims to be made although it neither says what is meant by 'any changes' nor specifies the application procedure to be followed; we understand that the European Commission may provide guidance in the future.
Article 13(5) provides for authorising health claims based on newly-developed scientific evidence and/or which include a request for the protection of proprietary data to the EU list. The process to be used is that set out in Article 18. The Regulation does not define what is meant by 'newly-developed scientific evidence'. Our understanding is that, in this context, a claim based on newly-developed scientific evidence may be a claim that has never been made before (e.g. the claim for water-soluble tomato concentrate authorised in Commission Decision 2009/980/EU) or it may be a claim based on evidence that has become available since 31 January 2008 – the date by which Member States had to submit lists of candidate claims according to Article 13(2) of the Regulation. Therefore, the Article 18 process may now be used to submit health claims other than those referring to disease risk reduction or to children's development and health; for example, a new application for a claim which received a negative opinion from EFSA after submission under Article 13(2), and for which relevant information has come to light since 31 January 2008, could be submitted by this route.
6.4 Reduction of disease risk claims and claims referring to children's development and health (Articles 14, 15, 16 and 17)
The Regulation requires disease risk reduction claims and claims which refer to children's development and health to be authorised prior to use, and specifies a procedure for such authorisations. Once authorised, a claim will be added to the list of authorised health claims in the EU Register and can be used on any product that meets the conditions of the Regulation and the conditions of use specified. In order to get the claim authorised an application with supporting information may be submitted to the Department of Health (DH). The Department is required to acknowledge receipt in writing within 14 days and, working with the applicant if necessary, to ensure the application is valid i.e. that it includes all the elements referred to in Article 15(3) of the Regulation. The Department will then forward the application to EFSA for its assessment. EFSA will then make the information available to other Member States and the Commission and a summary available to the public.
The Regulation requires that an application contains the following information about the claim:
(a) the name and address of the applicant
(b) the nutrient or other substance, or the food or the category of food, in respect of which the health claim is to be made and its particular characteristics
(c) a copy of the studies, including, where available, independent, peer-reviewed studies, which have been carried out with regard to the health claim and any other material which is available to demonstrate that the health claim complies with the criteria provided for in this Regulation
(d) where appropriate, an indication of the information which should be regarded as proprietary accompanied by verifiable justification (note: see section 6.5 for claims based on proprietary data)
(e) a copy of other scientific studies which are relevant to that health claim
(f) a proposal for the wording of the health claim for which authorisation is sought including, as the case may be, specific conditions for use
(g) a summary of the application (which EFSA will make public)
Once EFSA has received an application, it has 5 months to give an opinion. EFSA has the option to request further information about the application if necessary. If EFSA requests any further information the overall time limit will be extended by up to 2 months. In order to get a quick response and approval it is important to submit a well prepared dossier that includes all relevant information. EFSA has produced extensive guidance on this and to how it assesses health claim applications and these can be found in EFSA's guidance for applicants.
If EFSA gives a positive opinion on the claim its opinion will contain details of the applicant, the claim and the nutrient or other substance it refers to, a proposal for the wording of the claim and where necessary any conditions or restrictions on use, including compulsory warnings. The opinion, whether negative or positive, together with details about the reasoning for EFSA's opinion will go to the Commission and Member States and will be made available to the public. The public and the applicant will have 30 days to comment.
The Commission has 2 months to refer the opinion and claim to Standing Committee (comprising representatives of all Member States, chaired by the Commission). The Standing Committee will consider the claim and decide whether it is necessary and appropriate to amend the list of authorised health claims.
Approved claims will be added to the authorised list of health claims in the EU Register, together with any conditions of use. If the claim is rejected it will be added to the EU Register together with the reasons for the rejection.
A flowchart of this process can be found in appendix 4.
6.5 Health claims based on new or emerging science or proprietary data (Article 18, 15 and 16)
Health claims based on new or emerging science or health claims based on proprietary data are required to be authorised prior to use and the Regulation specifies a procedure for such authorisations. In order to get the claim authorised an application with supporting information may be submitted to DHSC. DHSC will acknowledge receipt in writing within 14 days and, working with the applicant where necessary, will ensure the application is valid. DHSC will then send the application to EFSA for assessment and to other Member States and the Commission for information.
Article 13(5) requires the dossier to contain the following information about the claim:
(a) the name and address of the applicant
(b) the nutrient or other substance, or the food or the category of food, in respect of which the health claim is to be made and its particular characteristics
(c) a copy of the studies, including, where available, independent, peer-reviewed studies, which have been carried out with regard to the health claim and any other material which is available to demonstrate that the health claim complies with the criteria provided for in this Regulation
(d) where appropriate, an indication of the information which should be regarded as proprietary accompanied by verifiable justification
(e) a copy of other scientific studies which are relevant to that health claim
(f) a proposal for the wording of the health claim for which authorisation is sought including, as the case may be, specific conditions for use
(g) a summary of the application
(h) reasons for the request
Once EFSA has received a valid application it has 5 months to give an opinion. EFSA has the option to request further information about the application if necessary. If EFSA requests any further information the overall time limit will be extended by 1 month, with the applicant required to submit the requested information within 15 days. EFSA will forward its opinion to the Commission, Member States and the applicant as well as making it public. The applicant and members of the public have 30 days to make comments to the Commission.
The Commission then has 2 months to decide if the claim should be authorised. The Commission will take into account EFSA's opinion, relevant provisions in EU law, any other relevant considerations and will consult with Member States in reaching its view. Approved claims will be added to the authorised list of health claims in the EU Register, together with any conditions of use. If the claim is rejected it will be added to the EU Register together with the reasons for the rejection.
A flowchart of this process can be found in appendix 4.
Once added to the list of authorised health claims in the EU Register, the claim will be available for use on any product that meets with the requirements of the Regulation and any conditions of use specified. If, however, any of the supporting scientific data or other information has been granted data protection it cannot be used by any other applicant for 5 years. This is reliant on:
a) the scientific data or other information being designated as proprietary by the applicant when the application is made
b) the prior applicant having exclusive right of reference to the proprietary data at the time the prior application was made
c) the health claim not being able to be authorised without the submission of the proprietary data by the applicant
This aims to protect proprietary data, but will also, to a certain extent, protect particular claims as the Regulation requires manufacturers to be in a position to scientifically justify any claims they make. It does not stop the same claim being submitted with another scientific justification by another food business operator. Also, if during this 5-year period, the Commission decides the claim could be authorised without the protected information, it can decide to make that information available.
6.6 EU guidance on the application process and tools for small businesses
The Commission adopted Regulation 353/2008, which establishes rules for applications for authorisation of Article 13(5) and Article 14 health claims, on 19th April 2008; this was amended by Commission Regulation 1169/2009.
The Regulation also requires the Commission and EFSA to produce guidance to assist food business operators in the preparation and presentation of the application for scientific assessment (Article 15 (5)), particularly small businesses, where "tools" for their assistance are also required. EFSA has published scientific and technical guidance for the preparation and presentation of an application for authorisation of a health claim.
In addition to this, EFSA has published general guidance for stakeholders on the evaluation of Article 13(1), 13(5) and 14 health claims.
You should also read the answers to frequently asked questions which can be found in section 10.13.
Section 7: future control of nutrition and health claims
7.1 Introduction
Several of the Regulation's requirements do not apply now, but will apply to the use of nutrition and health claims in future. This section gives details about these controls and significant dates associated with them.
7.2 Nutrient profiles (Article 4)
To prevent the use of claims misleading consumers about the true nutritional composition of the food, Article 4 requires the Commission, on advice from EFSA, to establish nutrient profiles for foods and food groups. Nutrient profiles should have been adopted by 19 January 2009 but this deadline has not been met. Use of authorised nutrition and health claims on foods failing the nutrient profile may be restricted, as explained below. Under transitional arrangements food business operators are not required to comply with the requirements associated with nutrient profiles until 24 months after the date of adoption.
Nutrient profiles will set certain nutritional criteria that a product must meet to make claims. When establishing nutrient profiles the Regulation requires EFSA to give advice and the Commission to consult stakeholders before adoption. It also requires the following to be taken into account when the profiles are developed:
- the quantities of certain nutrients, such as fat, salt and sugar, and other substances in the food
- the role, importance and contribution of the food in the diet
- the overall composition of the food including any nutrients that have been scientifically recognised as having an effect on health
What claims can be made will depend on the extent to which a product complies with the profile:
- meets the profile: nutrition and health claims can be made, if they comply with the other requirements of the Regulation
- fails on one nutrient: No health claim can be made. Nutrition claims can only be made if the statement "high [name of nutrient that fails the profile] content' is also made. This must be done in close proximity to, and with the same prominence as the nutrition claim. For example, a food high in sugar might carry the claim "low fat" only if the statement "high sugar content" is made. Article 4 specifies that this would have to be in the same field of vision as the claim and in the same size of typeface
- fails on more than one nutrient: no nutrition or health claim can be made, except for certain reduced claims. Article 4(2) exempts foods making "reduced" claims from having to respect the nutrient profile, but only where the reduced claim relates to a nutrient that fails the profile. For example if a product fails the profile due to its fat and sugar content, it cannot make a nutrition or health claim except "reduced fat" or "reduced sugar"
The Regulation allows for further exemptions to the application of nutrient profiles to be set during their development.
You should also read the answers to frequently asked questions which can be found in section 10.14.
7.3 Labelling requirements for health claims (Article 10)
Article 10 requires additional statements to be made in the labelling (or if there is no labelling, in the presentation and advertising) of products that make health claims. It is our view that these do not become requirements until the health claim has been authorised for use. Below is an outline of these additional labelling statements:
- include a statement indicating the importance of a varied and balanced diet and a healthy lifestyle
- include information about the quantity of the food and pattern of consumption required to obtain the claimed beneficial effect
- where appropriate, include a statement addressed to persons who should avoid using the food. Specific statements may be included within the conditions of use for a particular claim
- an appropriate warning for products likely to present a health risk if consumed to excess. Specific statements may be included within the conditions of use for a particular claim
In our view the requirements in Article 10(3), which apply to claims about general, non-specific benefits of a nutrient or food to overall good health or health-related well-being only apply once the EU Register of authorised claims is adopted (see section 6.1 for more details).
Section 8: when do I need to comply with the regulation?
8.1 Introduction
Some of the requirements of the Regulation do not take immediate effect. Below is information about the transitional periods and key dates by which you will need to comply with the various requirements of the Regulation.
8.2 Transitional periods (Article 28 and 29)
The Regulation came into force on 19 January 2007. This is the date on which the Regulation officially became law and the date by which other time periods and transitional periods in the Regulation are based.
The requirements of the Regulation applied from 1st July 2007. After this date any products put on the market carrying a claim must meet the requirements of the Regulation, unless there are specific transitional measures in place. Nutrition claims in use before 1 January 2006 and not in the Annex of authorised claims could continue in use until 19 January 2010, subject to the provisions of the Food Labelling Regulations 1996 (as amended) and the Food Safety Act 1990. Nutrition claims new to the market from 1 January 2006 had to comply with the Regulation or be taken off the market by the product's expiry date or no later than 31 July 2009, whichever was the sooner.
Further information about transitional periods and when specific elements of the Regulation will take effect is given in the table below. Other than the transitional period explained above, it is our view that the transitional periods relate to the claim, rather than the product making the claim. During the transitional periods UK legislation will continue to apply. Details of this legislation are given in appendix 3.
