Veterinary Medicines Directorate annual report and accounts 2021 to 2022
Published 25 October 2022
1. Annual Report and Accounts 2021/22
Presented to the House of Commons pursuant to Section 7(3)(c) of the Government Resources and Accounts Act 2000
Ordered by the House of Commons to be printed 25 October 2022
HC 473
© Crown copyright 2022
ISBN 978-1-5286-3413-7 E02755278 10/22
2. Performance Report
3. Overview
Chief Executive’s Statement
The Veterinary Medicines Directorate (VMD) is the UK regulator of veterinary medicines and policy lead body responsible for issues concerning the use and manufacture of veterinary medicines in the UK.
The VMD’s aim is to protect public health, animal health and the environment and promote animal welfare by assuring the safety, quality and effectiveness of veterinary medicines. The VMD supports the Defra objectives on public and animal health, the promotion of a sustainable, competitive and safe food supply chain, and growing the rural economy.
Through the end-to-end regulatory oversight of inspecting, authorising, monitoring and enforcing we facilitate availability of safe and effective medicines to prevent and treat diseases and improve welfare in animals. In doing so we ensure that this is not at the expense of human health or the environment. Veterinary medicines are important to ensure a viable livestock and fish-farming industry and healthy lives for companion and working animals.
VMD is the lead policy maker and adviser to ministers on veterinary medicine regulation and residues surveillance. The VMD also leads activity across government on antimicrobial resistance (AMR) in animal health. This year we continued to work closely with the veterinary profession, livestock industry and other stakeholders across the public and private sectors to implement the Government’s 2019-2024 AMR strategy (20-year vision on AMR and the 5-year action plan).
The VMD had a very successful year, delivering the Business Plan within the budget, while maintaining services acknowledged as excellent, and maintaining a high staff engagement index. We have retained whole business ISO 9001:2015 certification, and ISO 27001:2013 for Information Security Management Systems.
The international collaboration opportunities available since leaving the EU have started to be realised through membership at international fora and through new agreements in place with other global regulators.
Over the course of the year we have continued to respond, as per last year, to the impacts and restrictions of the Covid-19 pandemic and adapting to the new version of normal. The skills, capabilities and wellbeing of the VMD staff has been a high priority, to ensure we have the right resources to deliver our outcomes.
The path to a successful year however has been affected by significant challenges both in relation to staff recruitment and retention and in relation to the continuing uncertainty of the situation in Northern Ireland.
4. About the Veterinary Medicines Directorate
4.1 Purpose
The VMD is the UK competent authority for veterinary medicines regulation. We seek to ensure maximum availability of safe and effective medicines for prevention and treatment of diseases and improved welfare in all animal species. We also ensure that the medicines pose minimal possible risk to human health and the environment. The breadth of our functions in support of our purpose are presented below.
The Overview section of this report summarises our organisation, objectives, key risks to the achievement of our objectives, and how we have performed during the year.
4.2 We are responsible for:
Government:
- servicing, developing and implementing government policy and legislation on veterinary medicines, including medicated feed and antimicrobial resistance (AMR)
- supporting ministers through briefing and advice on correspondence and Parliamentary Questions
- collaborating with government departments and agencies, and stakeholder groups
- working internationally
- enforcement of the Veterinary Medicines Regulations (VMR)
- commissioning veterinary medicines related Research and Development (R&D)
- leading on responsible antibiotic use and on AMR surveillance
Veterinary pharmaceutical industry:
- assessing, issuing and processing applications for or changes to, marketing authorisations (MAs) valid in the UK
- post-authorisation surveillance (pharmacovigilance) through the collation of suspected adverse event reports
- licensing and/or inspection of manufacturers, wholesale dealers and retailers of veterinary medicines
Food industry:
- Manage the programme for the surveillance of residues of veterinary medicines and banned substances in British livestock and animal produce, reporting of results and co‑ordinating follow-up action.
4.3 Future developments, performance and risk
Our key challenges throughout 2021/22 and our plans for meeting them have been outlined in the VMD’s Business Priorities section, of this report.
Key future developments and/or risks are the:
- UK’s regulatory independence from the EU and requirements for Northern Ireland
- economic climate affecting the veterinary pharmaceutical industry and the number of authorisation applications the VMD receives
- developing new international alliances on medicines regulation
- implementation of the new UK strategy on antimicrobial resistance
- continue broadening externally funded outreach work on developing medicines regulation and antimicrobial resistance control capability
- building our role and reputation as a training provider on all aspects of veterinary medicine regulation
- revision of the VMR as they have effect in Great Britain
4.4 Operating framework
We operate within an overall policy and financial framework determined by the Secretary of State for Defra, through the Parliamentary Under Secretary of State for Rural Affairs and Biosecurity. More information on our governance is set out in our Framework Document.
4.5 Going concern
The financial statements are prepared on a going concern basis. In common with other agencies in the Defra Group, the future financing of the VMD’s liabilities is to be met by future supply of Grant in Aid and the application of future income from fees and charges, both approved annually by Parliament. Approval for the amount required for 2022/23 has already been given and there is no reason to believe that future approvals will not be forthcoming.
The VMD is responsible for enforcing the Veterinary Medicines Regulations and the VMD continues to lead on activity across government on AMR in animal health. The UK government have committed to maintaining funding to implement the 2019-2024 AMR strategy and the 5-year action plan, both multi-year projects which confirms the VMD functions are necessary and should continue to be delivered and that the VMD is best placed to carry out these functions.
5. Performance Summary
We thoroughly monitor our financial performance and continue to seek efficiency while maintaining our standards of performance. We achieved our 2021/22 targets, while maintaining our fees at or below the rates set in the Veterinary Medicines Regulation 2013 applicable to the veterinary pharmaceutical industry or SI No: 2945 applicable to the food industry charges.
In 2021/22 the VMD delivered regulatory services to the veterinary pharmaceutical industry (VPI) to a total cost of £6.01m (2020/21: £5.98m). These services are for authorisations and inspections work, costs are recovered through fees and charges to industry. The cost of regulation of the food industry is £3.79m (2020/21: £3.68m), these were recovered through charges levied on abattoirs and other food processors. We achieved our cost recovery targets for the year.
Our total operating expenditure for the financial year was £27.64m (2020/21: £24.68m), funded by operating income of £11.22m (2020/21: £10.63m) resulting in a comprehensive net expenditure of £16.43m (2020/21: £14.05m) to be funded by grant-in-aid from Defra. The statement of financial position on 31 March 2022 shows taxpayers’ equity of £3.63m (2020/21: £6.82m).
In the year, £7.67m (2020/21: £5.34m) was utilised, inclusive of non-cash and notional recharges for our activities on policy, enforcement, management of the R&D, AMR programmes and activities to support our international capacity building objectives.
Extra funding was made available for ongoing VMD activities to support the EU Transition, to ensure smooth continuation of medicines availability and over-sight at the point of EU Exit and to explore the opportunities that arise from exit. Resources to support EU transition work were £11.10m (2020/21: £9.42m), including £1.53m (2020-21: £1.87m) capital investment, for the development of IT systems.
The government signed agreements with ferry operators to provide freight capacity, to mitigate the risk of disruption as the UK and EU adjust to new border processes after the transition period and as a result of the Covid travel requirements on hauliers. This ferry capacity made available an alternative supply route for companies moving prioritised products into the UK, which included veterinary medicines. The Department for Transport reported a cost of £0.88m (2020/21: £1.48m) for unused freight capacity to be recharged up to 31 March 2022.
5.1 Cash flow
Cash and cash equivalents have increased to £3.74m as of 31 March 2022 from £1.89m on 31 March 2021, an increase of £1.85m. In year, the VMD’s net cash draw down was £12.42m (2020/21: £11.9m). The net cash requirement under the gross control funding arrangement was £10.57m (2020/21: £13.9m).
We follow the principles of the Better Payment Practice Code in compliance with the Public Sector Payment Policy and paid 99.92% (2020/21: 99.3%) of undisputed invoices within five working days.
Business priorities and Key Performance Indicators (KPIs)
We successfully delivered against the majority our framework of Deliverables and KPIs through which we provide the best possible service to all our customers.
6. Performance Analysis
6.1 About the performance analysis
This report outlines our performance against our priorities for the financial year from 1 April 2021 to 31 March 2022. It gives examples of how we are achieving our aims and highlights important events from the year. It follows the structure of our Deliverables and KPIs for 2021/22 to show how we are meeting our objectives.
6.2 The Defra Strategy
A clear, shared framework is provided to staff across the whole group of Defra organisations (including non-ministerial departments, executive agencies, non‑departmental and other public bodies) in our Outcome Delivery Plan. Actions to achieve Defra’s strategic objectives are described in detail in the plan.
6.3 Our performance
We successfully delivered against the majority of our Deliverables and KPIs, the details of which are given against our business priorities below.
6.4 Business Priority 1 – Policy
Policy Lead on behalf of Defra for Veterinary Medicines and Antimicrobial Resistance (AMR)
Why are we doing this? The VMD has overall responsibility in the UK for veterinary medicines policy, and animal health aspects of antimicrobial resistance in England, in the broader context of Defra’s animal health and welfare responsibilities and the contribution this makes to safeguarding public health.
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1. | Publish consultation on revisions to the Veterinary Medicines Regulations (Q3). | Partially met | In December 2021, the VMD started a series of engagement sessions with stakeholders. This informal consultation set out our thinking around the changes to the VMR and was intended to invite early feedback from stakeholders ahead of the formal, public consultation. |
| 2. | Assess the evidence of environmental impacts of zinc oxide (Q2). | Yes | A report assessing the evidence of environmental impacts of zinc oxide has been completed. Additional work has, subsequently, been highlighted for which the funding has been agreed. |
| 3. | Establish a VMD Working Group (Q1) and identify evidence gaps and commission research to close gaps (Q4). | Yes | A formal Anthelmintic Resistance Working Group (ARWG) has been established and meets every two months. A successful equine AR stakeholder workshop was held and identified some AR research gaps which have been discussed and supported for funding at Research Programme Steering Group (RPSG). |
| 4. | Commission evidence to close evidence gaps on topical pet flea products’ contribution to environmental levels and impacts (Q2). | Yes | The VMD is funding a project with University of Sussex within our R&D programme on the impact of Veterinary Medicinal Products (VMPs) in the environment. This is specifically looking at the potential direct and indirect environmental exposure pathways for dog parasiticide products (containing imidacloprid or fipronil), in relation to the significance of veterinary use to levels of these seen in the environment. The project is due to complete with outputs from it in March 2023. |
| 5.i | Consider options on biodiversity impact assessment and develop a plan for incorporation into the authorisation process (Q3). | Yes | A report considering the options on biodiversity impact assessment has been produced. An internal plan has been developed to incorporate any appropriate options highlighted from the report into the authorisation process. |
| 5.ii | Explore authorisation routes for medicinal products that reduce greenhouse gas emissions, including liaison with the Food Standards Agency (FSA) (Q3). | Yes | Following meeting with FSA a report has been completed and outputs reported to the VMD Management Board (MB). |
| 5.iii | Provide funding to projects exploring development of medicines for pollinators. | Yes | The VMD is funding a project with the University of Aberdeen within our R&D programme which aims to provide impetus for the pharmaceutical industry to develop medicinal treatments for unmet clinical needs in bee health. This project covers a number of important diseases and pests that impact on bee health. This project is due to complete with outputs from it in March 2023. |
| 6. | Annual test of the VMD Veterinary Medicines Availability & Diseases Emergency Response (VADER) response exercise or for it to be stood up and staffed within 24 hours of need. | Yes | The VADER emergency response system was successfully tested in March. |
| 6.1 | Contribute to the work on global supply chains | Yes | The VMD participated in a project (with the Department for International Trade (DIT) and the Department of Health and Social Care (DHSC) analysing the manufacturing sites of raw materials and continues to contribute to cross-Government work on global supply chains. |
6.5 Business Priority 2 – Delivering effective regulation as an independent trading nation and operationalisation of the Northern Ireland Protocol
Why are we doing this? The UK has left the EU. As a consequence, we need to ensure that animal medicines availability in the UK is not compromised and that the UK remains attractive to the pharmaceutical industry for Marketing Authorisation applications and complying with all post authorisation regulations.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1 | Completed IT requirements (including testing and communications) and prepare authorisation processes (Q3). | Yes | A new IT system for pharmacovigilance and adaptations to other IT systems have been implemented to meet new legislative requirements. |
| 1.1 | Maintain regular liaison meetings with other government departments: Department of Agriculture, Environment and Rural Affairs (DAERA), Department of Health (DoH - Northern Ireland and Department of Agriculture, Food and the Marine (DAFM). | Yes | Regular monthly and quarterly meetings held with representatives from DAERA who also attended other relevant VMD meetings such as National Regulatory Committee (NRC). Meetings were also regularly held with DoH and the NI Chief Veterinary Officer (CVO) |
| 1.2 | Regular liaison and cooperation with The Health Products Regulatory Authority (HPRA) on authorisation and other policy aspects as they relate to NI. | Yes | A Memorandum of Understanding (MOU) has been established with the HPRA and meetings were held regularly throughout this reporting period. During each meeting there was a useful exchange on areas of common interest. |
| 2. | Regain membership of Veterinary Dictionary for Drug Related Affairs (VeDDRA), Working Group of Enforcement Officers (WGEO) and Vermont Biomedical Research Network (VBRN) and attain membership of Transatlantic Taskforce on Antimicrobial Resistance (TATFAR) (Q2). | Yes | We now fully participate in the VeDDRA group. A MOU has been signed between the VMD, UK and the EU VBRN (represented by the European Directorate for the Quality of Medicines & HealthCare (EDQM) for sharing of information related to batch release of Immunological Veterinary Medicinal Products (IVMP) and observership to the VBRN. WGEO - The UK has been invited to the Spring 22 meeting in Paris, with Observer Status. We attained membership of the TATFAR. |