You should also read the answers to frequently asked questions which can be found in section 10.15.
Table 1: summary of transitional periods and key dates
In some instances the Regulation does not provide exact end dates for transition periods but instead allows for periods following certain decisions. For example there is a 2-year transitional period for products to comply with the controls relating to nutrient profiles, following their adoption.
| Date | Requirements | Article reference |
|---|---|---|
| 1st July 2007 | Reduced and increased claims must comply with the conditions of use specified in the Annex and in Article 9. See section 5.4 for details. Nutrition labelling must be provided if a nutrition or health claim is made. If the claim relates to a nutrient or other substance not included in the nutrition labelling, it must be stated, together with the amount present, in the same field of vision as the nutrition labelling. See sections 5.2 and 6.1 for more information. Claims must not be made on alcoholic beverages containing more than 1.2% by volume of alcohol, with limited exceptions for reduced energy and alcohol content claims (see section 4.3). Health claims which suggest that health could be affected by not consuming the food cannot be made on food. Health claims which make reference to the rate or amount of weight loss cannot be made on food. Health claims which make reference to recommendations of individual doctors or health professionals cannot be made on food. Disease risk reduction claims cannot be made (this was also the case prior to the 1st July 2007) unless the claim has been authorised. Health claims referring to psychological and behavioural functions, slimming or weight control or a reduction in the sense of hunger or an increase in the sense of satiety or to a reduction of the available energy from the diet can only be made if they were in use, in compliance with national provisions, before 19th January 2007. |
28(1) |
| 19 January 2008 Applications for authorisation of certain health claims to trigger the transition period. |
Health claims referring to psychological and behavioural functions, slimming or weight control or a reduction in the sense of hunger or an increase in the sense of satiety or to a reduction of the available energy from the diet cannot be made on food unless they were used in compliance with national conditions before 19th January 2007 and an application for authorisation has been submitted. Inclusion in the UK list of health claims is sufficient to fulfil this requirement Claims referring to children's development and health cannot be made on food unless the claim was in use before 19th January 2007 and an application for authorisation has been submitted. |
28(6) |
| Date of adoption of the EU Register of health claims | Only health claims included in the EU Register of authorised health claims, or claims submitted for authorisation and awaiting a decision, can be made on food. Health claims referring to general, non-specific benefits of the nutrient to overall good health, such as 'good for you" must be accompanied by an authorised health claim. Health claims must be accompanied by additional labelling requirements, such as a statement indicating the importance of a varied and balanced diet and a healthy lifestyle. See section 7.3 for details. |
20 (EU Register), 28(5), 28(6), 10(3), 10(2) |
| 19 January 2010 | Only nutrition claims included in the Annex can be used on food and only on products that meet with the specific conditions of use. | 28(3) |
| 2 years following adoption of the nutrient profiles. | Products must comply with the nutrient profile in order to make nutrition or health claims. Details of these controls can be found in section 7.2. | 28(1) |
| 19 January 2022 | Trademarks and brand names in use before January 2005 and which could be construed as claims must be accompanied by an authorised health or nutrition claim. This applies to the use of the trademark or brand name and not to any other claims made on the product. | 28(2) |
The Regulation also puts in place transitional periods for:
- nutrition claims in the form of pictorial, graphic or symbolic representation that are used in accordance with specific conditions and criteria elaborated by national provisions
- health claims that refer to psychological and behavioural functions, slimming or weight control or a reduction in the sense of hunger or an increase in the sense of satiety or to a reduction of the available energy from the diet and which have been subject of evaluation and authorisation in a Member State
There were no claims in the UK that were eligible for these transitional periods.
Section 9: enforcement and compliance
9.1 Enforcement and compliance
The enforcement of food law in the UK is the responsibility of Local Authorities and, in some instances, port health authorities. In each of the UK countries a domestic Regulation or Statutory Instrument provides for enforcement of the Regulation; creates offences; puts in place penalties; and designates competent authorities.
Trading Standards Officers (TSOs) and Environmental Health Officers (EHOs) or any other authorised officer of the Local Authority (as appropriate) could initiate any legal proceedings in connection with a product that they consider to be in breach of the Regulations.
To ensure that your product complies with the Regulation we would strongly recommend you contact your local TSO or EHO for advice.
The ASA is the UK body responsible for advertisements in broadcast (TV and radio) and non-broadcast media. There are 2 advertising content codes: the Committee on Advertising Practice writes and maintains the non-broadcast advertising code (the CAP code) and the broadcast committee of advertising practice writes and maintains the TV and radio advertising standards code (the BCAP code). The ASA is the independent body responsible for administering those codes and is able to require advertisers and broadcasts to remove non-compliant claims. The advertising codes reflect the requirements of the Regulation.
In March 2011 the ASA's remit was extended to cover companies' own marketing communications on their own websites and in other, third-party space under their control e.g. advertiser-controlled pages on social network sites.
There is full information about the advertising codes and the ASA's remit on the ASA website.
9.2 Home Authority principle
The Home Authority principle allows local authorities to work with a business to provide consistent and coordinated Trading Standards and Food Enforcement Services across the UK. It assists those businesses that have outlets in more than one local authority and distribute goods and/or services beyond the boundaries of one local authority. Further information about the Home Authority principle can be found on the Local Government Association website.
9.3 Primary Authority scheme
The government's statutory Primary Authority scheme came into force on 6 April 2009 and introduces provisions for businesses, charities or other organisations that operate across more than one site to enter into a partnership agreement with a single authority for it to become that organisation's Primary Authority. The existing Home Authority principle will continue to operate across the UK, particularly in Scotland and Northern Ireland where the Primary Authority scheme does not extend to the devolved functions of food law enforcement. Further information about the Primary Authority scheme can be found on the government website.
You should also read section 10.16.
Section 10: questions and answers
10.1 Nutrition claims (section 3.2 of this guidance)
Q1. Do I have to use the exact wording of the nutrition claim as set out in the Annex of the Regulation?
Not necessarily, as explained in Recital paragraph 21 of the Regulation. The entries in the Annex give the most frequently used wording, but wording that means the same to the consumer may be used, subject to the same control. See section 3.2 for more information.
Q2. Does "contains calcium" mean the same as "source of calcium"?
The Annex of the Regulation states that where a "contains" claim is made for vitamins or minerals, the conditions of "source of [name of vitamin/s] and/or [name of mineral/s]" will apply.
Q3. Can I include statements such as "less than 5% fat"?
Our view is that use of the term "less" in relation to fat content would, in this case, have the same meaning for consumers as "low fat". The product would have to comply with the conditions of use for "low fat" claims specified in the Annex. See section 3.2 for more information.
Q4. Can I include statements such as "90g of fat per 100g"?
Depending on the context, this could be a factual statement. Please see section 3.2 for information on what is and what is not a nutrition claim.
Q5. Will the Regulation control front-of-pack labelling?
The Department of Health is of the view that front-of-pack (FOP) nutrition information, which aids consumer understanding of the nutrition declaration is a form of nutrition labelling rather than a claim because it gives the consumer an at-a-glance indication of the relative amounts of key nutrients in a product as well as an overview of its nutritional composition. Where the term 'low' or green colour coding in such labelling is used, it should comply with the criteria for low 'nutrient' established in the nutrition and health claims Regulation. Once the proposed new Food Information Regulation has been agreed, the Department of Health will update guidance on FOP nutrition labelling to reflect the new legislative requirements.
10.2 Nutrition or health claims (section 3.2 and 3.3 of this guidance)
Q6. Are claims such as "contains antioxidants" nutrition or health claims?
Following discussions at the European level, and publication of Commission guidance claims such as 'contains antioxidants', which refer to a function in the body, are defined as health claims and will need to be authorised via Article 13.
Q7. Is "probiotics and prebiotic fibre" a nutrition or health claim?
Following discussions at European level it has been agreed that claims, such as "probiotics and prebiotic fibre", refer to a function in the body, and are therefore defined as health claims and will need to be authorised via Article 13. 'Probiotic' could at the very least be considered a general, non-specific health claim (as referred to in Article 10(3) of the Regulation) and so will, in due course, have to be accompanied by a specific authorised health claim. In practice this is likely to mean accompanied by an authorised health claim for the specific probiotic strain in the food in question.
Q8. Is "detox" a nutrition or health claim?
In our view this is referring to a function in the body and would be a health claim. As detox could refer to a range of functions, whether this is a health claim that should be listed in the EU Register of authorised claims or is a claim referring to general, non-specific benefits of the nutrient or food for overall good health or health-related well-being, may depend on the nature of the product. Ultimately the claim will either have to be on the EU Register of authorised claims or be accompanied by a specific claim from the EU Register. It will be for you to decide which is the most suitable route for your specific detox claim.
Q9. Is "lowers cholesterol" a disease risk reduction claim?
Yes, to authorise "lowers cholesterol" claims, the Article 14 route must be used.
Q10. What type of claim is "Isotonic" or "Hypotonic"?
Depending upon the context, these could be nutrition or health claims. However, there is not an authorised nutrition claim for either term so they could not be used in this way. Relevant health claims would need to be authorised for these terms to be used as such.
Q11. Would it be acceptable to claim 'This product contains calcium which your baby needs for healthy bones' in an advertisement for a food supplement aimed at pregnant women? Would such a claim fall under Article 13 or Article 14(1)(b) of the Regulation?
Such a claim could be made if the evidence substantiating the claim showed that calcium intake during pregnancy, at the recommended dose level for the food supplement, was necessary for a baby to develop healthy bones. Whether it was authorised as an Article 13 or Article 14(1)(b) claim would depend on whether the substantiating evidence related to all life stages or only to the development and health of children, including the developing foetus.
10.3 Scope (section 3 of this guidance)
Q12. Do I need to comply with the Regulation if the name of the product required by the Food Labelling Regulations 1996 contains a claim, e.g. "fruit juice with added zinc"?
The Regulation controls voluntary nutrition and health claims made on foods and if a manufacturer chooses to advertise or label the food in such a way to emphasise a fortified ingredient, compliance with this Regulation may be necessary. However, this Regulation does not apply to statements or descriptions that are required to be present by other EU or national food legislation. To the extent that wording is necessary to comply with mandatory labelling requirements it is not subject to the Regulation. See section 3.11 for further information.
Q13. Does the Regulation cover claims such as suitable for diabetics, lactose free, gluten free or other claims aimed at consumers with specific disorders?
Recital paragraph 22 states that claims addressed to a group of consumers with specific disorders are not intended to be covered by the Regulation. However, please note that in June 2011 the European Commission adopted a proposal that would amend the rules on foods for particular nutritional uses therefore, such foods may be subject to the Regulation in the future.
The FSA has produced guidance on allergen management, which refers to 'free-from' claims. The FSA has also published guidance on Commission Regulation (EC) 41/2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten.
If allergens are present in the product there are requirements within the Food Labelling Regulations 1996 (as amended) to label their presence.
Q14. Does the Regulation cover additive claims such as "no colours or preservatives"?
In our opinion this claim could be viewed as an ingredient claim or as a nutrition claim, depending upon how it was presented. Please see section 3.2 for further information.
Q15. Does the Regulation cover claims relating to 5-a-day or fruit and vegetable content?