| 3.1 | Maintain monthly liaison meetings with the regulatory bodies with responsibility for veterinary medicines in the USA, Australia, Canada and New Zealand, establish at least one operational network (Q3), and have processes in place to accept joint marketing authorisation applications (Q4). | Yes | Meetings with the Quints group (USA, Australia, Canada, New Zealand and the UK) continued largely on a monthly basis throughout the year. Areas of common interest were discussed with information freely exchanged. A joint review process for significant variations submitted in one or more of the Quints regulatory jurisdictions was adopted. A joint review procedure with New Zealand has been agreed. An application has been submitted under the joint review process agreed with Canada. This has passed validation and is now in the initial assessment phase. Regular meetings with Swissmedic have also been arranged with a view to exchange information and perhaps agree a joint review process. |
| 3.2 | Complete agreement with the FDA (USA) for mutual recognition of GMP inspections (Q2). | Yes | The UK-US Mutual Recognition Agreement (MRA) was officially extended to include veterinary pharmaceuticals. |
| 3.3 | Establish a Memorandum of Understanding with at least two medicines national competent authorities (Q3). | Partially met | An MOU has been agreed with the HPRA. Work is ongoing to refresh the agreement we have with Veterinary Drugs Directorate and contact has been made with Canadian Centre for Veterinary Biologics to have a similar MOU in place with the biologics side. The VMD has also reached out to the Centre for Veterinary Medicines (USFDA) seeking their thoughts on agreeing an MOU in light of the enhanced Quints cooperation. A draft MOU has been proposed to cover our interactions with the MHRA highlighting particular areas where cooperation and information exchange would be of benefit. A draft MOU with Swissmedic has also been drafted. |
| 3.4 | Participate in trade and technical co-operation negotiations to secure agreements for collaborative working. | Yes | In collaboration with DIT, DHSC and Defra, we have secured commitments for co-operation on the regulation of veterinary medicines and AMR in both, the Australia and New Zealand Free Trade Agreement negotiations. |
| 4. | Establish regular liaison with the Coordination Group for Mutual Recognition and Decentralised Procedures (CMDv) chair, particularly for the operation of authorisation procedures that include Northern Ireland (Q1). | Yes | We are continuing to keep the lines of communication open and in place. Sessions with CMDv Member State representatives have taken place regarding variations and the Union Product Database as foreseen under the new EU legislation. Meetings have also taken place with EMA to discuss specific Pharmacovigilance related cases and Substance, Product, Organisation, Referential (SPOR) requirements. |
6.6 Business Priority 3 – Delivery of core regulatory services
A) Facilitate optimal availability and safe use of veterinary medicines
Why are we doing this? The VMD authorise veterinary medicines in the UK. Our work creates an environment that provides confidence and investment within the medicines industry and enables exports. It protects the food chain, human and animal health as well as the environment and biodiversity. It also ensures that unsafe medicines can be identified, and appropriate corrective action taken including, where appropriate, removal from the market.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1&2 | Monthly reporting against all Published Standards which set out the timelines and performance indicators for a range of key functions. Overall performance against published standards to be at or above the effective level (≥92% of performance indicators met) (Q4). | Yes | The expectations for the VMD’s performance (time and quality) in terms of handling applications, inspections and pharmacovigilance matters are set out in the published standards. Of 29 individual performance parameters, where 12 or more applications were received, 28 met the ‘excellent’ performance standard and one was rated as being effective. Overall, our performance related score was 98.85%. The independent Veterinary Products Committee (VPC) rated the quality of the VMD initial assessments for Marketing Authorisation applications as ‘substantial’ – the highest rating. |
| 1.2 | Formalise and publish a regulatory science strategy and plans to operationalise the strategy (Q3). | Yes | The Regulatory Science Strategy was published in November 2021 and launched at the VMD/VPC Open day. Action plans have been developed for each of the topic areas detailing deliverables for the next 5 years. |
| 2. | To review the requirements of vet practice inspections and identify improved approaches to securing required assurance (Q2). | Yes | Due to Covid-related restrictions, we developed a procedure for remotely assessing veterinary practice premises’ (VPPs’) compliance with the VMR. We also developed an ‘app’ to improve the efficiency of data capture during an inspection and production of the inspection report which will be implemented from Q1 2022-23. We regularly liaised with the Royal College of Veterinary Surgeons (RCVS) to share and compare our respective VPP inspection findings to ensure equivalence. |
| 3. | Report Pharmacovigilance findings to the Veterinary Products Committee and publish findings. Take proportionate action. | Yes | The Pharmacovigilance team have provided reports to the Veterinary Products Committee at each of their meetings, for animal, human and environment adverse events. See our pharmacovigilance annual review for 2020. During 2020, 88 products labels or Summary of Product Characteristics were improved based on pharmacovigilance data. |
| 4.1 | Meet the assessment and issuing of import certificates as detailed within the published standards | Yes | 99% of Special Import Certificate applications received for VMD assessment were approved or refused within 2-25 working days of receipt, as per the VMD’s published standards. |
| 4.2 | Report on availability issues and import patterns to the Veterinary Products Committee at each of its meetings. | Yes | The Supply Team have provided quarterly summary reports of all Special Import Certificates granted by the VMD for the information of the Veterinary Products Committee at each meeting. Additional information regarding key availability issues for UK veterinary medicines and consequential trends observed in Special Import Scheme activity have also been shared with members. |
| 4.3 | Enhance MAH reporting and wholesale dealer intelligence of availability and inform others as required (Q2). | Yes | We have continued to liaise with relevant stakeholders when availability issues became known. This included liaison with Marketing Authorisation holders, veterinary wholesale dealers and representatives from relevant veterinary bodies. A requirement for mandatory reporting of veterinary medicine shortages will be included in the VMR consultation. Information of key availability issues affecting UK veterinary surgeons have been published on the VMD’s ‘Known supply problems’ page. |
| 4.4 | Maintain an up-to-date list of alternative products for veterinary medicines identified as critical for the UK. | Yes | We have maintained a list of alternative products for UK authorised medicines considered of high importance to animal health and welfare for the purpose of supporting emergency response planning. |
| 4.5 | Work with the pharmaceutical industry to develop resilience within the supply chain (Q2). | Partially met | Feedback has been received from industry regarding what Government can do to help industry to build resilience. The VMD continues to work with industry on increasing resilience, both in the short-term and in the longer-term. |
| 5. | Respond to ‘High risk’ product defect reports within five working days and all others within ten working days. | Yes | All medium or low risk product defect reports have been responded to within 10 working days. Of the 52 product defect reports received, 2 were considered as high risk and were responded to within 5 working days. |
| 6. | Annual test of our emergency response capability (Q4). | Yes | Emergency response capability satisfactorily tested. For example, disaster recovery IT tests and all staff emergency communication tests. |
B) Surveillance, research and enforcement activities that influence the responsible, safe and effective use of veterinary medicines in the UK and protection from medicines residues in food
Why are we doing this? To detect unsafe products or activities and to take corrective action to ensure confidence in veterinary medicines, assist competitiveness, aid consumer confidence, assist with safety and help to ensure medicines, in particular antibiotics, are used responsibly to maintain effectiveness.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1. | Residues surveillance plan for 2021 delivered and results published (Q4); and publish summary results on a two-monthly basis. | Yes | The 2021 residues surveillance programme was delivered with over 99.7% of planned samples being collected. This is a great achievement given the challenging conditions posed by Covid-19 and Avian Influenza, which was a significant pull on sampling resources. The number of non-compliances identified remained low at 0.5% continuing the trend of recent years. |
| 2. | Establish resource to fulfil the function and agree an SLA with the Defra UK Office SPS Trade Assurance (UKOSPSTA) (Q1). | Partially met | Resourcing is in place and an SLA was agreed with UKOSPSTA. |
| 3.1 | Milestones and deliverables led by the VMD in the UK 5-year AMR national action plan achieved, namely: Implement a new veterinary pathogen surveillance programme (Q1). | Yes | Minimal inhibitory concentration (MIC) batch testing methods of veterinary respiratory pathogens was implemented in 2021 and the first data published in the UK-VARSS 2020 report. Programme to be expanded to other veterinary pathogens in 2022. |
| 3.2 | Annual report on antibiotic sales and antibacterial susceptibility data published (Q3). | Yes | UK-VARSS 2020 was published presenting antibiotic sales, usage and resistance data from 2020. |
| 4. | Publish summary data including cases handled, internet listings removed, enforcement notices served, and outcomes of successful prosecutions on a quarterly basis. | Yes | The Enforcement Newsletter was produced three times during this period and circulated widely to our enforcement stakeholders and partners. The newsletter focusses on particular issues relevant to veterinary medicines. As well as giving a summary of cases handled and internet listings removed, it also provides a link to enforcement notices published, prosecutions taken and Police Cautions issued. |
C) Effective customer and stakeholder engagement
Why are we doing this? To raise awareness of the work of the VMD and why it is important that veterinary medicines are properly regulated and used. To enable effective feedback on our work. To enable maximum utilisation of VMD datasets.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1 | Develop a new Communications and Stakeholder Engagement Strategy. Complete (Q1). | Yes | We have developed a new strategy that aims to deliver an improved approach to information sharing. It includes developing existing channels and identifying new opportunities to share more consistent, comprehensive, and appropriate communications and engagement with our stakeholders. |
| 1.2 | Successful delivery of 90% of 2021/22 priorities as set out in Action Plans under the Strategy | Yes | We have established the Vet Medicines Blog as a new channel to reach users of vet medicines and professional animal keepers. We have utilised online tools to engage with stakeholders more regularly, remotely. We have improved our gov.uk structure to make finding information and contacting us easier and we have already seen a reduction in commonly asked questions. |
| 1.3 | Establish a network of social media champions in the VMD to increase VMD social media impact by achieving: 10-15% increase in VMD followers on Twitter, Facebook and LinkedIn; 10-15% increase in posts on social media | Yes | We have focussed our approach to social media activity and collaborate widely across the VMD to create timely and informative content. Our social media following has increased by 60%. |
| 1.4 | 90% of feedback from Company meetings and open days and other stakeholder engagement activities give the VMD a positive rating for example for company meetings, at least 4 out of 5. | Yes | A total of 23 returns were received from companies after meetings, with an average score per question of 4.23 out of 5. |
| 2.1 | Fewer than ten negative feedback comments received on the accuracy and completeness of VMD‘s GOV.UK material. Ensure all new material meets accessibility standards. | Yes | All new gov.uk content meets accessibility standards according to our accessibility policy. We received 5 notifications of inaccurate content or technical faults which we have resolved. |
| 2.2 | At least quarterly liaison with NOAH and with other stakeholders in line with the VMD’s Strategy. | Yes | Meeting schedules with our stakeholders were completed. |
| 3 | Access to information requests: at least 95% cases responded to on time. | Yes | 100% of access to information requests responded to within deadline. |
| 4 | Continue to publish VMD datasets on the product information database and on data.gov.uk. | Yes | We continue to publish all information that we are able and is of interest. |
6.7 Business Priority 4 – To support the capacity building efforts of international regulatory authorities to ensure high quality veterinary medicines that are used safely and effectively to protect human and animal health and the environment
Why are we doing this? There is increasing international recognition of the importance of regulation of veterinary medicines driven by a combination of interest in stewardship and appropriate use of antibiotics as well as development of livestock business for low and middle-income countries. UK international action is expected to support the cross-government 20 year antimicrobial resistance (AMR) vision and 5 year AMR national action plan, and the UN Sustainable Development Goals. The expertise of the VMD to support capacity development is recognised by our designation (together with our partner agencies APHA and CEFAS) as a Food and Agriculture Organisation (FAO) AMR Reference Centre. Non-government funding is available to be accessed.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1 | Objectives and activities set out in the project initiation document, and/or if subsequently mutually amended, are achieved. Global directory of vet med regulators (Q1); draft benchmarking tool (Q2); harmonised marketing application form and common electronic submission portal criteria (Q4). | Yes | All of the objectives were met as set out in the project initiation document. The Global directory of veterinary medicines regulators now contains an entry for 142 countries. The common application form was agreed as was the draft benchmarking tool. The criteria for the electronic submission portal was also agreed and proof of concept is now being developed. |
| 2. | Three countries participate in VMD e-learning (Q4). | Yes | Ethiopia, Philippines and Nepal attended the VMD e-learning on residue surveillance. |
| 3.1 | Support the development of regulatory capability and resistance and use surveillance capability and capacity in five lower/middle income countries. | Partially met | Activities have been supported directly in four countries, and indirect support provided to a wider range of countries through participation in regional initiatives in south east Asia and east Africa (led by UN FAO and Galvmed respectively), as well as through support for the global initiative on pre-qualification of Foot and Mouth Disease vaccines led by UN FAO. |
| 3.2 | Establish in the UK a One-Health UK AMR Reference collaborative by coordinated governance of the UK based FAO, World Health Organisation (WHO) and World Organisation for Animal Health (OIE) reference centres for AMR (Q3) | Yes | Terms of reference for the Reference Centre collaboration have now been agreed across the participating centres. |
6.8 Business Priority 5 – Capacity and capability
To ensure funding streams are used efficiently to maintain capability and capacity to deliver business objectives in a sustainable and environmentally friendly way.
Why are we doing this?: To enable the VMD to deliver its business objectives by maintaining staffing and other support structures at a level that ensures the business is fit for purpose. Through risk management we aim to identify and respond to issues that could adversely affect the business. We seek continuous improvement to enable us to meet current and future business needs and to ensure we remain competitive alongside other National Competent Authorities.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1.1 | Internal Audit opinion to be “moderate” or better. | Yes | Government Internal Audit Agency (GIAA) gave us a Moderate Assurance rating. |
| 1.2 | External Audit assurance to report an unqualified opinion. | Yes | The external Auditors reported an unqualified opinion. |
| 2.1 | Delivery of 90% of targets set out in the IT strategy. | Yes | 90% of prioritised targets delivered. Some highlights include: Delivery of new Pharmacovigilance system, Cyber Security enhancements, Delivery of IT services required to support the Northern Ireland Protocol, changes to the Electronic application form to support submission of Marketing Authorisation variation applications, Digital Disaster Recovery enhancements and testing, continued enhancement of VMD Digital services and cloud infrastructure to support future IT delivery, continued alpha phase of new Special Imports digital service, virtual environment upgrades. |
| 2.2 | To achieve at least 95% uptime for VMD’s IT systems. | Yes | 98% uptime achieved for VMD IT systems |
| 2.3 | Develop and implement a new system for pharmacovigilance reporting (Q3). | Yes | A new pharmacovigilance system was purchased and implemented Q4, meeting the new VMR requirements for Northern Ireland (NI) and the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). |
| 3. | No serious risks on risk register materialise. | Yes | No serious risks on risk register materialised. |
| 4. | To maintain business certification against ISO 9001 and ISO 27001 by end Q3 2021. | Yes | Following the annual audit VMD retained its ISO certification for ISO 9001 and ISO 27001. |
| 5.1 | Maintain a top quartile staff engagement score in the 2021 Civil Service People Survey. | No | VMD dropped out of the top quartile. We are further analysing the results in order to improve staff engagement. |
| 5.2 | Training days per full-time equivalent (FTE) to be at least 5 days per year. | Yes | 5.85 training days completed per FTE. |
| 5.3 | Sickness absence – to maintain in 2021/22 the low number of days lost per FTE for short-term sickness and to perform well compared to Defra and wider public sector benchmarks for equivalent periods. | Yes | Whilst the average number of days lost to short-term sickness has increased from 0.6 in 2020/21 to 1.3 in 2021/22 the figure is still low when compared to the most recent wider public sector benchmarks available. |
| 5.4 | To prepare for possible changed working patterns (home/office) and practices following easement of covid-19 restrictions and further embed well-being support (Q2). | Yes | Completed through business continuity meetings (BCM). |
6.9 Business Priority 6 – Value for money
Achieve cost recovery and delivery of value for money.
Why are we doing this? To ensure that we can demonstrate to all customers how we achieve best value for money. To ensure an appropriate regulatory framework is in place that supports growth whilst providing appropriate safeguards to protect the food chain, human and animal health and the environment. To ensure that the costs of activities that are carried out to support delivery of our international capacity building objectives are adequately financed, outside our policy budget and industry fee structure.
Key Performance Indicators
| Reference | KPI | KPI Met | KPI Achievement |
|---|---|---|---|
| 1 | Cost recovery for charged for regulatory services to be within the range 100-102% of full cost recovery. | Yes | We recovered all our costs including cost of capital charge from the provision of services to industry. An analysis of the fees and charges to industry is provided within the Parliamentary Accountability and Audit Report section. |
| 2 | To have identified further reductions in regulatory burden. | Yes | One example is the ‘airgap’ mitigations for GB, which were required to minimise regulatory burdens to marketing authorisation holders - without making legislative changes - whilst respecting the legislation applying in GB and the EU, until such time the Veterinary Medicines Regulations 2013 (VMR) are amended. Other reductions in regulatory burden have been identified and will be implemented when we update the VMR. |
| 3 | Ensure that the 2020/21 budget reduction for policy work is met. Assurance from Defra. | Yes | The Policy budget reduction was met. |
| 4 | Pay 97% of all undisputed and valid invoices within 5 working days. | Yes | We achieved 99.92%. |
7. Social and Community Issues and Environmental Matters
7.1 Social and community issues
Defra group equality, diversity and inclusion strategy 2020 to 2024, which includes the VMD, aims to ensure that Defra is a great place to work and we deliver our aspirations to be an organisation with a diverse, open and inclusive culture.
We do this by: creating more inclusive cultures; building and sustaining a representative workforce; making the UK a great place to live for all; improving Equality Diversity and Inclusion capability and confidence; and communicating, raising awareness and reporting progress.
All our staff are required to complete training on:
- Security & Data Protection training
- Health and safety awareness
- Diversity and inclusion in the civil service
All our assessments of marketing authorisations include an environmental impact assessment to ensure that the use and disposal of veterinary medicines do not adversely affect the environment.
7.2 Environmental matters: sustainability report for 2021/22
For more information please see Defra’s Annual Report and Accounts – section headed: “Commentary on Sustainable Performance” and the Greening government ICT and digital services annual report, which covers the VMD.
Under the Greening Government Commitments, which sets out how we are working toward net zero by 2050, we have a commitment to reduce our greenhouse gas emissions, the amount of waste we generate and our water consumption. Defra’s Built Environment Sustainability Team (BEST) provides us with quarterly figures on each of the following categories.
Our baseline figures are based on our usage during 2017/18. The costs for our energy, water and waste disposal are part of the overall Defra Corporate Recharge costs and are not billed separately.
7.3 Sustainability Data
Energy consumption (1000 kWh)
| Baseline | 2021/22 | 2020/21 | 2019/20 | |
|---|---|---|---|---|
| Electricity | 271.7 | 223.9 | 212 | 293.6 |
| Gas | 36.7 | 29.6 | 27.9 | 34.9 |
Greenhouse gas emissions (tonnes CO2)
| Baseline | 2021/22 | 2020/21 | 2019/20 | |
|---|---|---|---|---|
| Scope 1: direct emissions | 67.6 | 66.6 | 16.1 | 74.6 |
| Scope 2: indirect emissions | 95.5 | 47.5 | 54.2 | 75.0 |
| Scope 3: domestic travel emissions | 8.9 | 4.9 | 4.9 | 14.9 |
| Total emissions | 172 | 119 | 75.2 | 164.5 |
We are on track to meet our target to reduce overall CO2 emissions by 50% to 88 tonnes by 2025. The main direct impact is our electricity and gas consumption. Due to the Covid-19 pandemic, and our new blended approach to working in the office, we continue to see lower than usual usage. Normally significant changes to consumption cannot be made without capital investment, for example to introduce more energy efficient heat sources.
Waste (tonnes)
| Baseline | 2021/22 | 2020/21 | 2019/20 | |
|---|---|---|---|---|
| Incinerated with energy recovery | 10.1 | 3.4 | 2.6 | 8.0 |
We have met our target to reduce waste by 15%, to 8.6 by 2025. Our main waste is paper and other office related materials. Due to the Covid-19 pandemic and our blended working, we have made greater progress towards working digital by default. We are already generating 60% less waste than our 2025 target.
The VMD building is located on a shared Defra site in Weybridge and all our waste is recycled through an energy recovery incineration system.
Water (m3)
| Baseline | 2021/22 | 2020/21 | 2019/20 | |
|---|---|---|---|---|
| Water consumption | 811 | 248 | 84.0 | 705 |
We have met our target to reduce water by 8%, to 746 by 2025. Our main water use is in the toilet facilities, which have ‘water pigs’ in the cisterns. We cannot do more to reduce toilet facility water usage without capital investment in new hardware. The two showers are already low volume units. We are already using 67% less waste than our 2024 target.
Travel: Flights emissions (tonnes CO2)
| Baseline | 2021/22 | 2020/21 | 2019/20 | |
|---|---|---|---|---|
| Carbon from UK flights | 3.3 | 0.4 | not recorded | not recorded |
| Carbon from international flights | Not recorded | 0.88 | not recorded | not recorded |
We have met our target to reduce domestic flights emissions by 30%, to 2.3 by 2025. Almost all of our UK travel, with the exception of travelling to Northern Ireland, is by train and many of our meetings are carried out online.
All our fleet cars are hybrid powered and are ultra-low emission vehicles.
Consumables and procurement
Our procurement decisions include sustainability considerations and, where possible, we select suppliers and award contracts in line with our sustainability commitments.
The only single use plastics we purchase are to enable the safe and secure collection of animal tissue and fluid samples for our residue surveillance work. These are made from recycled materials, where possible, and include vials, pots, and bags. There is currently no safe alternative to plastic, however, we review this annually as part of our procurement process.
Our paper use is very low as we work digitally by default. For the year 2021/22 we used 15 reams of paper and 36 notebooks (no data for previous years).
Abi Seager
Chief Executive
17 October 2022
8. Accountability Report
8.1 Corporate Governance Report
8.2 Director’s Report
Board, Executive Directors and Senior Officials
The VMD employs one Director in addition to the Chief Executive.
| Position | Position holder |
|---|---|
| Chief Executive | Peter Borriello CB until 9 September, then Abigail Seager |
| Director of Authorisations | Abigail Seager until 9 September, then Gavin Hall as Interim Director. |
| Chief Operating Officer | Mike Griffiths |
The notice period for Executive Directors and senior officials is 3 months.
The composition of the Management Board (including non-executive directors), which provides leadership for directing or controlling the major activities during the year is described within the Governance Statement in this report.
The Board members had no company directorships or other significant interests which conflicted with their management responsibilities in the financial year 2021/22.
Protecting personal data
There were no personal data-related incidents in 2021/22.
9. Statement of Accounting Officer’s Responsibilities
Under the Government Resources and Accounts Act 2000 HM Treasury has directed the VMD to prepare for each financial year a statement of accounts in the form and on the basis set out in the Accounts Direction.
The Accounts are prepared on an accruals basis and must give a true and fair view of the state of affairs of the VMD and of its income and expenditure, changes in taxpayers’ equity and cash flows for the financial year.
In preparing the Accounts the Accounting Officer is required to comply with the requirements of the Government Financial Reporting Manual (FReM) and in particular to:
- observe the Accounts Direction issued by HM Treasury, including the relevant accounting and disclosure requirements, and apply suitable accounting policies on a consistent basis
- make judgements and estimates on a reasonable basis
- state whether applicable accounting standards as set out in the Government FReM have been followed, and disclose and explain any material departures in the Accounts
- prepare the accounts on the going concern basis
- confirm that the Annual Report and Accounts as a whole is fair, balanced and understandable and take personal responsibility for the Annual Report and Accounts and the judgements required for determining that it is fair, balanced and understandable
The Accounting Officer for Defra has designated the Chief Executive of the VMD as Accounting Officer of the VMD. The responsibilities of an Accounting Officer, including responsibility for the propriety and regularity of the public finances for which the Accounting Officer is answerable, for keeping proper records and for safeguarding the Agency’s assets, are set out in Managing Public Money published by HM Treasury.
9.1 Preparation and audit of the Accounts
The Accounts have been prepared under a direction issued on 16 December 2021 by HM Treasury under Section 7(2) of the Government Resources and Accounts Act 2000 and are audited by the Comptroller and Auditor General.
Our income and expenditure was monitored under a gross control total by HM Treasury and was also incorporated into the Defra Resource Accounting total.
As the Accounting Officer, I have taken all steps that I ought to have taken to make myself aware of any relevant audit information and to establish that VMD’s auditors are aware of that information. So far as I am aware, there is no relevant audit information of which the auditors are unaware.
10. Governance Statement
The Accounting Officer is responsible for maintaining a system of internal control that supports the achievement of the Agency’s policies, aims and objectives, while safeguarding public funds and departmental assets. This is in accordance with the responsibilities assigned in the HM Treasury publication: Managing Public Money.
Assurance and audit findings in this governance statement overall confirm that we have complied with the governance arrangements effectively.
10.1 Governance framework
The VMD is an Executive Agency of Defra. We are the UK policy lead on veterinary medicines and AMR, as the national competent authority, are responsible for the implementation of all aspects of the VMD and related legislation.
The Agency is led by the Chief Executive Officer (CEO), who is accountable to the Secretary of State for Defra for our performance and operation and for the achievement of our business priorities in accordance with its targets and key deliverables.
The Secretary of State for Defra determines the overall policy and financial framework within which we operate and the Defra ownership function is exercised by the Defra Director General for Food, Farming and Biosecurity (DG FFaB). The DG FFaB provides advice and challenge on our strategic direction and performance to the VMD management. The CEO formally reports on Agency performance to Defra through the quarterly DG FFaB meetings.