In our view references to 5-a-day and the number of portions a product provides are not within the scope of this Regulation. This was discussed during negotiations and a statement clarifying this was included in the minutes of the June 2005 Council. However, the Consumer Protection from Unfair Trading Regulations 2008), the Food Safety Act 1990 and European Regulation 178/2002, which make it an offence to mislead consumers and give out false information, continue to apply. Claims relating to 5-a-day should conform with the government's criteria and advice on what constitutes a portion. If a health claim is made in addition to the reference to 5-a-day, such as "good for you because it contains one of your 5-a-day", use of the term "good for you" would come within the scope of the Regulation. For further information about the government's 5-a-day message, in particular what constitutes a portion, please see the NHS website.
Q16. Does the Regulation control government health messages?
No. As government health messages are not made in a commercial context they do not come within scope of the Regulation (Article 1) and do not have to meet with the requirements of the Regulation. This does not necessarily mean that they can be repeated by food business operators in a commercial context and enjoy the same exemption. More information can be found in section 3.3.
Q17. Does the Regulation control statements such as "digestive", "tonic water", or "light ale" which could be viewed as a claim, but are now seen by consumers as a category of food?
Under Article 1(4) descriptors, which have traditionally been used to indicate a class of foods or beverages, can be granted a derogation (exemption) and would then not have to comply with the requirements of the Regulation. For information on applying for a derogation please contact the Department of Health and Social Care (contact details can be found in appendix 1).
However, if the terms 'tonic' or 'digestive' were used not as traditional, generic descriptors but as health claims, they would have to comply with the requirements of the Regulation.
Q18. Does the Regulation cover generic health claims such as "good for you"?
Yes. Such a claim is likely to be viewed as a general reference to health under Article 10(3) of the Regulation. Article 10(3) claims do not have to be authorised and listed, so there is no requirement for a specific decision to be made at the Community level. Instead, these general references to health have to be accompanied by a specific health claim from the authorised list, explaining to consumers why the product is beneficial to health. See section 6.1 for further information.
Q19. Does the Regulation cover use of the term "superfood" on a product?
You will need to carefully consider how consumers would view this claim and the context in which it's made. The term "superfood" is generally seen as short-hand for some non-specific but clear benefit of the food it describes, and generally seen as a health benefit. While only courts can give a definitive interpretation, consumers have been given to expect some general health benefit from use of this term it. It is our view, therefore, that the term "superfood" is unlikely to be considered to be a specific health claim that would require authorisation and listing in its own right, but that it may be viewed to be a general reference to health under Article 10(3) of the EU Regulation 1924/2006 on nutrition and health claims made on foods. Article 10(3) claims do not have to be authorised and listed, so there is no requirement for a specific decision to be made at the Community level. Instead these are general references to health and have to be accompanied by a specific health claim from the authorised list, explaining to consumers why the product is beneficial to health, what makes it a "superfood".
Q20. Does the Regulation control claims made on vending machines?
In our view, if the claim relates to the products in the machine it would need to comply with the requirements of the Regulation. With regards to any labelling requirements, you must ensure that consumers have access to information that allows them to make an informed choice. In our view this information would need to be available before a purchase is made. This would apply to nutritional information that must accompany a claim.
Q21. Does the Regulation control claims about sports performance?
Such claims could be health claims and, if so, would need to meet with the requirements of the Regulation.
Q22. Does the Regulation control the use of claims about beauty?
Some attributes of beauty seem to be clearly linked to health, for example condition of skin, brightness of eyes. Other attributes may have no relation to health and would not be seen as health claims. However it would be important to consider whether the claim met the requirements of the Regulation to see if was within its scope e.g. it was linked to a function of the body. The Consumer Protection from Unfair Trading Regulations 2008, the Food Safety Act 1990 and European Regulation 178/2002, which make it an offence to mislead consumers and give out false information, continue to apply.
In some cases there may be a borderline between beauty and health. One example of a potential borderline claim is "glowing skin". Although there are no medical conditions associated with lack of glow, the average consumer may consider there to be an association with health, especially because we often talk of "glowing health". Distinguishing between beauty and health is an inexact science and is likely to come down to the individual claim and its presentation and context, taking into account consumer understanding.
Q23. Does the Regulation control the use of claims for branded food products and for generic foods?
The Regulation does not distinguish between branded food products and generic foods. It applies to nutrition and health claims made in a commercial context. Recital 4 of the Regulation states that this includes generic advertising and promotional campaigns. In our view this means that nutrition and health claims made in advertising and promotional campaigns, e.g. for a class of food such as milk, meat, potatoes, would fall within the scope of the Regulation.
10.4 Commercial communication (section 3.4 of this guidance)
Q 24. My business supplies food ingredients to manufacturers of foods that are designed to be delivered to the final consumer. If I make health claims about these ingredients in commercial communications directed at food manufacturers (e.g. catalogues) must these claims comply with the requirements of the Regulation?
Health claims made in these circumstances would not need to comply with the Regulation. However, a manufacturer of foods to be delivered to the final consumer would therefore need to be careful about using such claims and should check whether they comply with the Regulation. It would be helpful if business-to-business commercial communications included disclaimers advising that the information (health claims) therein should be checked for compliance with other legislation if being considered for use in the labelling or advertising of foods to be delivered to the final consumer.
Q25. I'm trying to decide if the information I want to give in a magazine article / on a website is a claim within a commercial communication. Section 3.4 mentions same field of view, what does this mean?
Ultimately decisions will need to be taken on a case-by-case basis. The Regulation does, however, place emphasis on consumer understanding and how consumers view the information presented to them. With this in mind, our view is if consumers could link a claim with a specific product or ingredient it is likely to be a commercial communication. In our view, this is more likely to be the case where the product and claim are seen together, at the same time and without turning the page, for example on the facing page of a (general interest) magazine.
If the claim and the related product or ingredient cannot been seen together, consideration should also be given to whether or not there is a direct link between the product and the claims being made, for example reference to a website. This is particularly relevant when we consider internet pages. In our view only claims which are on a separate internet site[footnote 3], and where there is no direct link to the purchase of products, would fall outside the scope of a commercial communication and outside the scope of the Regulation.
Q26. I've got an in-store magazine, does this count as a commercial communication?
In our view such publications are no different to other general interest magazines and it will depend on the type of information included and the way it is presented. For example if food retailer X produces an in-store magazine that gives consumers information about a healthy diet and includes an article on the benefits of oily fish, this alone would not appear to be a commercial communication; information given is unlikely to be construed as a claim and would not have to meet the requirements of the Regulation. However, if the article also mentions that oily fish or other products that consumers perceive as having the same health benefits (e.g. fish oil capsules) can be purchased in retailer X's shops or mentions a particular brand, this could be a commercial communication and any information given is more likely to be construed as a claim and would need to meet with the requirements of the Regulation. Similarly, if an advertisement for oily fish is made in the same field of vision as, or consumers could link the claims in the article with, a particular product it would more likely be a commercial communication and would need to comply with the requirements of the Regulation.
Q27. According to Recital 4 of the Regulation scientific publications may be considered to be non-commercial communications and, in that case, health claims in such publications would not be covered by the Regulation. If a scientific publication including a health claim is placed on a website promoting a food or food products does the health claim fall within the scope of the Regulation?
In this case, the health claim would fall within the scope of the Regulation if the website were viewed as a commercial communication. Please see the answer to the previous question.
Q28. Is a press release a commercial communication?
A communication between a company and the news media that will not be seen by consumers may not be considered commercial. However, if that communication or the information in it were, at any time, conveyed to consumers any claims would have to comply with the requirements of the Regulation. For example, if a press release was put on a website to promote a product or given out in a store where the product was being sold then it would be being used for commercial purposes and any nutrition or health claims in it would have to comply with the requirements of the Regulation.
Q29. What is meant by generic advertising in Recital 4 of the Regulation?
Recital 4 clarifies that the Regulation should apply to all nutrition and health claims made in commercial communications including generic advertising and promotional campaigns. In our view this means that nutrition and health claims made in advertising and promotional campaigns, e.g. for a class of food such as milk, meat, potatoes, would fall within the scope of the Regulation. However, the key consideration is whether or not the claim is made in a commercial context. Further guidance on what is a commercial communication is given in section 3.4.
Q30. Does use of the term 'medium fat' to describe cheese fall within the scope of the Regulation?
The Codex General Standard for Cheese sets criteria for using the term "medium fat" to describe cheeses that have no specific standard, and states that the content of fat in dry matter should be between 25-45%.
Q31. Does use of the term 'lean' to describe meat fall within the scope of the Regulation?
There is no legal definition of "lean", but Regulation (EC) 2076/2005 defines "lean mince meat" as mince containing no more than 7% fat. This only applies to this specific descriptor, therefore if mince is described in any other way, for example "lean meat", the law does not apply. There is also UK guidance, issued by the Association of Public Analysts (APA), on recommended fat levels for minced beef only. The APA advises that any "lean" minced beef should contain no more than 16% total fat whilst it is advised that "extra / super lean" minced beef should have a maximum 9% fat content. Public analysts have applied a maximum limit of 25% for "standard" minced beef which is widely established in case law.
10.5 Trademarks and brand names (section 3.6 of this guidance)
Q32. If I use a trademark or brand name that could also be a claim are there any controls on the positioning of the associated claim?
No. Although the Regulation does not specify where the accompanying claim should be made and there is no case law to inform an interpretation, it is our view that the claim should be clearly visible and legible.
Q33. Are there any controls on the type of claim I have to make to accompany the trademark/brand name?
Yes. The claim must be relevant to the trademark or brand name. Article 1(3) requires the claim to be in either the Annex of approved nutrition claims or the EU Register of authorised health claims and the product must meet the requirements to make the accompanying claim.
Q34. What if the trademark or brand name is in a television advert, can the accompanying claim be in the labelling?
Article 1(3) of the Regulation states that where the "trademark or brand name appearing in the labelling, presentation or advertising of the food … provided it is accompanied by a related nutrition or health claim in that labelling, presentation or advertising". This would appear to require that claim to appear with the trademark or brand name even where it is in an advert. However, this may depend on the circumstances and a test of reasonableness. Consult your Home Authority and the Advertising Standards Authority (ASA) for further guidance.
Q35. What happens if a product, with a brand name or trademark which could be a claim, does not meet with the nutrient profile?
In order to use a brand name or trademark that could be construed as a claim, Article 1(3) requires it be accompanied by an approved claim. The product must meet with the requirements of the Regulation to use the approved claim, including restrictions such as those based on nutrient profiles. If it cannot comply with these conditions it would not be able to use the brand name or trademark. For brand names or trademarks existing before 1 January 2005, the 15 year transitional period would apply before they would need to comply with this requirement (Article 28(2)).
Q36. When do I have to include the accompanying claim?
For trademarks or brand names in use before 1 January 2005 there is a 15 year transition period, in Article 28, for products to comply with these conditions. See section 8 for further details of the transitional periods.
Q37. What is meant by "trademarks or brand names existing before January 2005" in Article 28?
The Regulation does not define what is meant by "trademarks or brand names existing before January 2005". In some cases the trademark will have been registered before January 2005, showing that it existed. For brand names and other trademarks, manufacturers should be able to establish that it was in use on products before this date. We take the view that this interpretation is common across the EU, but in the case of exported products, it would be best to check with the importing country authorities.
Q38. Can I add products to the range and still use the trademark or brand name?