10.2 Committee structure
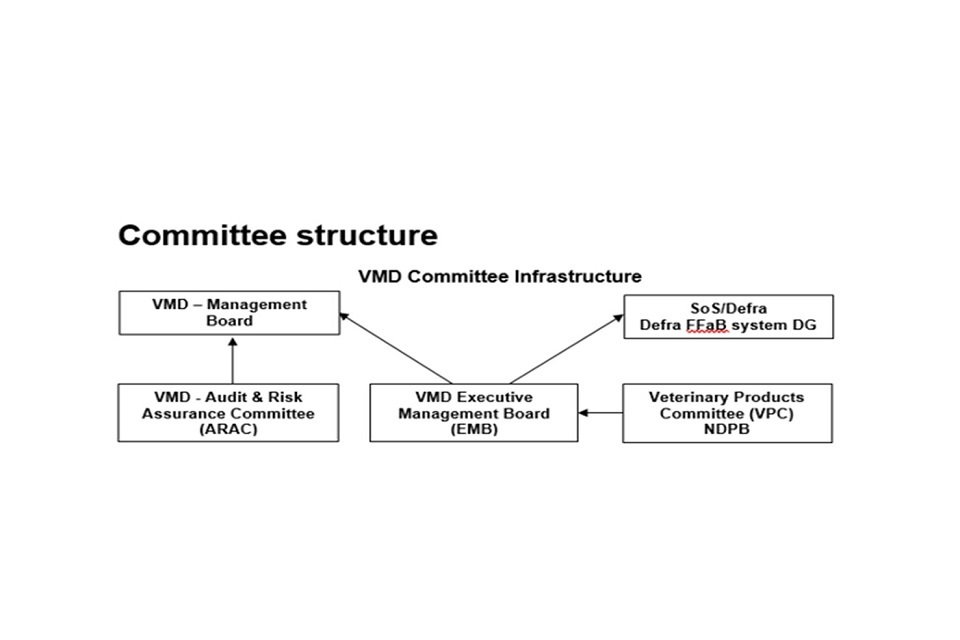
Committee Structure
The VMD Management Board (MB) meets quarterly and is the internal governance board providing advice, support and challenge on: the delivery of the key objectives; achieving value for money, and regularity and propriety in the administration and operation of the VMD.
Chaired by a Non-executive Director (NED) Julia Drown, the MB consists of the CEO, the Deputy CEO, the Chief Operating Officer (COO), the Head of Finance, the three other NEDs, David Catlow, Timothy Riley and Philippa Hardwick and the Defra FFaB system DG. Selected Heads of Teams, as appropriate and applicable to the tabled agenda, are also invited to attend. Minutes of MB meetings are published on GOV.UK.
The Audit and Risk Assurance Committee (ARAC), a sub-committee of the MB, also meets quarterly and provides advice to the CEO on the adequacy and effectiveness of the VMD’s governance and risk management frameworks. Chaired by a NED, Timothy Riley, and consisting of the other three NEDs, the Committee considers reports from a number of senior staff, Defra’s internal auditor (the Government Internal Audit Agency; GIAA) and the external auditor (the National Audit Office). Minutes of ARAC meetings are published on GOV.UK.
Executive Management Board (EMB) The EMB is formed of the CEO, the Deputy CEO (Director of Authorisations), and the Chief Operating Officer (Head of Business Support Division) who collectively form the Executive Team (the Directorate) that sets the direction for the Agency and has the overall authority to run the Agency on a day-to-day basis. The EMB also includes of all Heads of Divisions and Offices and others may be called upon to attend for specific agenda items.
Compliance with the Corporate Governance Code
The focus of HM Treasury’s Corporate Governance Code (CGC) is on ministerial departments and sets out the protocol, accountabilities and role of Departmental Boards. We apply the principles of the code, which requires that Boards operate according to recognised precepts of good corporate governance in business:
- leadership
- effectiveness
- accountability
- sustainability
It also requires that arrangements are in place for an annual evaluation of the effectiveness of the Board and for results of the evaluation to be acted upon.
The MB and ARAC assessed their effectiveness and the quality of the management information and performance data at each meeting and found both to be acceptable. A more formal assessment was carried out following their March 2022 meetings whereby Committee members and regular attendees of the Boards completed a questionnaire.
The review was very positive for the MB and confirmed that it has a clear understanding of its role, has the correct membership and is operating effectively. For ARAC, it was agreed that it is performing well and has a clear understanding of its assurance role. The results were discussed at the July 2022 meetings and some minor observations will be taken into the next period.
The EMB has formally assessed its compliance with the CGC and its effectiveness as evidenced by the delivery of the 2021/22 targets and key deliverables, and the results of the 2021 annual staff survey. The outcomes of the EMB are reported to staff through the weekly EMB Debrief and follow up email to all staff, and where appropriate Office Notices. To increase involvement and increase challenge from outside the Executive Team, individual Heads of Team are invited on a rolling basis for a month each to attend and contribute to the meeting.
Conflicts of interest
All VMD staff and Board members are required annually to declare interests which could emerge as a conflict of interest. There is a standing agenda item on declarations of interest at the start of every Board meeting and members who have declared a specific conflict leave the meeting during the discussion of that item. During 2021/22 no Board member conflicts of interest were identified. NED declarations of interest are published on GOV.UK.
The Veterinary Products Committee is an independent scientific advisory committee which advises the VMD on veterinary medicinal products and animal feed additives.
The VPC held meetings in May, September and February. Minutes of meetings and further information is published on GOV.UK. The Committee considered and gave advice to the VMD for an application to change the legal distribution category of an authorised product. It also considered the effects of climate change on the use of veterinary medicines and continued to monitor veterinary pharmacovigilance activities through the reports compiled by the VMD’s Pharmacovigilance team. In addition, the Committee held its virtual open day in November jointly with VMD.
The overall governance structure and associated assurance, as well as advice and challenge, are enriched by the VPC and discussions between the CEO and the Chief Veterinary Officer. We hold external certification to ISO 9001:2015 (Quality Management), which covers all our operational processes. We also hold external certification to ISO 27001:2013 (Information Security Management Systems).
Civil Service People Survey
Our Civil Service People Survey engagement index score (the survey’s measure of those areas that most shape our experience at work) is 63% (70% last year and 65% the year before).
Governance and control
Whistleblowing
We are committed to high standards, reinforced by the Civil Service Code, of integrity, honesty and professionalism in all that we do. We encourage all employees to use Defra’s Whistleblowing Policy if they need to raise a concern about a past, present or imminent wrongdoing within Defra/VMD; or any wrongdoings which conflict with the Civil Service Code. There have been no reports of whistleblowing in the past year.
Business critical models and quality assurance
An appropriate quality assurance framework is in place to assess business models relevant to the Agency. We obtain through MB and ARAC assurance that the associated risks are properly managed. There are no business models which currently fall within the definition ‘business critical models’ as set out by HM Treasury.
10.3 Audits
Following an independent audit by (SGS) in September 2021 the VMD’s continuing certification under ISO 9001:2015 (Quality Management) and ISO 27001:2013 (Information Security) was confirmed. Areas of the ISO 9001:2015 audit covered were: Scheduled & unscheduled inspections of premises of manufacturers and wholesale dealers of veterinary medicines; feed businesses SQP retailers and Veterinary practices; Research & Development Programme (R&D); Specific Manufacturing authorisations; and Special import scheme / export certificate scheme. This represents independent assurance that our systems and processes meet these internationally recognised standards.
For ISO 9001:2015 no non-conformities were found; and two opportunities for improvement were suggested and accepted.
For ISO 27001:2013 one major non-conformity and four minor non-conformities were identified and five opportunities for improvement were recommended. We accept these non-conformities and are working towards addressing them. We also accepted all the suggested opportunities for improvement.
Quality Management System
Our Quality Management System (QMS) ensures processes and procedures are documented and managed effectively. Trained VMD auditors, Defra Internal Auditors (GIAA) the National Audit Office (NAO) and SGS each provide us with assurance that processes are being followed and improvements are made on an ongoing basis. Our Quality Management System is certified to ISO 9001:2015.
Business continuity plans
We operate a Business Continuity Management system to ensure the operation of key activities in the event of a serious incident, including our off-site IT back-up systems. Two tests are carried out each year to check that all staff receive urgent business continuity information. The same method is used to notify staff of IT system issues which act as a further check that staff can be updated as required.
Information management and data security
We have an established governance structure to ensure that information assets are handled appropriately. To support the Information Systems Security Officer, the Agency’s IT Security Officer provides a focal point for Information Asset Owners to seek guidance on effective approaches to managing risk. Information data handling courses are embedded in induction processes and each year all staff are required to complete the Security & Data Protection training course.
Data security remains critical and is assured by the VMD’s maintenance of the Cabinet Office Security Standards and external certification to ISO 27001:2013.
There were no data security lapses that were deemed to be significant or critical during 2021/22.
We continued to be part of a wider Defra Data Protection Network to ensure our implementation of the General Data Protection Regulations (GDPR) reflected the latest thinking and practice. We met regularly to share knowledge as GDPR bedded-in and developed through custom and practice. We have also continued to work with our IT systems’ developers to ensure we applied the requirements of GDPR to our new systems.
Managing our risks and significant issues
Our primary role is in the authorisation of veterinary medicines, which is always based on assessing the benefit of medicines against their risks. Consequently, the very nature of our work is to examine risks, to reduce these to an acceptable level, and then to consider the residual risks against the benefits. This philosophy in managing risks is adopted in the approach to risk management across the organisation to identify key risks that could threaten the achievement of the VMD’s objectives. This process of risk management was in place for the year and up to the date of approval of the Annual Report and Accounts.
Our Strategic Risk Register and significant issues are regularly reviewed by the EMB, MB and ARAC and updated as necessary. The degree of risk is measured by considering the likelihood and impact of those risks and issues, and in 2021/2022 these were:
Covid-19 pandemic
- potential impaired ability to deliver our services, including VMD’s Global contracts
- impacts on VMD’s stakeholders and consequent implications for the VMD
Operational
- inadequate business continuity procedures
- inadequate IT services and associated communications
- reduction in funding
Reputational
- delays in delivering the new VMRs
- GB and NI regulatory frameworks diverging causing unintended increased burden on industry
- reduced confidence in veterinary medicines, food safety and/or the VMD
- risk of litigation
- failure to deliver on newly secured contracts with third sector funders for medicines regulation capability development work
Financial
- EU Exit and potential reduction in revenue
- Changes in Government policy reducing funding to AMR compromising the 5-year AMR Action plan
- failure to deliver statutory requirements
- overspending budget
- fraud
Staffing
- failure to attract and retain experienced professional staff for certain skills, particularly in buoyant veterinary medicines and IT sectors where demand for scarce experience and talent is high
- delays in Defra and Government Recruitment Service (GRS) processing candidates
Governance or structural changes
- imposed change to estate and/or other support services
Delivery by partners
- inability of partner organisations to deliver on our behalf
- decreasing resilience of others to deliver commissioned services
- inability of centralised government services to deliver support needed
Mitigation of risks
All of these were managed appropriately by the VMD through:
- careful workforce and succession planning
- providing first-class learning and development that develops talent for staff
- strong management focus on efficiencies
- efficient contract management
- efficient project management
- escalating unresolved business continuity issues with central Defra
- collaboration with central Defra, especially in relation to EU Exit
- ability to respond swiftly to Business Continuity issues – for example Covid-19 and IT enabling the whole business to work remotely
We also seek to identify risks that, while not significant enough to appear on the Strategic Risk Register, could still affect the successful outcome of our objectives. These risks are managed within individual business areas and are ‘owned’ by the respective Division or Office or Project Leaders who report progress to Directors at regular intervals. This includes a process for escalation to the Strategic Risk Register.
The Covid-19 pandemic and its implications have created uncertainty and additional pressures for a full delivery response for the Business. The most significant impact Covid-19 had was in the inspections side of our work, where it was not possible to do on-site inspections for large periods of the year, however, the risks of this were mitigated by carrying out remote inspections. For the remainder of VMD staff its impact was very limited, apart from the move away from office to remote and blended working.
The UK’s exit from the EU continued to be a high risk area, in particular, the uncertainty over our new relationship with the EU, for medicines regulation, and the ability to make the most of the opportunities and the impacts of the Northern Ireland Protocol.
Some of the specific actions we implemented and progressed to help control risks included:
- secured funds to cover additional pressures and new requirements as a result of leaving the EU
- dedicated work force planning
- operational readiness
- working with Defra, Department of Health and the Medicines and Healthcare products Regulatory Agency
Counter Fraud, Antibribery and anti-corruption matters
The VMD adheres the Defra Group policy in relation to fraud and its zero-tolerance approach to any instances of fraud, bribery & corruption, or other related illegal activities, including money laundering, terrorist financing and insider trading. In simple terms, ‘fraud’ encompasses several criminal offences, typically involving the use of deception, to obtain a benefit or cause detriment to individuals or organisations.
All staff must follow VMD’s Fraud Response and Procedure plan that provides the direction on how we deal with potential fraud and describes the process that will be followed in setting up, handling, and managing investigations. This document is reviewed annually by Audit and Risk Assurance Committee members. All cases of suspected fraud, bribery and corruption are thoroughly investigated and dealt with appropriately.
Every individual has the responsibility to report suspected fraud and VMD encourages anyone who has reasonable suspicious of fraud or malpractice to report them. The Executive Management Board is particularly committed to ensuring staff feel empowered, supported and protected should they need to raise any areas of concern.
Our Fraud Risk Management Strategy is aligned to the Defra Group Counter Fraud Strategy and anti-bribery and corruption policy. These apply to all VMD staff whether permanent or temporary. The policies are regularly reviewed for relevance and clarity.
There was no instance of potential fraud, bribery and corruption identified within VMD in 2021-22 (2020-21: Nil).
Human rights disclosure
The VMD has an obligation to ensure that all its actions respect the human rights of those who work for the Agency, and for whom they provide services. There has not been any litigation against the Agency alleging a breach of the Human Rights Act 1998 during 2021-22 (2020-21: Nil).
Internal audit arrangements
The Government Internal Audit Agency has been responsible for providing VMD’s internal audit service. Internal auditors carry out their work in line with the Annual Internal Audit Plan that is informed by our risk profile and approved by the ARAC on an annual basis. Internal auditors complete their Internal Audit responsibilities using a methodology that is aligned to Public Sector Internal Audit Standards. Reports are issued making recommendations for improvements where appropriate.