Yes. The transitional period applies to the trademark or brand name and not the products that it is used on.
Q39. I'm setting up a partnership scheme with a health-related charity, can I put their heart shaped logo on my products?
Yes, but as consumers are likely to view this in the same way as a health claim, you must comply with the Regulation. If the partnership scheme is intended for fundraising purposes only and the logo is not intended to imply that the food has a particular benefit to health, this should be clearly stated so consumers are not misled. Please see section 3.7 for further details.
10.6 Where the claim is made: healthy catering (section 237 of this guidance)
Q40. I want to indicate healthy options on my menu, do I need to comply with the Regulation?
Generally speaking, menus are likely to be considered commercial communications so statements on a menu such as "low fat" or "healthy" or any logos or pictures that would have the same meaning to consumers would have to comply with the Regulation. However, see section 3.8 about diet codes on menus for hospital in-patients.
Q41. I use a heart logo on my menu to indicate options that are low fat. Is this allowed?
Yes, as long as you make it clear to customers what the heart logo means. On its own, a heart logo could imply a wide range of different things to different customers and could indicate that some sort of benefit to heart health would result from eating that dish –and this would be a health claim. If the heart logo is being used to denote dishes that are low in fat then you should make sure a key explaining this clearly appears prominently on the menu.
Q42. What is the legal position regarding food bought by caterers from catering suppliers? Can I use claims made in my supplier's catalogue about the food I have bought?
Regulation (EC) 1924/2006 applies mainly to claims about foods supplied to the final consumer, as opposed to transactions between one business and another. However, as an exception to this, the Regulation does apply in relation to foods intended for supply to restaurants, hospitals, schools, canteens and similar mass caterers. The Regulation affects claims made in any "commercial communication", such as labelling, advertising and promotional campaigns, and would include claims made in a catering supplier's catalogue or directory, whether on-line or printed. This means that if you, as a caterer, for example purchased product X from a food supplier's catalogue which stated " Product X is a mix of dried sweetened cranberries, jumbo raisins and dried sweetened blueberries (a natural source of antioxidants and helps to maintain a healthy digestive system)", this contains a claim that should be compliant with the Regulation and you would need to satisfy yourself that it is before repeating it in connection with food that you serve to your customers.
At the time of writing, there are no such authorised health claims to help maintain a healthy digestive system. Until there are, claims may be used as long as they comply with current rules on the labelling, advertising and presentation of foods. However, once there are some claims in the EU list, claims may only be used if they have been authorised or have been submitted for authorisation and are still being considered. Authorised claims may be used on any product that meets the relevant conditions of use. Rejected claims may no longer be used on foods.
10.7 Prohibited claims: on alcoholic beverages (section 4.3 of the guidance)
Q43. Most claims on alcohol are prohibited, but does this include food supplements that contain more than 1.2% alcohol by volume?
No. Recital paragraph 13 of the Regulation draws a distinction between these products and alcoholic beverages. The product would need to comply with the definition of a supplement in Regulation 2(1)(a) of the Food Supplement Regulations 2003 and would need to comply with the other requirements of the Regulation on nutrition and health claims made on food.
10.8 prohibitions: reference to recommendations of doctors or health professionals (section 4.5 of this guidance)
Q44. What is meant by recommendations of individual doctors or health professionals?
Article 12(c) prohibits, in commercial communications, health claims which make reference to recommendations of individual doctors or health professionals or associations other than national associations of medical, nutrition or dietetic professionals and health-related charities. The Regulation does not define 'recommendation' however Oxford Dictionaries online defines 'recommendation' as 'a suggestion or proposal as to the best course of action, especially one put forward by an authoritative body'. In our view it is a useful definition in the context of Article 12(c) of the Regulation particularly if it is taken to refer to an authoritative individual as well as to an authoritative body.
Q45. What is a health professional?
In the context of the Regulation, we take the view that this would include anyone who is presenting themselves, or is understood by the consumer, as having expertise in the field of health or nutrition.
Q46. Will the Regulation control advice given by doctors and health professionals to patients?
The Regulation does not cover non-commercial communications such as independent advice given by dieticians, doctors, in-store pharmacies and health centres to patients. For more advice on what is a commercial communication please see section 3.4
Q47. Can a doctor or health professional recommend a product if a health claim isn't made?
In our opinion it would be permitted, but care needs to be taken about context and presentation to ensure that consumers are not misled. Remember too, that the Regulation applies to commercial communications and does not apply to non-commercial communications. Professional codes of practice, such as the Health Professions Council's Standards of Conduct, Performance and Ethics, or the British Dietetic Association's code should be adequate guides in distinguishing conduct on the borders of the professional and the commercial. At present, there are few authorised health claims, therefore remember that the current legislation requires that any health claim should not mislead consumers and anyone making a health claim should be able to substantiate it.
Q48. Can claims make reference to recommendations of individual doctors or health professionals if they are speaking on behalf of a charity or medical association?
If it is clear that the recommendation or endorsement is that of the national association of medical, nutrition or dietetic professionals or health related charity, Article 11 would apply. Otherwise, Article 12 would apply.
Q49. Can a doctor or health professional provide general healthy eating advice if it is not linked to a branded product?
The Regulation only applies to commercial communications. It will therefore depend whether the general healthy eating advice is in a commercial communication and can be construed as a claim making reference to a recommendation of a doctor or health professional. Only in these cases would the prohibition in Article 12(c) apply. See section 3.4 for more information about what is and what isn't a commercial communication.
Q50. Will individual doctors or health professionals be able to write in a commercial communication about the relationship that exists between a food category, a food or one of its constituents and health?
As explained above, the Regulation prohibits a very specific type of health claim. When writing in commercial communications, whether these be product labels, in-store leaflets or advertising copy, care would need to taken to use health claims only from the authorised list of claims, once this is available, when describing the relationship that exists between a food category, a food or one of its constituents and health.
Q51. Can a doctor or health professional recommend a branded product that is also making a heath claim?
In our view this could be permitted. Any recommendation should not be presented in a way that can be construed as an unauthorised health claim, or as misleading to the consumer. If, in a commercial communication, a health professional recommends a product which also bears an authorised health claim, the 2 components should ideally be separated in presentation to the consumer in case the 2 read together makes an unauthorised health claim because, for example, a possible implication is created that the claim is referring to the recommendation. Context is likely to be very important here and discussion with enforcement authorities prior to the claim being made is recommended.
Remember, any health claim that is made must comply with the requirements of the Regulation outlined in sections 4 and 6.
Q52. Does the Regulation prohibit doctors and health professionals using health claims to recommend branded products in presentations to peers or industry?
The Regulation controls claims made in commercial communications. As long as a communication from doctors or health professionals to their peers was not deemed to be a commercial communication and as long as those receiving the communication were not being addressed as the final consumers of the products for which health claims were being made, then such claims would be outside the scope of the Regulation.
Q53. Can I refer to research conducted by a doctor or health professional on my product label?
In our view this would be permitted, as long as this isn't in the form of a recommendation and any associated claims comply with the requirements of the Regulation. For example, you could say "research conducted by Doctor X / institute X shows that calcium is good for your bones" as a generic statement, but on a product containing calcium this would be an implied health claim. There would therefore have to be an authorised claim, such as "calcium is good for your bones", the product must comply with the requirements for making such a claim, and the labelling comply with the other general requirements in sections 4 and 6.
Q54. I am a dietitian and write commercial communications about the health benefits of foods containing a particular ingredient for food companies marketing products containing that ingredient; separately, I am paid by a daily newspaper to write about the health benefits of the same ingredient. Does the Regulation apply to any health or nutrition claims I use in the press articles?
Recital 4 of the Regulation indicates that "non-commercial communications and information in the press and in scientific publications" are outside the scope of the Regulation. So, as a general rule, a newspaper article will not be covered by the Regulation. However, see paragraph 32 above for circumstances when a newspaper article might be considered a "commercial communication".
Q55. As a health professional, how can I communicate emerging science including 'new', unauthorised health claims to consumers?
If you are communicating through a commercial communication and use any health claims then they must comply with the Regulation. Health claims used in a non-commercial communication would be outside the scope of the Regulation.
Q56. If a health professional speaks about underpinning science at a food brand-related event would any health claims they use be covered by the Regulation?
If such communication were 'commercial' any health claims used would have to comply with the rules in the Regulation. The source of any payment received by such speakers would need to be taken into account in deciding whether claims were being made in a commercial context.
Q57. Can I use a celebrity endorsement?
Celebrity endorsements do not appear to fall within the scope of the prohibition in Article 12(c) (unless the celebrity is a doctor or health professional). However, any nutrition or health claim made in a commercial context would need to comply with the requirements of the Regulation in the same way as any other nutrition or health claim. You could seek advice from the Advertising Standards Authority.
10.9 General Requirements (section 4.8 of this guidance)
Q58. How will "…in a form that is available to be used by the body…" in Article 5(1) be applied?
Article 6 of the Regulation requires manufacturers to be in a position to justify the use of the claim. This will require evidence to show that the product contains a significant quantity and that it is available to the body. For example, making a "high in iron" claim on spinach could be misleading to consumers because, while spinach contains iron, it also contains other substances that make it harder for the body to absorb the iron.
Q59. What is a significant amount?
Where possible this will be as defined by legislation, for example significant amounts for vitamins and minerals are defined in Directive 90/496/EEC (amended by Directive 2008/100/EC) and the corresponding Schedule 6 of the Food Labelling Regulations 1996. Where significant amounts are not defined by legislation, food business operators are required by Article 5 and 6 of the Regulation to justify the use of the claim. You may want to consider looking at any levels set for any corresponding nutrition claims in the Annex or health claims in the EU Register of authorised health claims, once adopted.
Q60. Is what would constitute a significant amount different for foods intended exclusively for children?
It is our opinion that when making claims on products intended exclusively for children, like follow-on formulae, processed cereal-based foods and baby foods, labelling reference values for children as defined in specific legislation for these products (currently Directive 2006/141/EC and Directive 2006/125/EC) should be taken into account when considering what constitutes a significant amount.
Q61. What is meant by the "average consumer"?
The Regulation does not formally define the average consumer, but Recital paragraph 16 refers to previous adjudications by the European Court of Justice in this area. It will ultimately be for the courts to decide if a claim is understood by the average consumer. As a guide, the average consumer is someone who is reasonably well-informed and reasonably observant and circumspect. The Regulation does highlight that the concept of the average consumer should take into account social, cultural and linguistic factors and also consider consumers whose characteristics make them particularly vulnerable to misleading claims. It also takes into account products that are aimed at particular groups of the population.
The Unfair Commercial Practices Directive has the same general definition of the average consumer (the average consumer, who is reasonably well informed and reasonably observant and circumspect, taking into account social, cultural and linguistic factors). As this is major horizontal EC consumer protection legislation, the courts may take this definition into account if asked to consider more general issues as to whether a consumer is likely to be misled by a claim.
10.10 Criteria to make claims (section 5.2 of this guidance)
Q62. What about claims that relate to a vitamin or mineral that's not in the Annex of Directive 90/496/EEC on nutrition labelling of foodstuffs and where no significant amount is defined?
The general principle to be followed when deciding what is a significant amount is what the scientific substantiation indicates is necessary for the desired effect, and what contribution to the diet the product making the claim would make.
Directive 90/496/EEC on the nutrition labelling for foodstuffs states that only vitamins and minerals contained in the annex may be included as part of the nutrition panel on food labels. Directive 2008/100/EC amends Directive 90/496/EEC and amongst other things updates the annex of vitamins and minerals and sets associated RDAs. These RDAs must be taken into account when considering what constitutes a significant amount to make a claim.
Article 3 of Regulation 1924/2006 requires food business operators to ensure that any claims made are not false, ambiguous or misleading and should provide consumers with access to a full nutritional breakdown of the product so they can make an informed choice. Where a vitamin or mineral claim is made consumers should have information available about the amount of that vitamin or mineral in the product.
Q63. If the criteria to make a claim are different under the Food Labelling Regulations 1996, which applies?
For claims that are in the Annex of the claims Regulation, you will need to comply with those criteria (Article 8(1)). The general transitional period allowed foods placed on the market or labelled prior to 1 July 2007 to be marketed until their expiry date, but no later than 31 July 2009 (Article 28(1)).
The Regulation does put in place other transitional periods and in some cases refers to national legislation applying in the interim. In these cases the Food Labelling Regulations will apply.
Full details of the interactions between the Food Labelling Regulations and the claims Regulation can be found in appendix 3.
Q64. To make, for example, a "source of calcium" claim, how much calcium does there need to be in each portion of my product?
Article 5 of the Regulation requires a significant amount of the nutrient or other substance for which a claim is made to be present in the quantity of the food that can reasonably be expected to be consumed.
For "source of [name of vitamin/s] and/or [name of mineral/s]" claims, the Annex of the Regulation refers food business operators to Directive 90/496/EEC when considering what constitutes a significant amount. This Directive states that 15% of the recommended daily allowance supplied by 100g or 100ml should be taken into consideration when deciding what constitutes a significant amount. Our interpretation is that this is intended as a guide, since the claims Regulation requires there to be a significant amount present in the amount that can reasonably be expected to be consumed. Ultimately you must ensure that consumers would not be misled by any claims you make. Therefore if you make a claim about the calcium content of a food, the consumer should get 15% of the RDA for calcium, from the amount they could reasonably be expected to consume.
Q65. What does "quantity of the product that can reasonably be expected to be consumed" mean?
This will need to be judged on a case-by-case basis taking into account the type of product. As an example, it is our view that it would be unreasonable to require consumers to eat 100g of margarine a day in order to consume the levels of the nutrient needed to make the claim; however, for bread this would be a more acceptable expectation. Again, it is important to remember that this requirement aims to protect consumers from misleading claims and to ensure a significant level of consumption where a claim is made. If a 250ml bottle of fruit juice states that it "contains vitamin C", is part of a balanced diet where there will be other sources of vitamin C and it is reasonable to expect a consumer to drink that amount as a serving, 15% of the RDA should be present in the 250ml. By contrast, a food presented as a single source of nutrition may need to contain the full daily requirement.
In situations where it is difficult to assess the amount of food that could reasonably be expected to be consumed, for example products such as milk, we would consider it reasonable to ensure that a significant amount is present per 100g or 100ml.
The frequency of consumption should also be considered, especially where claims are based on longer consumption. For example, it could be reasonable to make a health claim based on 5 portions of fruit and vegetables a day, whereas for oily fish it may need to be on a weekly basis. It is a requirement of Article 10(2(b)) that health claims are accompanied by a statement indicating the quantity of food and pattern of consumption required to obtain the claimed effect. In our view this only becomes a requirement following adoption of the Community Register of health claims, likely to be in January 2010.once the health claim has been authorised for use in the EU.
Q66. If my product is sold as a solid, but consumed as a liquid, and I want to make a claim, which condition, per 100g or per 100ml, would apply?
Article 5(3) states that the Regulation applies to the food ready for consumption in accordance with the manufacturer's instructions and so the criteria for a liquid – per 100ml – will apply in this case.
Q67. If my product could be sold as a liquid or a solid (e.g. a yoghurt) and the conditions vary per 100g and per 100ml, with which do I comply?
The reason certain nutrition claims have different criteria for solids and liquids is to take account of different consumption patterns. For example consuming a 330ml can of drink is very different to consuming 330g of food. Bearing this in mind, which criteria should apply will depend on the nature of the product and the levels of consumption and decisions would need to be made on a case-by-case basis. You should consult your Local Authority for further advice on what criteria should be applied to your product.
Q68. If I make a claim in a TV advert, do I have to provide nutrition labelling and if so how do I do it?
The Regulation refers to Directive 90/496/EEC on nutrition labelling for foodstuffs for the requirements on nutrition labelling. This Directive already requires nutrition labelling to be provided where a nutrition claim is made and states what information must be presented and in what format. In the UK this is implemented by the Food Labelling Regulations 1996. The Regulation requires the same conditions that apply to nutrition claims to also apply to health claims, although group 2 nutrition labelling should be provided in these cases. The Regulation also exempts non-prepacked foodstuff put up for sale to the final consumer, to mass caterers, a foodstuff packed at point of sale at the request of the purchaser or pre-packed with a view to immediate sale, from providing nutrition labelling.
If you make a claim in a TV advert, that falls within the scope of the nutrition and health claims Regulation (see section 3), you do not have to provide the nutrition labelling in the advert, but it must appear on the labelling/packaging of the product advertised.
Q69. What method do I need to use to measure fibre in order to make a claim?
Commission Directive 2008/100/EC (amending Directive 90/496/EEC) introduces a legal definition of 'fibre'. During the negotiation of Directive 2008/100/EC, the Commission was requested by Member States to coordinate discussions about methods of analysis for fibre and to develop EU guidance to assist in the implementation of the new definition. The Commission has initiated work on this and the UK will continue to engage with the Commission and other Member States on this to ensure a consistent application of the new definition, for the purposes of food labelling, across the European Union. We will of course ensure that all stakeholders are kept fully informed of any further developments.
Q70. The conditions for "no added sugar" claims includes "…or any other food used for its sweetening properties". What does this mean?
This will have to be looked at on a case-by-case basis and will depend on the nature of the product, why ingredients are used and how it is labelled. The name of the product is likely to indicate why the other food is present – as a defining ingredient or as a sweetener. For example, in a cranberry juice drink, the use of concentrated grape juice is usually to sweeten the product and is not included in the name; whereas in a mango and apple juice drink, the presence of apple juice is indicated in the name and is not added to sweeten the product (the sweetening effect is likely to be negligible with sweet mango juice).
Q71. How much sugar has to be present to trigger the requirement to state "CONTAINS NATURALLY OCCURING SUGARS" on a product making a "no added sugar" claim?
The Regulation does not specifically mention how much sugar should be present to trigger the use of this statement. The Regulation does however, define any product with no more than 0.5g of sugar per 100ml or per 100g as "sugar free". Taking this into consideration it is our view that only products that contain more than 0.5g of naturally present sugar per 100ml or per 100g should make the statement "CONTAINS NATURALLY OCCURING SUGARS".
Q72. Current legislation on spreadable fats allows "low fat" to be used under conditions not permitted by this Regulation – which applies?
Recital 8 clarifies that products that meet the conditions of Regulation (EC) No 2991/94 laying down standards for spreadable fats, can make "low fat" claims that meet the criteria in that legislation. It also states in the recitals of the nutrition and health claims Regulation that the spreadable fats legislation should be adapted to the provisions of the Regulation on nutrition and health claims as soon as possible. Regulation (EC) No 2991/94 has been repealed and replaced by Regulation (EC) No 1234/2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products. However, this maintains the specific criteria for the use of certain nutrition claims on spreadable fats laid down by Regulation (EC) No 2991/94. Until the criteria for claims on spreadable fats are brought in line with Regulation (EC) No 1924/2006 food business operators should continue to comply with the criteria set out in Regulation (EC) No 1234/2007.
10.11 Claims not in the annex of permitted nutrition claims (section 5.3 of this guidance)
Q73. "Diet" is not mentioned in the Annex, can I still use this claim?
In our opinion, using "diet" to distinguish one product from another is likely to be seen by consumers to mean the same as "light/lite", and would need to meet the conditions of use for this claim. However in another context – that of weight control or loss, this would be a health claim.
Q74. Omega-6 and omega-9 claims are not in the Annex. Can I use these?
For a product to make the claim e.g. "contains omega-6 fatty acids" or "contains omega-9 fatty acids" the Regulation requires the product to comply with the conditions of use for the claim "contains [name of nutrient or other substance]" which is in the Annex.
Q75. Can I use GI (glycaemic index) claims?
GI claims would be covered by the Regulation and would need to be submitted for authorisation and added to the list of permitted health claims. For information on getting health claims onto the EU Register of authorised claims please see section 6.
Q76. Can I use low carb claims?
Low carb claims are not in the Annex of permitted nutrition claims and therefore can no longer be used.
Q77. "Half fat" is not in the Annex, can I use this claim?
It is our view that "Half fat" is likely to mean the same to the average consumer as a reduced fat claim and products would need to meet with the requirements to make a claim in this category. See section 5.4 for more details. The general rules in the Consumer Protection from Unfair Trading Regulations 2008), the Food Safety Act 1990 and European Regulation 178/2002 which make it an offence to mislead consumers and give out false information, will apply. In this case it will need to have half the fat of the original (50% less).
Q78. Can I make statements such as "90% fat free"?
No. The Annex to the Regulation specifically prohibits this type of claim.
Q79. Can I make multivitamin claims or claims such as "contains vitamins and minerals"?
Although the Annex contains the claims "source of [name of vitamin/s] and/or [name of mineral/s]" and "high in [name of vitamin/s] and/or [name of mineral/s]", it does not contain a non-specific vitamin or mineral claim, such as "source of vitamins and minerals". Therefore, in our view this claim can no longer be used.
Q80. How do vitamin and mineral claims relate to multi-vitamin / -mineral supplements?
It is a legal requirement of Food Labelling Regulations 1996 that foods are labelled with a name and to the extent that wording is necessary to comply with mandatory labelling requirements it is not subject to Regulation 1924/2006. See section 3.11 for further information.
In addition, it is a requirement of Directive 2002/46/EC relating to food supplements that supplements state the names of the categories of nutrients or other substances that characterise the product. It may be that this includes statements about the vitamin or mineral content. In this case, it is a mandatory labelling requirement to present this information and does not need to comply with the requirements of the claims Regulation, although the product would need to comply with the requirements of Directive 2002/46/EC. Any additional statements about the vitamin or mineral content that are not required by that Directive would have to comply with the requirements of the claims Regulation.
Q81. What about energy claims?
The Annex of the Regulation already includes nutrition claims for low energy, reduced energy and energy-free and products will need to meet the compositional requirements listed, as well as the general requirements of the Regulation, in order to make these claims. Claims that relate to a "source of energy" or "high in energy" or "gives you energy" may also be controlled by the Regulation. To decide how the Regulation controls these claims, consideration needs to be given to whether the claim is a nutrition or health claim. In our view if the claim relates to the calorie content of the food, and consumers would view it as such, it would be a nutrition claim. The Annex of permitted nutrition claims does not contain a specific "high in energy" claim or generic "high [name of nutrient]" claim therefore such claims can no longer be used.
However, if the claim refers to, or would be considered by consumers as relating to, the feeling of energy it provides following consumption, it is a health claim and would have to meet with the requirements in section 6. If the feeling of energy is due to a particular ingredient e.g. caffeine then, in our view, it would be preferable to make this clear to consumers. If appropriate, this could be achieved by the use of an authorised health claim.