Progress on all agreed actions are reported back to (and any extensions, if required, are approved at) ARAC. These four reports were issued during 2021/22:
- Enforcement Activity – overall rating “Limited”
- Cyber Security – overall rating “Moderate”
- Stakeholder Engagement with other Government Bodies – overall rating “Moderate”
- Key Financial Controls - Accounts Payable and Accounts Receivable – overall rating “Substantial”
In their Annual Report, which offers their opinion on the adequacy and effectiveness of risk management, control and governance, the Head of Internal Audit Opinion is one of “Moderate Assurance”.
VMD staff who have been trained as auditors have carried out internal Quality Management System (QMS) audits against the ISO 9001:2015 standard of the following processes:
- Management of temporary staff - focussing on key aspects of their relationship with their Line Manager, the training process and performance reporting
- Departing staff (leaving the organisation)
Whilst no significant internal control problems have been identified during the year, we continually strive to improve our procedures and processes and to manage risk.
11. Remuneration and Staff Report
Service contracts
The Constitutional Reform and Governance Act 2010 requires Civil Service appointments to be made on merit, on the basis of fair and open competition. The Recruitment Principles published by the Civil Service Commission specify the circumstances when appointments may be made or not.
Unless otherwise stated below, the officials covered by this report hold appointments which are open ended. Early termination, other than for misconduct, would result in the individual receiving compensation as set out in the Civil Service Compensation Scheme.
Further information about the work of the Civil Service Commission can be found on the Civil Service Commission website.
Remuneration Policy
The remuneration of the Senior Civil Service (SCS) is set by the Prime Minister following independent advice from the Senior Salaries Review Body (SSRB). The Cabinet Office advises departments in March or April each year of the Government’s response to the SSRB recommendations and produces guidance for departments and network bodies to follow.
Defra develops the SCS Reward Strategy within the Cabinet Office Framework, ensuring that the overall pay awards for the SCS are within the cost ceiling allowed.
Members of the SCS, excluding the Permanent Secretary, are eligible to be considered for individual levels of bonus as non-pensionable, non-consolidated variable pay (NCVP), based on their performance. NCVP is paid in the financial year after that in which it was earned. NCVP values, informed by each individual’s appraisal grade, are paid within Cabinet Office guidelines.
The COO receives an annual salary paid in accordance with the standard Veterinary Medicines Directorate Staff Pay Agreement negotiated through collective bargaining with recognised trade unions. Their performance is monitored and reviewed through the VMD’s Performance Management System.
Remuneration – salary, benefits-in-kind and pensions (audited)
The following sections provide details of the remuneration and pension interests of the VMD’s Directors and COO.
| Officials | Salary | Bonus payments | Pension benefits (1) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| 2021/22 | 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | |
| £’000 | £’000 | £’000 | £’000 | £’000 | £’000 | £’000 | £’000 | |
| SP Borriello (2) Chief Executive to 9 Sep 2021 | 50-55 | 120-125 | - | 5-10 | 107 | 85 | 160-165 | 215-220 |
| A Seager (3) Director of Authorisations to 9 Sep 2021 and Chief Executive from 10 Sep 2021 | 80-85 | 70-75 | 5-10 | 10-15 | 66 | 34 | 155-160 | 115-120 |
| G Hall (4) Interim Director of Authorisations from 10 Sep 2021 | 35-40 | - | 0-5 | - | 34 | - | 70-75 | - |
| M Griffiths (6) Chief Operating Officer | 65-70 | 20-25 | 0-5 | 0-5 | 26 | 9 | 90-95 | 30-35 |
| P Green (5) Director of Operations | - | 40-50 | - | 0-5 | - | 19 | - | 70-75 |
(1) The value of pension benefits accrued during the year is calculated as (the real increase in pension multiplied by 20) plus (the real increase in any lump sum) less (the contributions made by the individual). The real increases exclude increases due to inflation or any increase or decreases due to a transfer of pension rights.
(2) SP Borriello retired from his post as the Chief Executive in September 2021. Salary figures are quoted for the period 1 April 2021 to 10 September 2021. The full year equivalent banding is the same as prior year.
(3) A Seager was appointed Chief Executive on 10 September 2021 having been Director of Authorisations during the year from 1 April 2021. The full year equivalent banding is £90k to £95k.
(4) G Hall was appointed interim Director of Authorisations on 10 September 2021. Salary figures are quoted for the period from 10 September 2021 to 31 March 2022. The full year equivalent banding is £70k to £75k.
(5) P Green left the post of Director of Operations on 24 November 2020.
(6) M Griffiths took up post as Chief Operating Officer from 25 November 2020. The 2021/22 salary figures are quoted for the period from 25 November 2020 to 31 March 2021. The full year equivalent banding is £65,000 to £70,000
Salary
‘Salary’ includes gross salary; overtime; reserved rights to London weighting or London allowances; recruitment and retention allowances; private office allowances, and any other allowance to the extent that it is subject to UK taxation. This report is based on accrued payments made by the Agency and thus recorded in these accounts.
Benefits-in-kind
The monetary value of benefits-in-kind covers any benefits provided by the employer and treated by HM Revenue and Customs as a taxable emolument. None of the Directors received any benefits-in-kind in 2021/22 (2020/21: nil)
Bonuses
Bonuses are based on performance levels attained and are made as part of the appraisal process. Bonuses paid in 2021/22 relate to performance in the prior financial year, comparative bonuses for 2020/21 relate to the 2019/20 performance.
Fair pay disclosure (audited)
Reporting bodies are required to disclose the percentage change from the previous financial year for both salary and performance pay in respect of the highest paid director and the average percentage change in respect of employees of the organisation taken as a whole.
| 2021-22 | 2020-21 | |||||
|---|---|---|---|---|---|---|
| Salary | Bonus | Total | Salary | Bonus | Total | |
| Annualised band of highest paid Director remuneration £’000 | 120 - 125 | 0 - 5 | 120 - 125 | 120 - 125 | 5 - 10 | 130 - 135 |
| Median employee remuneration | 40,377 | 366 | 40,790 | 40,806 | 223 | 41,014 |
| Percentage change from previous year | Salary | Bonus | Total |
|---|---|---|---|
| Highest paid director (based on midpoint of band) | 0% | -100% | -6% |
| Median employee remuneration | -1% | 64% | -0.55% |
Median employee remuneration includes permanent and temporary staff.
Reporting bodies are required to disclose the relationship between the remuneration of the highest paid director in their organisation and the top to median, lower quartile and upper quartile staff pay multiples within the organisation’s workforce. Employees are ranked based on their total FTE remuneration from low to high. The employee remuneration placed at the 25th, 50th and 75th percentile point of the ranking is disclosed.
The full-time equivalent annualised total banded remuneration of the highest paid Director and the interquartile ranges (25%, 50% and 75%) member of staff excluding the highest paid Director are as shown in the following table:
| 2021-22 | 2020-21 | |||||
|---|---|---|---|---|---|---|
| 25th percentile of other staff | Median of other staff | 75th percentile of other staff | 25th percentile of other staff | Median of other staff | 75th percentile of other staff | |
| Highest paid director remuneration (mid-point of pay band) | £122,500 | £132,500 | ||||
| All employees (excluding highest paid director) Total pay and benefits | £28,719 | £38,751 | £53,719 | £28,002 | £37,070 | £53,985 |
| All employees (excluding highest paid director) salary component only | £27,963 | £38,751 | £53,195 | £28,002 | £37,564 | £53,873 |
| Total pay ratio | 4.3 | 3.2 | 2.3 | 4.7 | 3.5 | 2.5 |
The highest paid staff member is not necessarily the highest paid employee. During the year, on a full year equivalent basis, an agency staff received the highest remuneration. The difference to the highest paid director is in the range £0 - £500. (2020/21: no employees received remuneration in excess of the highest paid Director). Remuneration ranged from £20,978 to £123,000 (2020/21: £20,366 to £131,000).
The highest paid Director in 2020/21 was the CEO who retired during 2021/22 and whose remuneration was 6% less due to changes in performance and bonus pay in 2021/22. There is a decrease of 0.55% in the average remuneration for employees from prior year, taken as a whole (excluding the highest paid director).
Total remuneration includes salary, non-consolidated performance-related pay and benefits-in-kind. It does not include severance payments, employer pension contributions and the cash equivalent transfer value of pensions.
There have been no ex-gratia payments or amounts paid during the year in respect of compensation to former senior managers or to third parties for services of a senior manager.
None of the VMD Directors or senior officials have held any company directorships or other significant interests during the year that, in the opinion of the Directors or senior official, may conflict with their management responsibilities.
No employer contributions were made to partnership pension accounts during 2021/22 or 2020/21 in respect of the VMD’s Directors.
Civil service pensions
Pension benefits are provided through the Civil Service pension arrangements. From 1 April 2015 a new pension scheme for civil servants was introduced – the Civil Servants and Others Pension Scheme or alpha, which provides benefits on a career average basis with a normal pension age equal to the member’s State Pension Age (or 65 if higher). From that date all newly appointed civil servants and the majority of those already in service joined alpha. Prior to this, civil servants participated in the Principal Civil Service Pension Scheme (PCSPS). Further details about the Civil Service pension features and benefits can be found in the resource accounts of the Cabinet Office: Civil Superannuation, www.civilservicepensionscheme.org.uk.
These statutory arrangements are unfunded with the cost of benefits met by monies voted by Parliament each year and increased annually in line with Pensions Increase legislation.
Other arrangements include money purchase pensions known as a ‘partnership’ are available as an alternative for employees joining on or after the 1 October 2002. The employer makes an age-related basic contribution of between 8% and 14.75% into a stakeholder pension product chosen by the employee from a panel of providers. The employee does not have to contribute but where they do make contributions, the employer will match these up to a limit of 3% of pensionable salary (in addition to the employer’s basic contribution). Employers also contribute a further 0.5% of pensionable salary to cover the cost of death in service and ill health retirement lump sum benefits.
The pension figures quoted for officials in this report show combined pension earned in all schemes as appropriate.
Cash equivalent transfer values
A Cash Equivalent Transfer Value (CETV) is the actuarially assessed capitalised value of the pension scheme benefits accrued by a member at a particular point in time. The benefits valued are the member’s accrued benefits and any contingent spouse’s pension payable from the scheme. A CETV is a payment made by a pension scheme or arrangement to secure pension benefits in another pension scheme or arrangement when the member leaves a scheme and chooses to transfer the benefits accrued in their former scheme.
The pension figures shown relate to the benefits that the individual has accrued as a consequence of their total membership of the pension scheme, not just their service in a senior capacity to which disclosure applies. These figures also include the value of any pension benefit in another scheme or arrangement which has been transferred to the Civil Service pension arrangements and any additional pension benefit accrued as a result of buying additional pension benefits at their own cost. CETVs are worked out in accordance with The Occupational Pension Schemes (Transfer Values) (Amendment) Regulations 2008 and do not take account of any actual or potential reduction to benefits resulting from Lifetime Allowance Tax which may be due when pension benefits are taken.
Real increase in CETV (audited)
This reflects the increase in CETV that is funded by the employer. It does not include the increase in accrued pension due to inflation, contributions paid by the employee (including the value of any benefits transferred from another pension scheme or arrangement) and uses common market valuation factors for the start and end of the period.
Senior Management Pension in £’000
| Officials | Accrued pension at pension age as at 31/3/2022 | Real increase in pension and related sum at pension age | CETV at 31/3/2022 | CETV at 31/3/2021 | Real Increase in CETV |
|---|---|---|---|---|---|
| SP Borriello Chief Executive to 10 Sep 2021 | 45 - 50 plus lump sum of 0 | 5.0 – 7.5 plus lump sum of 0 | 793 | 696 | 90 |
| A Seager Chief Executive from 10 Sep 2021 | 25 - 30 plus lump sum of 0 | 2.5 – 5.0 plus lump sum of 0 | 327 | 272 | 37 |
| M Griffiths (1) Chief Operating Officer | 15 - 20 plus lump sum of 0 | 0 - 2.5 plus lump sum of 0 | 217 | 194 | 13 |
| G Hall Director of Authorisations | 40 – 45 plus a lump sum of 45 – 50 | 0 – 2.5 plus a lump sum of 0 – 2.5 | 772 | 712 | 28 |
(1) As a result of a retrospective update to salary data, the closing balance in the previous year for CETV as at 31 March 2021 was updated from £162k to £194k
External Management Board members (audited)
Membership details of the Management Board are detailed in the Governance Statement in this report. The Non-executive members also form the ARAC. The following salaries and benefits-in-kind were paid to the external members:
| Non- executive members | Salary (as defined above) £’000 | Benefits-in-kind (1) to the nearest £100 | Total £’000 | |||
|---|---|---|---|---|---|---|
| 2021/22 | 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | |
| D Corner to July 2021 | 0 – 5 | 0 – 5 | - | - | 0 – 5 | 0 – 5 |
| J Drown | 0 – 5 | 0 – 5 | 100 | 100 | 0 – 5 | 0 – 5 |
| D Catlow | 0 – 5 | 0 – 5 | - | 700 | 0 – 5 | 0 – 5 |
| T Riley from May 2021 | 0 – 5 | - | - | - | 0 – 5 | - |
| P Hardwick from May 2021 | 0 – 5 | - | - | - | 0 – 5 | - |
(1) Benefits-in-kind relate to reimbursement of home to office travel and subsistence.
12. Staff Report
Staff numbers
At 31 March 2022 we employed 172 permanent staff (168.4 FTE) and 15 temporary staff (14.6 FTE) supplied by employment agencies. The average number of full-time equivalent permanent and temporary staff during the year and an analysis of staff-in-post (headcount) as at 31 March 2022 by gender are shown below.