As discussed in section 3.2, only nutrition claims that refer to the beneficial nutritional properties of a food are controlled by the Regulation. Therefore, consideration also needs to be given to the context of any nutrition claim and the target audience. For example, it is our opinion that on a ready meal for average family consumption "high in calories" is unlikely to be claiming a beneficial nutritional property; however, on a drink aimed at athletes "high in energy" could be.
Q82. Can I claim 'contains energy'?
In our view this would not be a permitted nutrition claim. The conditions of use for the 'contains' nutrition claim state that it can be used to refer to a food's content of a nutrient or another substance. The definition of 'nutrition claim' in Article 2.2.4 mentions energy, nutrients and other substances separately, indicating that 'energy' is not seen as either a nutrient or as an 'other substance'.
Q83. Can I claim 'increased energy'?
In our view, this would not be a permitted nutrition claim since the conditions of use for the 'increased' claim refer only to 'nutrients'.
Q84. Does the Regulation control claims such as "contains wholegrain" or "does not contain hydrogenated fat"?
It may be possible to differentiate between a nutrition claim and an ingredient claim. Highlighting the presence, reduced content or absence of a nutrient or other substance is clearly covered in the definition of nutrition claim. However, ingredients might be listed in addition to the ingredients list or name of the product for good reason and in this context may fall outside the scope of the Regulation. Please see section 3.2 for further information.
Q85. How do I get a claim added to the Annex?
The Regulation does not contain a specific application process for submitting nutrition claims, and it is our understanding that applications should be made directly to the Commission, rather than via a Member State. A decision about inclusion on the list would then be taken by Member States at Standing Committee and would be based on any opinion EFSA may give. For more information please contact the European Commission. It would also be helpful if the Department of Health were kept informed of applications. Contact details can be found in appendix 1.
10.12 Criteria to make comparative claims (section 5.4 of this guidance)
Q86. The Annex of the Regulations requires products making reduced claims to have a 30% reduction compared to a similar product, but Article 9 requires the comparison to be with a range of products, which applies?
So that consumers are not misled reduced claims should be compared to a range of similar products on the market. This is to prevent a situation where, for example, a product making a reduced sugar claim has more sugar than the majority of similar products on the market. The European Commission has produced guidance to interpretation on this. The comparison must be between the product bearing the claim and a range of other products from the same category, which do not have a composition which allows them to bear a claim, including foods of other brands. In some cases it may only be possible to compare with one product, or within a manufacturer's range the comparator may be the 'standard' product in the range. However, that one product should be representative of other products on the market. For example, to make a reduced sugar claim on lemonade the comparison could be to the full sugar version of the same brand, provided the full sugar version has comparable sugar levels to other lemonades on the market that cannot make a nutrition claim.
Q87. Article 9 requires the difference to be stated, how do I do this?
This can be expressed as either a percentage or an absolute value and an average can be used. More information can be found in section 5.4.
Q88. It is a requirement that reduced energy and light claims are accompanied by an indication of the characteristics which make the food reduced in its total energy. What does this mean (section 5.4)?
In order to meet with this requirement the presentation of the product must explain to consumers how the energy content has been reduced. For example, if the energy has been reduced as a result of the sugar content being lowered this should be made clear to consumers, and in this case the condition that it must be at least 30% less does not apply. In addition, "reduced" and "light" claims have to comply with Article 9, which requires the difference in the quantity of a nutrient or other substance to be stated. This has been discussed at the European level and there is agreement that a single indication can fulfil the requirements of both Article 9 and the conditions for using a "light" claim. For example, a label stating "light – 50% less sugar".
Q89. What happens if I make a reduced claim and the comparative product is taken off the market?
The Regulation requires products making reduced claims to have a 30% reduction compared to similar products on the market. If this condition cannot be met the reduced claim should not be made.
Q90. How can I indicate that my product is reformulated if I cannot make a 'reduced' claim?
At the 13 October 2011 meeting of the Standing Committee on the Food Chain and Animal Health EU member states agreed a European Commission proposal to authorise the nutrition claim "now contains X% less [energy, fat, saturated fat, sodium/salt and/or sugars]. Once the Commission Regulation authorising the claim comes into force, the claim and associated conditions of use will be added to the list of authorised nutrition claims in the EU Register of nutrition claims.
Q91. Are claims comparing fruit juice to milk, such as "as much calcium as a glass of milk" or supplements to fruit, such as "contains as much vitamin C as an orange" also controlled by Article 9?
Since this type of claim is not included in the Annex it may no longer be used.
10.13 Health claims (section 6.1 of this guidance)
Q92. Will I have to use the exact wording of the permitted health claim?
The European Commission has indicated that the Regulation will not control the exact wording of health claims covered by Article 13 and Article 14. We therefore anticipate that there will be some flexibility over wording, within conditions where deemed necessary. The European Commission has committed to produce guidance on how to ensure authorised health claims are used in accordance with the Regulation.
Best practice
Q93. If I say "good for you" where does the accompanying claim have to be made?
Although the Regulation does not specify where the accompanying claim should be made and there is no case law to inform an interpretation, it is our view that it should be made clear to consumers why the product is good for them.
Q94. Who in the Commission makes decisions?
The Commission may take decisions under delegated powers ("comitology") after an opinion from EFSA and after discussion in and an opinion from the Standing Committee, in accordance with Article 25. The Standing Committee is comprised of representatives from each of the Member States and also of the Commission, and decisions are made by qualified majority voting. Some decisions may also be subject to scrutiny by the European Parliament and referred to the European Council. If either of these bodies feel the decision goes beyond the delegated powers, or does not comply with the aim of the Regulation or the decision is not proportionate, it can be overturned.
For Article 13(5) claims that have received a favourable opinion from EFSA, decisions are made by the Commission alone and there is no vote.
Q95. Who is the competent Authority in the UK?
The Department of Health is the competent Authority in the UK for the nutrition and health claims Regulation. For matters of local enforcement, the local food authority (including Port Health Authority where relevant) is the competent authority.
Q96. Will a claim ever be taken off this list?
Article 19 of the Regulation does allow for claims to be modified, suspended or revoked based on a further opinion by EFSA. If EFSA, the Commission, or a Member State request that a claim be reconsidered, EFSA will issue a further opinion. This opinion will be made public and the applicant or any member of the public will have 30 days to comment. As with all claims, the Standing Committee will consider if the approved wording or conditions of the claim should be changed, based on EFSA's assessment.
Q97. How long will approval of claims based on new or emerging science take?
Under Article 18 EFSA has 5 months to produce an opinion once it has received a valid dossier. This timeframe may be extended by one month if additional information is required. The applicant will have 15 days to submit the required information. If EFSA's opinion is in favour of the claim the Commission will have 2 months to make a decision. During this time the Commission will consult with Member States. See appendix 4 for further details.
Q98. If the claim I wish to make has already been rejected can I still apply?
Yes. The Regulation does not restrict the resubmission of claims, however in order to get a positive opinion you would need to consider what amendments or changes would need to be made to the application, including the submission of new evidence.
Q99. How will protected data be indicated?
The Regulation does not specify how protected data should be indicated. Implementing rules for the application process (Article 15)[footnote 4] and EFSA guidance require protected data – confidential and proprietary data – to be kept separate within the application. This would allow separation of protected data in the case of disclosure. In the case of the health claim for water-soluble tomato concentrate, the studies containing protected data are clearly identified in the Commission Decision authorising the claim.
10.14 Nutrient profiles (section 7.2 of this guidance)
Q100. Will nutrient profiles apply to all foods including supplements?
Until the nutrient profiles are agreed it is not possible to tell how they will apply to specific products or food groups. The Commission has indicated that they agree with the interpretation that food supplements should be subject to an exemption from the application of nutrient profiles.
Q101. What will happen if I make a claim, but when the nutrient profiles are adopted my product fails?
Products that fail the nutrient profile cannot make health claims. It will depend on how the product fails the profile if and how nutrition claims can be made. Full details of the conditions associated with nutrient profiles can be found in section 7.2. Products that fail the nutrient profile will have 2 years, following adoption of the profiles, to comply with these conditions. Until the end of the transitional periods the product must comply with the other requirements of the Regulation to make the claim.
10.15 Transitional periods (section 782 of this guidance)
Q102. Is the transitional period in Article 28(1), which refers to nutrient profiles, different from the general transitional period?
Yes. This transitional period allows products, which do not meet with the nutrient profiles, 2 years before they must comply with the controls associated with the profile.
Q103. What happens if the nutrition claim I want to use is not in the list of authorised nutrition claims?
Since 19 January 2010, nutrition claims which are not in the Annex can no longer be used (Article 28(3)). If you would like a claim added to the Annex you should contact the European Commission.
Q104. Does the transitional period for trademarks and brand names apply to the product or the brand name or trademark?
It applies to the trademark or brand name. If you are using a trademark or brand name that is also a nutrition or health claim, and it was in use prior to 1st January 2005, it can continue to be used until 19 January 2022. Any additional claims would need to comply with the requirements of the Regulation.
Q105. What if my claim doesn't make it onto the list of health claims based on generally accepted scientific data and I haven't submitted a dossier under the route for claims based on new or emerging science, can I still use the claim?
If the EU Register of authorised health claims has been adopted and the decision has been reached that the claim, as submitted, should not be included it will no longer be permitted for use on food. A further application can be submitted, under Article 13(5), and if a positive decision is reached, it would then be permitted for use on food.
Q106. Why is there no transitional period for disease risk reduction claims and claims referring to children's development and health?
Transitional periods are in place to provide time for products and claims currently in use to come into line with the requirements of the Regulation. Under current legislation, disease risk reduction claims are not permitted to be made on food and so no transitional period was needed.
The controls on claims referring to children's development and health were added late during negotiations to the same provision for disease risk reduction claims and as a result did not have an associated transitional period. This has now been rectified with the adoption of EU Regulation 109/2008. This opens the transition period in Article 28(6) to claims referring to children's development and health.
Q107. What if I want to bring a new claim on to the market after 1st July 2007, but before the list of health claims is authorised?
This will depend on the nature of the claim. If the claim refers to the role of a nutrient or other substance in the growth development and functions of the body, Article 28(5) allows new claims to be used until there is a decision on the authorised list. This applies both to claims that have previously been used on food and claims that have never been used. Unlike other transitional periods this does not require an application for authorisation to be made. During this period the claim would need to comply with the general requirements of the Regulation (see section 5) and with any national rules that are in place.
After 1st July 2007 new health claims which refer to psychological and behavioural functions, slimming, weight control, reduction in the sense of hunger, an increase in the sense of satiety, a reduction of the available energy from the diet, children's development and health and disease risk reduction claims will not be permitted unless authorised.
Q108. Once the list of claims is adopted there doesn't appear to be a transitional period to allow food business operators time to make the necessary changes?
Disease risk reduction claims and claims referring to children's health and development which have been rejected up to now have mostly been given transition periods of 6 months. It is likely that claims submitted under the procedure in Article 13(2) and not authorised will also be granted 6-month transition periods. The relevant Commission Regulations will give details of any such transition periods.
Q109. Which claims can go through the procedures outlined in Article 28(6)(a) of the Regulation?