We comply with equal opportunities legislation and departmental policy in relation to disabled employees, and Defra’s policies on equal opportunities and health and safety at work.
Staff recruitment
Following a Civil Service Commission (CSC) annual ‘Recruitment Compliance’ visit in January, the CSC observed excellent compliance with the CSC’s Recruitment Code.
The average FTE number of persons employed during the year was as follows (audited):
| Staff | Permanently employed staff | Temporary staff | 2021/22 Total | 2020/21 Total |
|---|---|---|---|---|
| Scientific | 48 | 3 | 51 | 54 |
| Administrative | 116 | 9 | 125 | 122 |
| Staff engaged on capital projects | 1 | - | 1 | 1 |
| Total staff | 165 | 12 | 177 | 177 |
The number of staff-in-post (headcount) by gender as at 31 March 2022 was as follows:
| Staff | Male | Female | 2021/22 Total | Male | Female | 2020/21 Total |
|---|---|---|---|---|---|---|
| Directors on the Board | 1 | 1 | 2 | 1 | 1 | 2 |
| Officials on the Board | 1 | - | 1 | 1 | - | 1 |
| SCS grade or equivalent (excluding senior officials on the Board) | - | - | - | 1 | - | 1 |
| Other - Scientific | 22 | 29 | 51 | 24 | 26 | 50 |
| Other - Administrative | 40 | 78 | 118 | 41 | 72 | 113 |
| Total staff | 64 | 108 | 172 | 68 | 99 | 167 |
Staff turnover
The agency uses Department Turnover (staff leaving the Civil Service or a particular department) as defined in the civil service turnover guidance in calculating its staff turnover.
The agency’s turnover is calculated as the number of leavers within the year divided by the average of staff in post over the period. The average staff in post is calculated as the average of headcount over the period. Leavers include retirements, death in service, end of appointments, as well as dismissals and resignations and leavers under compulsory and voluntary redundancies.
The staff turnover during the year was 11.9% (2020/21: 9.6%).
Early departure costs (audited)
Redundancy and other departure costs are paid in accordance with the provisions of the Civil Service Compensation Scheme, a statutory scheme made under the Superannuation Act 1972. Exit costs are accounted for in full in the year of departure or earlier where a demonstrable commitment exists.
For all staff, there were no early departures in 2021/22 (2020/21: nil).
For all staff, there were no compulsory exits in 2021/22 (2020/21: nil).
Staff costs (audited) in £’000:
| Staff costs consist of the following: | Permanently employed staff | Temporary staff | 2021/22 Total | 2020/21 Total |
|---|---|---|---|---|
| Wages and salaries | 7,055 | 710 | 7,765 | 8,022 |
| Social security costs | 796 | - | 796 | 783 |
| Other pension costs | 1,898 | - | 1,898 | 1,896 |
| Gross total staff costs | 9,749 | 710 | 10,459 | 10,701 |
| Less amounts charged to capital projects | (1) | - | (1) | (72) |
| Sub-total as reported in the Statement of Comprehensive Net expenditure | 9,748 | 710 | 10,458 | 10,629 |
| Less recoveries in respect of outward secondments | (331) | - | (331) | (215) |
| Net total staff costs | 9,417 | 710 | 10,127 | 10,414 |
Pensions
Pension benefits provided through the Civil Service pension arrangements are unfunded multi-employer defined benefit scheme and we are unable to identify our share of the underlying assets and liabilities. The Scheme Actuary valued the scheme as at 31 March 2016. You can find details in the resource accounts of the Cabinet Office: Civil Superannuation, www.civilservicepensionscheme.org.uk.
For 2021/22, employers’ contributions of £1,876,481 were payable to the PCSPS (2020/21: £1,834,551) at one of four rates in the range 26.6% to 30.3% (2020/21: 26.6% to 30.3%) of pensionable earnings, based on salary bands. The Scheme Actuary reviews employer contributions usually every four years following a full scheme valuation. The contribution rates are set to meet the cost of the benefits accruing during 2021/22 to be paid when the member retires and not the benefits paid during this period to existing pensioners.
Employees can opt to open a partnership pension account, a stakeholder pension with an employer contribution. Employers’ contributions of £27,064 (2020/21: £26,668) were paid to one or more of the panel of three appointed stakeholder pension providers.
In addition, employer contributions of £820, 0.5% of pensionable pay (2020/21: £811) were payable to the PCSPS to cover the cost of the future provision of lump sum benefits on death in service or ill-health retirement of these employees.
Contributions due to the partnership pension providers at the balance sheet date were £2,224 (2020/21: £2,135). Contributions prepaid at that date were nil.
No individuals retired early on ill-health grounds during the year and therefore no additional pension liabilities have been accrued for this purpose.
Sickness absence data
The total full-time equivalent days lost through staff sickness absence in the year was 761 compared to 372 in 2020/21. The average working days lost per employee during the year was 4.6 compared to 2.3 in 2020/21.
Short term sickness absences of 10 days or less increased from 0.6 days per FTE in 2020/21 to 1.3 days per FTE in 2021/22.
Tax arrangements of public sector appointees
As part of HM Treasury’s review of tax arrangements of public sector appointees, departments and their arms-length bodies are required to publish information in relation to the number of off payroll engagements costing over £245 per day that were in place as at 31 March 2022.
| Number of existing engagements as of 31 March 2022 | 28 |
|---|---|
| Of which: | |
| Number that have existed for less than one year at time of reporting | 8 |
| Number that have existed for between one and two years at time of reporting | 10 |
| Number that have existed for between two and three years at time of reporting | 5 |
| Number that have existed for between three and four years at time of reporting | 3 |
| Number that have existed for four or more years at time of reporting | 2 |
The majority of our contractors are engaged in developing new UK veterinary authorisation platforms to replace the EU platforms we lost access to post transition. Once operational we expect system maintenance to be carried out in-house and demand on IT contractors should reduce accordingly.
For all off-payroll appointments engaged at any point during the year ended 31 March and earning at least £245 per day.
| Number of appointments in force during the time period | 56 |
|---|---|
| Of which: | |
| Number determined as in-scope of IR35 | 25 |
| Number determined as out-of-scope of IR35 | 31 |
| Number of engagements reassessed for compliance or assurance purposes during the year | 43 |
| Number of engagements that saw a change to IR35 status following the review | 43 |
| Number of engagements where the status was disputed under provisions in the off-payroll legislation | 0 |
| Number of engagements that saw a change to IR35 status following review | 0 |
Off-payroll engagements of Board members and/or senior officials with significant financial responsibility between 1 April 2021 and 31 March 2022.
| Number of off-payroll engagements of Board members, and/or senior officials with significant financial responsibility | nil |
|---|---|
| Total number of individuals on-payroll and off-payroll that have been deemed “board members, and/or senior officials with significant financial responsibility” | Board members/senior officials x 4 (1 CEO, 2 Directors, 1 senior official) Non-Executive Directors x 5 |
Employment Legislation (IR35)
Employment Legislation (IR35), introduced in April 2017, requires public sector bodies to assess off-payroll workers employment status for tax and makes them liable for ensuring the correct tax is applied. VMD uses HM Revenue and Customs’ own Check of Employment Status for Tax tool (CEST) as well as expertise from the Defra tax team and accompanying guidance to make those assessments.
During the year, VMD reassessed all its current contractors following an HMRC enquiry into Defra’s compliance with off-payroll working (IR35) rules. The reassessment resulted in £4.02m owed to settle the tax liability for the period 2018-19 to 2021-22. A payment on account was made to HMRC in April 2022 for £1.31m and the final payment for £2.71m was made in July 2022 after an agreement with HMRC on the total liability owed. The total payment of £4.02m includes interest charges of £0.14m.
Consultancy and temporary staff expenditure:
| £’000 | 2021/22 | 2020/21 |
|---|---|---|
| Consultancy expenditure | 2,190 | 3,464 |
| Temporary staff expenditure | 710 | 734 |
| Total | 2,900 | 4,198 |
Consultants are engaged when it is better value for money to do so on specific programme work and when specialised skills are required. Expenditure on temporary staff has provided additional resources to meet short term needs to support priority projects and cover for the backlog in filling vacancies.
Employee involvement
We encourage staff involvement in our activities through a variety of channels including: a VMD intranet; topic meetings; day-to-day line management contacts; diverse membership of project teams, and regular meetings reviewing progress against the Business Plan and risk. Office Notices and the intranet are used to disseminate information. An annual staff meeting to review the work of the past year and expected key future issues is presented by the CEO. There are also quarterly all staff update meetings led by EMB where all staff can participate. We work with Defra on wellbeing activities and staff have access to both occupational health and employee assistance services. Trade Union membership and representation is in accordance with Defra’s policies.
Health and safety
Due to mainly low risk activities and the size of the organisation we continue to use the policies and advice services from Defra’s Safety, Health and Wellbeing team. 1 minor (hazardous) work-related incident was reported by employees during 2021/22 (2020/21: none) and changes have been made to address the cause.
13. Parliamentary Accountability and Audit Report (Audited)
Regularity of expenditure
We have considered all our activities during the year and confirm that they are in accordance with the legislation authorising them.
Sources of funding and associated costs – reconciliation to the Statement of Comprehensive Net Expenditure
| Sources of funding and associated costs £’000 | Income | Expenditure | Net Income or Defra Funding |
|---|---|---|---|
| Industry Fees and Charges | |||
| Veterinary pharmaceutical industry | 6,014 | 6,014 | - |
| Food Industry | 3,790 | 3,790 | - |
| Sub-total – industry fees and charges | 9,804 | 9,804 | - |
| Other external income | |||
| International | 559 | 559 | - |
| Recovery of costs for secondments out | 331 | 331 | - |
| Other income | 18 | 18 | - |
| Sub-total – other external income | 908 | 908 | - |
| Government funded activities | |||
| Services for government (1) | 504 | 5,192 | (4,688) |
| Research and development | - | 1,529 | (1,529) |
| Trade and transition | - | 9,569 | (9,569) |
| Government secure freight contract | - | 875 | (875) |
| Food industry residues in honey | - | 40 | (40) |
| Defra budget funding requirement | 504 | 17,205 | (16,701) |
| Less cost of capital charge (2) | - | (275) | 275 |
| Statement of Comprehensive Net Expenditure | 11,216 | 27,642 | (16,426) |
(1) Services for Government include: Policy; enforcement; AMR programmes. A grant from the Fleming Fund (£378k); an EU grant (£56k); and a contribution from the devolved administrations (£70k) has been received in 2021/22, contributing to the cost of the AMR programme.
The VMD is a Gross Controlled Agency which means the VMD receives an allocated expenditure budget for Defra work not funded by industry.
(2) The VMD is required to include a notional cost of capital charge in the calculation of fees and charges. In line with HM Treasury guidance, this figure is excluded from the results presented in the Financial Statements.
Fees and charges
Our fees and charges are set in statute. Our objective for charging is to ensure that we recover our costs for delivering the service. In assessing performance against this target a notional cost of capital charge is recorded in addition to the costs included in the Statement of Comprehensive Net Expenditure. The table below sets out the amount of income we have received and associated costs for the different areas of service which we provide to industry.
| 2021/22 | Income £’000 | Cost £’000 | Net Income £’000 | Cost Recovery % |
|---|---|---|---|---|
| Veterinary pharmaceutical industry | 6,014 | 6,014 | - | 100 |
| Food industry | 3,790 | 3,790 | - | 100 |
| Total | 9,804 | 9,804 | - | 100 |
| 2020/21 | Income £’000 | Cost £’000 | Net Income £’000 | Cost Recovery % |
|---|---|---|---|---|
| Veterinary pharmaceutical industry | 5,983 | 5,983 | - | 100 |
| Food industry | 3,675 | 3,675 | - | 100 |
| Total | 9,658 | 9,658 | - | 100 |
Losses and special payments
Managing Public Money requires a statement showing losses and special payments by value and type to be shown where they exceed £300,000 in total, and those individually that exceed £300,000.
Losses may relate to: cash and stores losses; book-keeping losses; losses arising from failure to make adequate charge for the use of public property or services; fruitless payments, and claims abandoned as well as frauds. Special payments may relate to extra contractual, extra statutory, and ex gratia payments and compensation.
VMD reassessed all its current contractors because of an HMRC enquiry into Defra’s compliance with off-payroll working (IR35) rules in relation to contingent labour in 2019. The IR35 tax liability payable to HMRC between 2018-19 and 2021-22 is £4.02m including interest charges. VMD agreed the total liability payable with HMRC in July 2022. A payment on account of £1.31m was made in April 2022 and final payment £2.71m in July 2022.
Contingent liabilities
There were no contingent liabilities as at 31 March 2022 (31 March 2021: nil).
Remote contingent liabilities
In addition to contingent liabilities reported within the meaning of IAS 37, Provisions, Contingent Liabilities and Contingent Assets, the Agency discloses, for parliamentary reporting and accountability purposes, liabilities for which the likelihood of a transfer of economic benefit in settlement is too remote to meet the definition of a contingent liability. As at 31 March 2022 there are nil to report (31 March 2021: Nil).
Abi Seager
Chief Executive
17 October 2022
14. The Certificate and Report of the Comptroller and Auditor General to the House of Commons
Opinion on financial statements
I certify that I have audited the financial statements of the Veterinary Medicines Directorate for the year ended 31 March 2022 under the Government Resources and Accounts Act 2000.
The financial statements comprise: the Veterinary Medicines Directorate’s:
- Statement of Financial Position as at 31 March 2022;
- Statement of Comprehensive Net Expenditure, Statement of Cash Flows and Statement of Changes in Taxpayers’ Equity for the year then ended; and
- the related notes including the significant accounting policies.
The financial reporting framework that has been applied in the preparation of the Veterinary Medicines Directorate’s financial statements is applicable law and UK adopted international accounting standards.
In my opinion, the financial statements:
- give a true and fair view of the state of the Veterinary Medicines Directorate’s affairs as at 31 March 2022 and its net operating cost for the year then ended; and
- have been properly prepared in accordance with the Government Resources and Accounts Act 2000 and HM Treasury directions issued thereunder.
Opinion on regularity
In my opinion, in all material respects, the income and expenditure recorded in the financial statements have been applied to the purposes intended by Parliament and the financial transactions recorded in the financial statements conform to the authorities which govern them.
Basis for opinions
I conducted my audit in accordance with International Standards on Auditing (UK) (ISAs UK), applicable law and Practice Note 10 Audit of Financial Statements of Public Sector Entities in the United Kingdom. My responsibilities under those standards are further described in the Auditor’s responsibilities for the audit of the financial statements section of my certificate.
Those standards require me and my staff to comply with the Financial Reporting Council’s Revised Ethical Standard 2019. I have also elected to apply the ethical standards relevant to listed entities. I am independent of the Veterinary Medicines Directorate in accordance with the ethical requirements that are relevant to my audit of the financial statements in the UK. My staff and I have fulfilled our other ethical responsibilities in accordance with these requirements.
I believe that the audit evidence I have obtained is sufficient and appropriate to provide a basis for my opinion.
Conclusions relating to going concern
In auditing the financial statements, I have concluded that the Veterinary Medicines Directorate’s use of the going concern basis of accounting in the preparation of the financial statements is appropriate.
Based on the work I have performed, I have not identified any material uncertainties relating to events or conditions that, individually or collectively, may cast significant doubt on the Veterinary Medicines Directorate’s ability to continue as a going concern for a period of at least twelve months from when the financial statements are authorised for issue.
My responsibilities and the responsibilities of the Chief Executive as Accounting Officer with respect to going concern are described in the relevant sections of this certificate.
The going concern basis of accounting for the Veterinary Medicines Directorate is adopted in consideration of the requirements set out in HM Treasury’s Government Financial Reporting Manual, which require entities to adopt the going concern basis of accounting in the preparation of the financial statements where it anticipated that the services which they provide will continue into the future.
Other Information
The other information comprises information included in the Annual Report but does not include the financial statements nor my auditor’s certificate and report. The Chief Executive as Accounting Officer is responsible for the other information.
My opinion on the financial statements does not cover the other information and, except to the extent otherwise explicitly stated in my certificate, I do not express any form of assurance conclusion thereon.
In connection with my audit of the financial statements, my responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the financial statements or my knowledge obtained in the audit or otherwise appears to be materially misstated.
If I identify such material inconsistencies or apparent material misstatements, I am required to determine whether this gives rise to a material misstatement in the financial statements themselves. If, based on the work I have performed, I conclude that there is a material misstatement of this other information, I am required to report that fact.
I have nothing to report in this regard.
Opinion on other matters
In my opinion the part of the Remuneration and Staff Report to be audited has been properly prepared in accordance with HM Treasury directions made under the Government Resources and Accounts Act 2000: .
In my opinion, based on the work undertaken in the course of the audit:
- the parts of the Accountability Report subject to audit have been properly prepared in accordance with HM Treasury directions made under the Government Resources and Accounts Act 2000;
- the information given in the Performance and Accountability Reports for the financial year for which the financial statements are prepared is consistent with the financial statements and is in accordance with the applicable legal requirements.
Matters on which I report by exception
In the light of the knowledge and understanding of the Veterinary Medicines Directorate and its environment obtained in the course of the audit, I have not identified material misstatements in the Performance and Accountability Report.
I have nothing to report in respect of the following matters which I report to you if, in my opinion:
- I have not received all of the information and explanations I require for my audit; or
- adequate accounting records have not been kept by the Veterinary Medicines Directorate or returns adequate for my audit have not been received from branches not visited by my staff; or
- the financial statements and the parts of the Accountability Report subject to audit are not in agreement with the accounting records and returns; or
- certain disclosures of remuneration specified by HM Treasury’s Government Financial Reporting Manual have not been made or parts of the Remuneration and Staff Report to be audited is not in agreement with the accounting records and returns; or
- the Governance Statement does not reflect compliance with HM Treasury’s guidance.
Responsibilities of the Chief Executive as Accounting Officer for the financial statements
As explained more fully in the statement of Responsibilities of the Chief Executive as Accounting Officer for the financial statements, the Chief Executive as Accounting Officer is responsible for:
maintaining proper accounting records;
- the preparation of the financial statements and Annual Report in accordance with the applicable financial reporting framework and for being satisfied that they give a true and fair view;
- ensuring that the Annual Report and accounts as a whole is fair, balanced and understandable;
- internal controls as the Chief Executive as Accounting Officer determines is necessary to enable the preparation of financial statement to be free from material misstatement, whether due to fraud or error; and
- assessing the Veterinary Medicines Directorate’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the Chief Executive as Accounting Officer anticipates that the services provided by the Veterinary Medicines Directorate will not continue to be provided in the future.
Auditor’s responsibilities for the audit of the financial statements
My responsibility is to audit, certify and report on the financial statements in accordance with the Government Resources and Accounts Act 2000.
My objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue a certificate that includes my opinion. Reasonable assurance is a high level of assurance but is not a guarantee that an audit conducted in accordance with ISAs (UK) will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these financial statements.
Extent to which the audit was considered capable of detecting non-compliance with laws and regulations including fraud
I design procedures in line with my responsibilities, outlined above, to detect material misstatements in respect of non-compliance with laws and regulations, including fraud. The extent to which my procedures are capable of detecting non-compliance with laws and regulations, including fraud is detailed below.
Identifying and assessing potential risks related to non-compliance with laws and regulations, including fraud
In identifying and assessing risks of material misstatement in respect of non-compliance with laws and regulations, including fraud, we considered the following:
- the nature of the sector, control environment and operational performance including the design of the Veterinary Medicines Directorate’s accounting policies, key performance indicators and performance incentives.
- Inquiring of management, the Veterinary Medicines Directorate’s head of internal audit and those charged with governance, including obtaining and reviewing supporting documentation relating to the Veterinary Medicines Directorate’s policies and procedures relating to:
- identifying, evaluating and complying with laws and regulations and whether they were aware of any instances of non-compliance.
- detecting and responding to the risks of fraud and whether they have knowledge of any actual, suspected or alleged fraud; and
- the internal controls established to mitigate risks related to fraud or non-compliance with laws and regulations including the Veterinary Medicines Directorate’s controls relating to the Veterinary Medicines Directorate’s compliance with the Government Resources and Accounts Act 2000, Managing Public Money .
- discussing among the engagement team and involving relevant internal specialists, including on property , regarding how and where fraud might occur in the financial statements and any potential indicators of fraud.
As a result of these procedures, I considered the opportunities and incentives that may exist within Veterinary Medicines Directorate for fraud and identified the greatest potential for fraud in the following areas: revenue recognition, posting of unusual journals, complex transactions, bias in management estimates. In common with all audits under ISAs (UK), I am also required to perform specific procedures to respond to the risk of management override of controls.
I also obtained an understanding of the Veterinary Medicines Directorate’s framework of authority as well as other legal and regulatory frameworks in which the Veterinary Medicines Directorate operates, focusing on those laws and regulations that had a direct effect on material amounts and disclosures in the financial statements or that had a fundamental effect on the operations of the Veterinary Medicines Directorate. The key laws and regulations I considered in this context included Government Resources and Accounts Act 2000, Managing Public Money, employment, taxation and pensions legislation.
Audit response to identified risk
As a result of performing the above, the procedures I implemented to respond to identified risks included the following:
- reviewing the financial statement disclosures and testing to supporting documentation to assess compliance with provisions of relevant laws and regulations described above as having direct effect on the financial statements;
- enquiring of management, the Audit and Risk Committee concerning actual and potential litigation and claims;
- reading and reviewing minutes of meetings of those charged with governance and the Board and internal audit reports;
- in addressing the risk of fraud through management override of controls, testing the appropriateness of journal entries and other adjustments; assessing whether the judgements made in making accounting estimates are indicative of a potential bias; and evaluating the business rationale of any significant transactions that are unusual or outside the normal course of business; and
- in addressing the risk of fraud in revenue recognition we have performed focused testing on income recognised across the year end.
I also communicated relevant identified laws and regulations and potential fraud risks to all engagement team members including internal specialists and remained alert to any indications of fraud or non-compliance with laws and regulations throughout the audit.
A further description of my responsibilities for the audit of the financial statements is located on the Financial Reporting Council’s website at: www.frc.org.uk/auditorsresponsibilities. This description forms part of my certificate.
Other auditor’s responsibilities
I am required to obtain evidence sufficient to give reasonable assurance that the income and expenditure reported in the financial statements have been applied to the purposes intended by Parliament and the financial transactions conform to the authorities which govern them.
I communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that I identify during my audit.
Report
I have no observations to make on these financial statements.
Gareth Davies Date: 20 October 2022 Comptroller and Auditor General
National Audit Office
157-197 Buckingham Palace Road
Victoria
London
SW1W 9SP
15. Financial Statements
16. Statement of Comprehensive Net Expenditure
for the year ending 31 March 2022
| Comprehensive Net Expenditure £’000 | Note | 2021/22 | 2020/21 |
|---|---|---|---|
| Revenue from contracts with customers | 2 | 10,363 | 10,181 |
| Other operating income | 2 | 853 | 448 |
| Total operating income | 11,216 | 10,629 | |
| Staff costs | 3 | (10,458) | (10,629) |
| Purchase of services | 4 | (7,019) | (7,737) |
| Non-cash costs | 4 | (2,126) | (1,497) |
| Other operating expenditure | 4 | (8,039) | (4,812) |
| Total operating expenditure | (27,642) | (24,675) | |
| Net operating expenditure | (16,426) | (14,046) | |
| Other comprehensive expenditure | |||
| Items that will not be reclassified to net operating costs: Net gain on revaluation of property, plant and equipment | 873 | 65 | |
| Comprehensive net expenditure for the year ending 31 March 2022 | (15,553) | (13,981) |
All income and expenditure are derived from continuing operations.
The notes section form part of these accounts.
17. Statement of Financial Position
as at 31 March 2022
| Financial Position £’000 | Note | 2021/22 | 2020/21 |
|---|---|---|---|
| Non-current assets | |||
| Property, plant and equipment | 5 | 6,442 | 5,891 |
| Intangible assets | 6 | 6,953 | 6,410 |
| Total non-current assets | 13,395 | 12,301 | |
| Current assets | |||
| Trade and other receivables, contract assets | 7 | 2,255 | 3,705 |
| Cash and cash equivalents | 8 | 3,738 | 1,893 |
| Total current assets | 5,993 | 5,598 | |
| Total assets | 19,388 | 17,899 | |
| Current liabilities | |||
| Trade and other payables, contract liabilities | 9 | (9,204) | (5,403) |
| Total current liabilities | (9,204) | (5,403) | |
| Total assets less current liabilities | 10,184 | 12,496 | |
| Non-current liabilities | 9 | (141) | (135) |
| Total assets less total liabilities | 10,043 | 12,361 | |
| Taxpayers’ equity and other reserves | |||
| General fund | 3,633 | 6,824 | |
| Revaluation reserve | 6,410 | 5,537 | |
| Total equity | 10,043 | 12,361 |
The notes section form part of these accounts.
Abi Seager
Chief Executive and Agency Accounting Officer
17 October 2022
18. Statement of Cash Flow
for the year ended 31 March 2022
| Cash Flows £’000 | Note | 2021/22 | 2020/21 |
|---|---|---|---|
| Cash flows from operating activities | |||
| Net operating expenditure | (16,426) | (14,046) | |
| Adjustments for non-cash transactions arising in the year | 4 | 2,126 | 1,497 |
| Decrease /(Increase) in trade and other receivables | 7 | 1,450 | (1,205) |
| Increase in trade and other payables | 9 | 3,807 | 1,783 |
| Net cash outflow from operating activities | (9,043) | (11,971) | |
| Cash flows from investing activities | |||
| Purchase of property, plant and equipment | 5 | (43) | - |
| Purchase of intangible assets | 6 | (1,489) | (1,927) |
| Net cash outflow from investing activities | (1,532) | (1,927) | |
| Cash flows from financing activities | |||
| Supply current year | 12,420 | 11,900 | |
| Net financing | 12,420 | 11,900 | |
| Net increase/(decrease) in cash and cash equivalents | 1,845 | (1,998) | |
| Cash at the beginning of the year | 8 | 1,893 | 3,891 |
| Cash at the end of the year | 8 | 3,738 | 1,893 |
The notes section form part of these accounts.