Article 28(6)(a) specifies that it is only available for claims which have been the subject of evaluation and authorisation in a Member State and fall into one of the following categories of claims:
- claims which refer to psychological and behavioural functions, are based on generally accepted scientific evidence, and are well understood by the average consumer
- claims which refer to slimming or weight-control or a reduction in the sense of hunger or an increase in the sense of satiety or to the reduction of the available energy from the diet, which are based on generally accepted scientific evidence and are well understood by the average consumer
- claims which refer to children's development and health
Q110. What does "health claims not authorised under this procedure may continue to be used for 6 months" (Article 28(6)(b)) mean?
Article 28(6)(b) applies to Article 13(1)(b) and (c) claims and claims referring to children's development and health. In our opinion, the intention of this transition period is to allow, for example, stocks of labels to be used up. A label bearing an unapproved claim still on the market after this 6 month period would become illegal. The 6 month period begins once the Standing Committee decision on the claim has been adopted and this has entered into force. However, in order to be sure about transition periods relating to particular claims it would be advisable to check the text of relevant Commission Regulations.
Q111. What does "used in compliance with national provisions" in Article 28(6)(first paragraph) mean? If I wish to rely on this provision to use a qualifying health claim in the UK should the claim have been used in accordance with UK national provisions prior to 19 January 2007 or could it have been legally used elsewhere in the EU prior to that date?
For qualifying health claims, the transition periods in Article 28(6) essentially maintain the conditions that applied before the Regulation came into force. Before the provisions of this harmonising Regulation came into force, non-harmonised, national provisions applied in different EU member states (MS). It therefore makes sense that national provisions in one MS should not have any bearing on what conditions apply in another MS during the transition periods set out in Article 28.
10.16 Enforcement (section 9 of this guidance)
Q112. I want to make a claim and I've read the legislation and this guidance. Is there anything else I need to do?
Food law enforcement in the UK is the responsibility of local authorities which are also a source of advice and guidance to businesses. It's always a good idea to check any particular claims that you want to make with your relevant local Trading Standards Department at your local authority in the UK where someone will be able to advise you about whether, in their opinion as the enforcement authority, you are complying with the law. Contact details of local Trading Standards Departments in the UK can be found on the Trading Standards Institute website .
A further resource is GOV.UK, which provides advice and support to businesses in the UK.
Appendix 1: sources of information
For further information about food safety please visit the Food Standards Agency website.
For further information about healthy eating advice please see NHS Choices.
For further information about the enforcement of food law please visit the website of Local Government Regulation (formerly known as LACORS).
For further information about what is considered a medicine and their control please visit the Medicines and Healthcare products Regulatory Agency website.
For further information and advice on the Regulation or these guidance notes please contact:
Nutrition Legislation Team
Obesity, Food and Nutrition Branch
Population Health Directorate
Department of Health and Social Care
39 Victoria Street
London SW1H 0EU
Tel: 020 7972 4340
E-mail: nutritionlegislation@dhsc.gov.uk
Appendix 2: terms used in this guidance
| Term | What it means |
|---|---|
| The Annex | The list of permitted nutritional claims and the associated conditions of use. These are in the Annex to the Regulation. |
| Authorised claim | A claim that has been assessed, approved and added to the list of permitted claims. |
| Claim | Any message or representation, which is not mandatory under Community or national legislation, including pictorial, graphic or symbolic representation, in any form, which states, suggests or implies that a food has particular characteristics. |
| Commission | European Commission |
| Community | European Community |
| EU Register | Centralised source of information about the Regulation, including the list of permitted nutrition and health claims. |
| EU Register of authorised health claims | Centralised list of authorised health claims. Once adopted only health claims on this list, or claims awaiting a decision, can be made on food. |
| DHSC | Department of Health and Social Care |
| Disease risk reduction claims | A health claim that states, suggests or implies that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease. |
| Dossier | The document containing information relevant to the application for authorisation of a claim. |
| EFSA | European Food Safety Authority (referred to as the Authority in the Regulation). |
| EU | European Union |
| FSA | Food Standards Agency |
| Food business operators | The natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control. |
| Health claim | Any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health. |
| Nutrient | Protein, carbohydrate, fat, fibre, sodium, vitamins and minerals listed in the annex to Directive 90/496/EEC (as amended), and substances which belong to or are components of one of those categories. |
| Nutrient profile | A set of nutritional criteria a product must meet to make a claim. |
| Nutrition claim | Any claim which states, suggests or implies that a food has particular beneficial nutritional properties due to the energy it provides, provides at a reduced or increased rate, does not provide or the nutrients or other substances it contains, contains in reduced or increased proportions or does not contain. |
| Other substance | A substance other than a nutrient that has a nutritional or physiological effect. |
| PARNUTS | Products for particular nutritional uses, which fall under Directive 2009/39/EC. |
| Scope | The products and type of claim the Regulation controls. |
| Standing Committee | European Commission's Standing Committee on the Food Chain and Animal Health. |
| Transitional period | A period of time set by the Regulation, during which its requirements will not apply, wholly or in part. |
Appendix 3: UK legislation controlling claims
Overarching legislation
The Food Safety Act 1990 and the General Food Regulations 2004, which enforce the food safety provisions of European Regulation 178/2002, make it an offence to falsely describe a food or provide misleading information regarding its nature, substance or quality. All claims need to comply with this legislation.
The Food Labelling Regulations 1996 (as amended)
Part III, Regulation 40 and 41, Schedule 6 and Schedule 8 of the Food Labelling Regulations 1996 put in place the following requirements on claims;
Claims that a food has the property of preventing, treating or curing a human disease or any reference to such property are prohibited;
Nutrition labelling is compulsory on any product for which a nutrition claim is made;
Schedule 6 sets specific criteria a product must meet to make certain nutrition claims, all of which are included in the Annex of permitted claims in the new European Regulation, which will now apply;
Schedule 8 sets criteria for other claims including claims relating to alcohol content;
The Food Labelling Regulations can be found online.
Where there may be any inconsistencies, Regulation 1924/2006 will in general take precedence over the Food Labelling Regulations 1996 and food business operators will need to ensure they comply with its controls. Further details of particular instances where 1924/2006 takes precedence and timescales associated with the new controls are given in Table 6 below.
Joint Health Claims Initiative (JHCI)
The JHCI was a tripartite alliance representing the interests of the consumer movement, the food industry and food law enforcement officers and developed a code of practice on health claims. While the list of authorised health claims, required by the Regulation, is still to be adopted we would still advise manufacturers intending to make health claims to follow the Code of Practice on Health Claims during the associated transitional periods.
Table 2: regulation 1924/2006 versus food labelling regulations
| 1924/2006Article | Food Labelling Regulations (as amended) regulation | Which applies | Timing |
|---|---|---|---|
| Article 1(2): requirements for non-prepacked foodstuffs | Regulation 29(1)(a): requirement for non-prepacked foods from vending machine | Although 1924/2006 will apply to non-prepackaged foodstuffs in general Regulation 29(1)(a) will continue to apply to non prepacked foodstuffs from vending machines | 1st July 2006 |
| Article 2: definition of nutrition claim | Regulation 2: definition of a nutrition claim | 1924/2006 applies. | 31 July 2009 |
| Article 4(3): Claims relating to alcohol content | Schedule 8, part 1: alcohol free, dealcoholised, low alcohol, non-alcoholic | The Food Labelling Regulations continue to apply | No timescale – continuous |
| Article 5(3): nutrition and health claims shall refer to the food ready for consumption in accordance with the manufacturers instructions | Schedule 6, part II, point 8: claims which depend on another food | 1924/2006 will take precedence in these cases. As well as complying with Article 5(3) claims must also comply with Article 3 and should not be false, ambiguous or misleading. | 19 January 2010 |
| Annex : Low Energy | Schedule 6, part II, point 2: low energy Schedule 8: low calorie on soft drinks | 1924/2006 applies. | 31 July 2009 |
| Annex: Reduced Energy | Schedule 6, part II, point 2: reduced energy | 1924/2006 applies to claims made on all foods including sweeteners. | 31 July 2009 |
| Annex: Source of protein | Schedule 6, part II, point 3: source of protein | 1924/2006 applies. | 31 July 2009 |
| Annex: High Protein | Schedule 6, part II, point 3: rich excellent source of protein | 1924/2006 applies. | 31 July 2009 |
| Annex: Source of [name of vitamin/s] and/or [mineral/s] | Schedule 6, part II, point 4: source of [name of vitamin]Schedule 6, part II, point 5: source of [name of mineral] | 1924/2006, to be eligible to use the claim a product must contain a significant amount of the vitamin or mineral. For those listed in Directive 90/496/EEC on nutrition labelling of foodstuffs this will be 15% of the RDA. Products will not be required to contain one sixth of the RDA or provide information about the % of the RDA contained in one serving, as required by the Food Labelling Regulations. However, the nutrition labelling requirements in Schedule 7 of the Food Labelling Requirements still apply. | 31 July 2009 |
| Annex: High in [name of vitamin/s] and/or [mineral/s] | Schedule 6, part II, point 4: rich or excellent source of [name of vitamin] Schedule 6, part II, point 5: rich or excellent source of [name of mineral] | 1924/2006. To be eligible to use the claim a product must contain at least twice the significant amount of the vitamin or mineral. For those listed in Directive 90/496/EEC on nutrition labelling of foodstuffs this will be 30% of the RDA. Products will not be required to contain 50% of the RDA or provide information about the % of the RDA contained in one serving, as required by the Food Labelling Regulations. However, the nutrition labelling requirements in Schedule 7 of the Food Labelling Requirements still apply. | 31 July 2009 |
| Annex – no claim | Schedule 6, part II, point 4: rich or excellent source of vitaminsSchedule 6, part II, point 5: rich or excellent source of minerals | Generic claims "contains vitamins" or "contains minerals" are not in the annex of permitted nutrition claims therefore they cannot be used. | 19 January 2010 |
| Annex – no claim | Schedule 6, part II, point 6: claim relating to the presence or absence of cholesterol | Claims relating to cholesterol content are not in the annex of permitted nutrition claims therefore they cannot be used. | 19 January 2010 |
| Annex – no claim | Schedule 8, part 1: starch reduced | The claim "starch reduced" is not in the annex of permitted nutrition claims therefore it cannot be used. | 19 January 2010 |
Appendix 4: flowcharts
How to make a claim
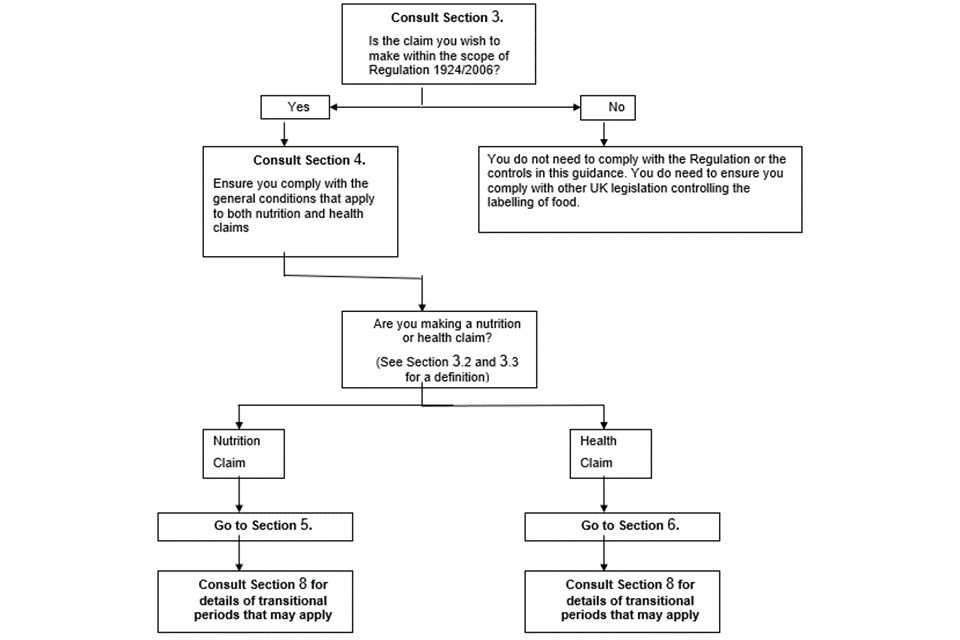
Text alternative to the flowchart
Is the claim you wish to make within the scope of Regulation 1924/2006?
No: You do not need to comply with the regulation or the controls in this guidance. You do need to ensure you comply with other UK legislation controlling the labelling of food.
Yes:
- make sure you comply with the general conditions that apply to both nutrition and health claims
- consult section 4
- see next question
Are you making a nutrition or health claim? (see section 3.2 and 3.3 for a definition)
If you’re making a nutrition claim:
- go to section 5
- consult section 8 for details of transitional periods that may apply
If you’re making a health claim:
- go to section 6
- consult section 8 for transitional periods that may apply
Do I need to comply with the Regulation?
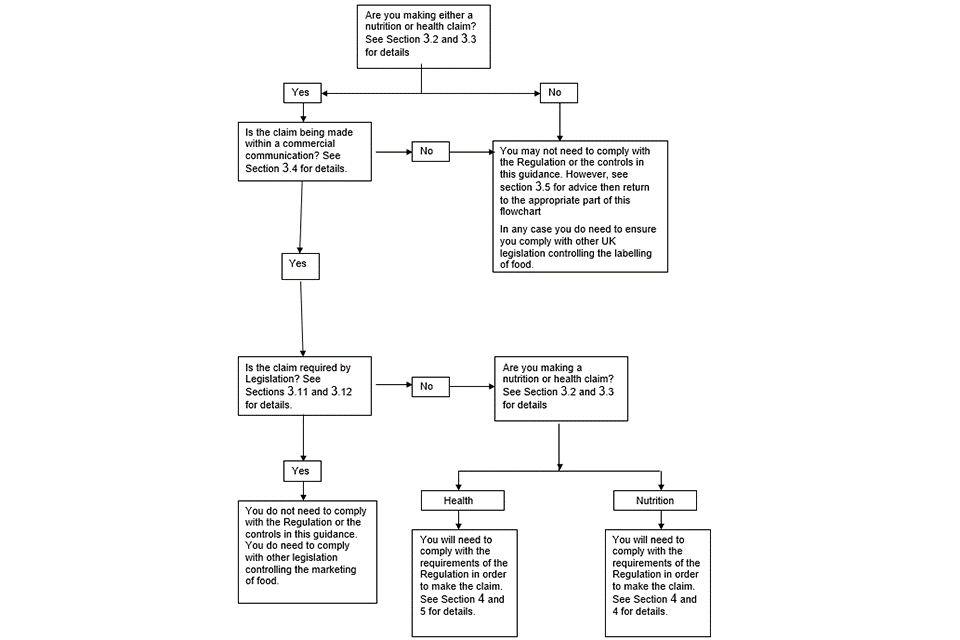
Text alternative to the flowchart
Are you making either a nutrition or health claim? (See section 3.2 and 3.3 if you need more details to answer this question.)
No: You may not need to comply with the regulation or the controls in this guidance. However, see section 3.5 for advice. Then return to the appropriate part of these questions. In any case you do need to ensure you comply with other UK legislation controlling the labelling of food.
Yes: see next question.
Is the claim being made within a commercial communication? (See section 3.4 if you need more details to answer this question.)
No: You may not need to comply with the regulation or the controls in this guidance. However, see section 3.5 for advice then return to the appropriate part of these questions. In any case you do need to ensure you comply with other UK legislation controlling the labelling of food.
Yes: see next question.
Is the claim required by legislation? (See sections 3.11 and 3.12 if you need more details to answer.)
Yes: You do not need to comply with the regulation or the controls in this guidance. You do need to comply with other legislation controlling the marketing of food.
No: see next question.
Are you making a nutrition or health claim? (See section 3.2 and 3.3 for more information.)
If you’re making a health claim, you will need to comply with the requirements of the regulation in order to make the claim. See section 4 and 5 for details.
If you’re making a nutrition claim, you will need to comply with the requirements of the regulation in order to make the claim. See section 4 and 5 for details.
The process of authorisation of health claims based on new or emerging science (timings as set out in the Regulation)
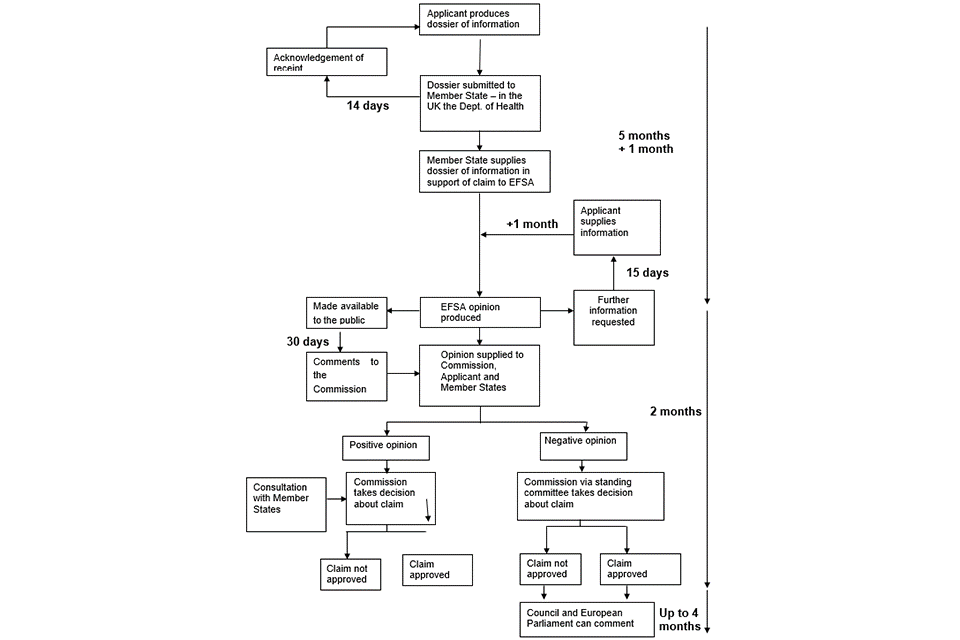
Text alternative to the flowchart
Step 1: the applicant produces the dossier of information.
Step 2: the dossier is submitted to the member state (in the UK this is the Department of Health and Social Care).
Acknowledgement of the reciept of the dossier can take up to 14 days.
Step 3: the member state supplies the dossier of information in support of the claim to EFSA.
Step 4: an EFSA opnion is produced.
Step 5: the opinion is supplied to the commission, applicant and member states.
If the opinion is positive:
- the commission takes a decision about the claim with consultation with member states
- the claim is either approved or not approved (step 6)
If the opinion is negative:
- the commission through the standing committee takes a decision about the claim
- the claim is either approved or not approved (step 6)
- the Council and European parliament can comment
Steps 1 to 4 can take up to 6 months.
Steps 4 to 6 can take up to 2 months.
If the opinion is negative, from the decision to the end of the period when the Council and European parliament can comment is up to 4 months.
The process of authorisation of disease risk reduction claims and claims referring to children's development and health (timings as set out in the Regulation)
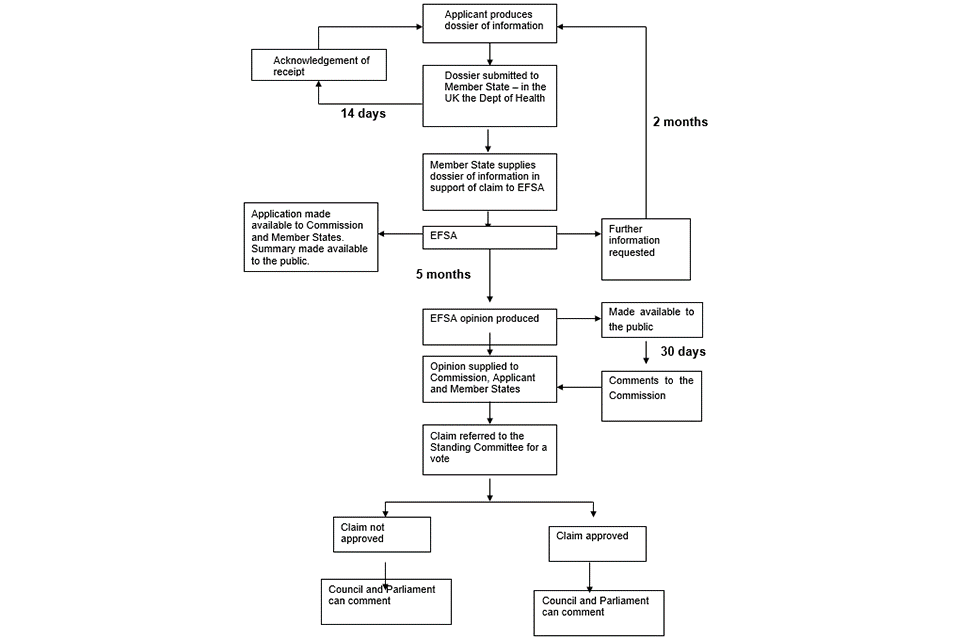
Text alternative to the flowchart
Step 1: the applicant produces the dossier of information.
Step 2: the dossier is submitted to the member state (in the UK this is the Department of Health and Social Care).
Acknowledgement of the receipt of the dossier can take up to 14 days.
Step 3: the member state supplies the dossier of information in support of the claim to EFSA. EFSA either requests further information. If further submission of information is requested, it can take up to 2 months for member state to resubmit information to EFSA.
Step 4: EFSA makes the application available to commission states and member states. A summary is made available to the public.
Step 5: An EFSA opinion is produced. This can take up to 5 months. The opinion is made public and then up to 30 days afterwards EFSA submits comments to the commission.
Step 6: the opinion is supplied to the commission, applicant and member states.
Step 7: the claim is referred to the standing committee for a vote.
Step 8: The claim is either approved or not approved. In both cases the Council and Parliament can comment.
-
When the Food Safety Act 1990 is referred to throughout this guidance, this should also be taken to include the Food Safety (Northern Ireland) Order 1991 in Northern Ireland. ↩
-
The rules on nutrition labelling are currently being reviewed in the proposed new Food Information Regulation. The final text of the Regulation is likely to be agreed at the end of 2011. ↩
-
This is the practice of the Medicines and Health products Regulatory Agency. ↩
-
Regulation (EC) No 353/2008 establishes implementing rules for applications for authorisation of health claims as provided for in Article 15 of Regulation (EC) No 1924/2006. These rules cite the EFSA guidance to be used when making applications. ↩