19. Statement of Changes in Taxpayers’ Equity
for the year ended 31 March 2022
| General | Revaluation | Total | ||||
|---|---|---|---|---|---|---|
| Changes in Taxpayers’ Equity £’000 | Note | Fund | Reserve | Reserves | ||
| Balance at 1 April 2020 | 8,279 | 5,472 | 13,751 | |||
| Changes in taxpayers’ equity for 2020/21 | ||||||
| Net Parliamentary Funding | 11,900 | - | 11,900 | |||
| Comprehensive net expenditure for the year | (14,046) | - | (14,046) | |||
| Non-Cash adjustments: | ||||||
| Defra corporate recharges | 4 | 527 | - | 527 | ||
| Defra Investigation Services | 4 | 118 | - | 118 | ||
| Auditors’ remuneration | 4 | 46 | - | 46 | ||
| Movements in Reserves: | ||||||
| Net gain on revaluation of property, plant and equipment | 5 | - | 65 | 65 | ||
| Total recognised income and expense for 2020/21 | (1,455) | 65 | (1,390) | |||
| Balance at 31 March 2021 | 6,824 | 5,537 | 12,361 | |||
| Changes in taxpayers’ equity for 2021/22 | ||||||
| Net Parliamentary Funding | 12,420 | - | 12,420 | |||
| Comprehensive net expenditure for the year | (16,426) | - | (16,426) | |||
| Non-cash adjustments | ||||||
| Defra corporate recharges | 4 | 582 | - | 582 | ||
| Defra Investigation Services | 4 | 176 | - | 176 | ||
| Auditors’ remuneration | 4 | 57 | - | 57 | ||
| Movements in Reserves: | ||||||
| Net gain on revaluation of property, plant and equipment | 5 | - | 873 | 873 | ||
| Total recognised income (expense) for 2021/22 | (3,191) | 873 | (2,318) | |||
| Balance as at 31 March 2022 | 3,633 | 6,410 | 10,043 |
The notes section form part of these accounts.
20. Notes to the Accounts
20.1 1. Statement of Accounting Policies
The financial statements have been prepared in accordance with the 2021/22 Government Financial Reporting Manual (FReM) and the Accounts Direction issued by HM Treasury. The accounting policies contained in the FReM apply International Financial Reporting Standards (IFRS), as adapted or interpreted for the public sector context. Where the FReM permits a choice of accounting policy, the accounting policy that is judged to be most appropriate to the particular circumstances of the VMD, for the purpose of giving a true and fair view has been selected. The particular policies adopted by the VMD are described below. They have been applied consistently in dealing with items which are considered material in relation to the accounts.
20.2 1.1 Accounting convention
These accounts have been prepared on an accrual basis under the historical cost convention, modified to account for the revaluation of property, plant and equipment. The accruals basis of accounting means reporting income and expenditure when it is incurred rather than when it is received or paid. The financial statements are based on the going concern principle.
20.3 1.2 Going concern
The financial statements cover the activities of the VMD and are prepared on a going concern basis. These statements comply with the principles laid out in the 2021/22 Government Financial Reporting Manual FReM issued by HM Treasury on IAS 1 interpretation of going concern for the public sector sponsored entities. The VMD is an Executive Agency of the Department for Environment Food & Rural Affairs (DEFRA) and the department has agreed 2022-23 budget for the Agency’s continuation beyond 2022-23. The going concern disclosures on page 3 of the Annual Report detail in full the basis on which the Accounting Officer considers it appropriate to prepare these Accounts on a going concern basis.
20.4 1.3 Significant Judgements and Estimation Uncertainty
In the preparation of financial statements VMD is required to make estimates and assumptions that affect the amounts reported of assets and liabilities, the disclosure of contingent assets and liabilities and the reported amount of income and expenditure. All estimates are based on knowledge of current facts and circumstances, assumptions concerning past events, and forecast of future events.
In the process of applying the accounting policies VMD has made the following judgements, which have the most significant effect on the amounts recognised in the financial statements.
Contract Liabilities: The Agency is responsible for managing the progress of, and income earned from, scientific assessments. Individual assessments may span across more than one financial year and the preparation of the financial statements requires the Agency to determine, based on an evaluation of the terms and conditions of the arrangements, that it fully and accurately reflects the completeness of any contract liabilities in this regard by reference to the stage of completion of any ongoing assessments. (The revenue measurement model is reported in Note 2).
Land and Buildings: Due to the VMD property being located on and interlinked with the Weybridge Estate laboratory complex, the land and building asset valuation is based on this being a ‘specialised building’ using the Depreciated Replacement Cost valuation method according to the RICS guidance. There is no active market for VMD property or interlinking Weybridge Estate. See note 1.3 below.
Non-current Assets/Depreciation: The Agency carries its non-current assets at fair value as stated in note 1.4 below. The charge for depreciation for each non-current asset is based on an estimate of its useful life.
20.5 1.4 Property, plant and equipment and Intangible assets
Freehold Land and Buildings
Land and Buildings are subject to professional valuation at no more than five yearly intervals. These are carried out by professionally qualified independent valuers, (appointed by Defra) who adhere to the principles outlined in the Royal Institute of Chartered Surveyors (RICS) Red Book. The last professional valuation was completed in March 2020 with a valuation date of 31 March 2020. These assets are stated at fair value, which is valued at Depreciated Replacement Cost applying to specialist buildings. Depreciated Replacement Cost is defined as “the current cost of replacing an asset with its modern equivalent asset less deductions for physical deterioration and all relevant forms of obsolescence and optimisation”. Between professional valuations, annual desk top revaluations are conducted by Defra’s appointed valuer, considering prevailing local and national indices and local knowledge.
Non-property assets costing £10,000 or more on a grouped or individual basis, where there is an expected useful economic life of more than one year, are carried in the Statement of Financial Position at fair value, using appropriate indices provided by the Office for National Statistics.
Losses on revaluation are charged to the Revaluation Reserve to the extent that gains have been recorded previously and otherwise to the Statement of Comprehensive Net Expenditure.
Intangible assets
Intangible assets are defined as identifiable non-monetary assets without physical substance. These comprise software licences and internally developed software, including assets under construction.
The Agency holds various software licences, which were capitalised at purchase cost where this exceeded capitalisation thresholds. Such assets are only revalued where it is possible to obtain a reliable estimate of their market value.
Internally developed computer software includes capitalisation of internal IT employee costs on projects. The Agency does not hold any intangible assets with an indefinite useful life. The capitalisation threshold is generally £10,000. When fully operational in the business, internally developed computer software is stated at the depreciated purchase cost.
Depreciation and amortisation
Depreciation and amortisation are provided at rates estimated to write-off the valuation of property, plant and equipment, software development and licences on a straight-line basis over the estimated useful life of the asset. Componentisation has been adopted for the VMD’s freehold building asset, with each component capitalised and depreciated separately. Estimated useful lives, component values and residual values are revised annually.
Assets are depreciated over the following timescales:
| Freehold land | Not depreciated |
|---|---|
| Freehold buildings (1) | 0 - 60 years |
| Furniture, fittings and office equipment | 0 - 15 years |
| IT Hardware | 0 - 5 years |
| IT Software development and licences | 5 -10 years |
- (1) The residual life of the VMD building is 35 years as of 31 March 2022
Impairment
Impairments are recognised when the recoverable amount of non-current assets falls below their carrying amount. An impairment review is carried out on an annual basis.
Impairment losses that arise from the consumption of economic benefit or reduction of service potential is recognised as an expense in the SOCNE. However, if the loss relates to a revalued asset, any balance on the revaluation reserve (to the extent that a balance exists) is transferred to the general fund.
Impairment losses that arise from a change in market value is firstly offset against a revaluation reserve for the asset and any amount in addition to this is recognised as an expense in the SoCNE.
Assets under construction
Assets under construction are shown at the accumulated cost with the depreciation commencing when the asset is completed and brought into service.
20.6 1.5 Research and development
Expenditure on R&D is treated as an operating cost in the year in which it is incurred and taken to the SoCNE.
20.7 1.6 Operating income
As a Gross Accounting Agency, activity for Defra is not invoiced or reported as income, but an authority to spend is delegated to the VMD along with deliverable objectives. The Net Parliamentary Funding is recorded as a movement in Taxpayers’ Equity.
20.8 1.7 Revenue from contracts with customers
Revenue from contracts with customers is recognised in accordance with IFRS 15 as adapted and interpreted for the public sector in the Government Financial Reporting Manual (FReM) from 2018-19.
VMD recognises revenue from contracts with customers in accordance with the five-stage model set out in IFRS 15. The core principle of IFRS 15 is that an entity recognises revenue to depict the transfer of services to customers in a way that reflects the consideration to which the entity expects to be entitled to in exchange for services.
A contract asset is an entity’s right to consideration in exchange for goods or services that the entity has transferred to a customer when that right is conditioned on something other than the passage of time (for example, the entity’s future performance). A contract liability is an entity’s obligation to transfer goods or services to a customer for which the entity has received consideration (or the amount is due) from the customer.
Revenue from contracts with customers comprises fees and charges for services provided to industry or contractually entitled income for services provided to market customers. This revenue is measured based on the consideration specified in a contract with a customer. The Agency recognises revenue from contracts with customers in accordance with the five-stage model set out in IFRS 15.
Significant judgements are required to assess the timing of revenue recognition based on the satisfaction of performance obligations. A performance obligation is a promise to deliver a good or service (or series of substantially the same good or service). Details of Agency’s main performance obligations, how and when they are satisfied, and the determination of transaction prices, is detailed in Note 2.
20.9 1.8 Pensions
Pension benefits are provided through the civil service pension arrangements, full details of which can be found in the Remuneration Report.
Although the PCSPS and the CSOPS, known as alpha, are unfunded defined benefit schemes, in accordance with explicit requirements in the FReM, the VMD account for the schemes as if they were defined contribution plans. Costs of the elements are recognised on a systematic and rational basis over the period during which it benefits from employees’ services by payment to the schemes of amounts calculated on an accruing basis. Liability for payment of future benefits is a charge on the schemes. The PCSPS and alpha pension schemes undergo a reassessment of the contribution rates by the Government Actuary at four-yearly intervals. In respect of defined contribution schemes, the VMD recognises the contributions payable for the year.
20.10 1.9 Defra Notional Recharges
A number of services are provided centrally by Defra and the cost is shown as notional recharges. They are charged against the SoCNE by virtue of an interdepartmental non-cash adjustment via the General Fund, with Core Defra recording the associated credit. Defra service recharges comprises legal, human resources, estates, corporate strategy and investigation and enforcement services.
20.11 1.10 Value Added Tax (VAT)
Most of the VMD’s activities are outside the scope of VAT and, in general, output tax does not apply. Input VAT can be recovered on certain contracted-out services. Irrecoverable VAT is charged to the relevant expenditure category or, if appropriate, capitalised with additions to non-current assets. Where output tax is charged or input tax is recoverable, the amounts are stated net of VAT.
20.12 1.11 Apprenticeship Levy
The Apprenticeship Levy was introduced in April 2017, requiring employers with a pay bill of over £3 million each year to pay the levy. The expense element of the apprenticeship levy is recorded against social security costs, within the staff costs note. If bodies utilise the levy for training expense, a notional charge is recognised. The corresponding credit element is recorded against grant income. Amounts are recognised on an accruals basis.
20.13 1.12 Financial instruments
VMD holds few financial instruments. Financial assets comprise of receivables that are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Receivables are initially recognised at fair value and subsequently held at amortised cost after an appropriate provision for expected credit loss. Financial liabilities comprise trade and other payables, and other financial liabilities. They are initially recognised at the fair value of consideration received, less directly attributable transaction costs. They are subsequently measured at amortised cost.
20.14 1.13 Cash and Cash Equivalents
Cash and cash equivalents comprise cash in hand and current balances with banks.
20.15 1.14 General Fund
The net operating result for each year is transferred from the SoCNE to the General Fund. The General Fund represents the value of the VMD’s net assets less liabilities as at 1 April 1991, which is the date from which the first Accounts Direction became effective, plus subsequent external funding movements, plus the accumulated net operating result transferred from the SoCNE.
20.16 1.15 Revaluation Reserve
The Revaluation Reserve represents the unrealised cumulative balance of indexation and revaluation adjustments to non-current assets.
20.17 1.16 Leases
All payments under operating leases are charged to the SoCNE. An operating lease is a lease other than a finance lease. A finance lease is one which transfers substantially all the risks and rewards of ownership to the lessee. The Agency does not have any finance leases.
20.18 1.17 Impending application of newly issued standards not yet effective
IAS 8, Accounting Policies, Changes in Accounting Estimates and Errors, requires disclosures in respect of new IFRSs, amendments and interpretations that are, or will be applicable after the reporting period. There are a number of IFRSs, amendments and interpretations that have been issued by the International Accounting Standards Board that are effective for future reporting periods. Those with relevance to the Agency are outlined below. The Agency has not adopted any new IFRS standards early.
HM Treasury have agreed implementation of IFRS 16 ‘Leases’ will be effective from 1 April 2022. IFRS 16 results in lessees accounting for most leases within the scope of the standard in a manner similar to the way in which finance leases are currently accounted for under IAS 17 ‘Leases’. Lessees will recognise a right of use (‘ROU’) asset and a corresponding financial liability on the balance sheet. The asset will be amortised over the length of the lease, and the financial liability measured at amortised cost. Lessor accounting remains substantially the same as under IAS 17. The Agency expects to adopt the standard using a modified retrospective approach where the cumulative effect of initially applying it is recognised as an adjustment to the opening balance of retained earnings and comparatives are not restated.
The Agency will apply this standard as adapted and interpreted by the Government Financial Manual from the effective date. Leases with a lease term over 12 months and above £5k will have an impact on the Statement of Financial Position for 2022-23 onwards. The impact on 2022-23 Statement of Financial Position is estimated to be between £80k to £85k. There will be no adjustments for leases where the lease term ends within 12 months of the date of initial application.
20.19 2. Operating income
| 2021/22 | 2020/21 | |||
|---|---|---|---|---|
| Revenue from contracts with customers | £’000 | £’000 | ||
| Veterinary pharmaceutical industry | ||||
| Authorisations | 2,559 | 2,097 | ||
| Graded Annual and Fixed Fees | 2,502 | 3,130 | ||
| Inspections | 953 | 756 | ||
| Food industry | 3,790 | 3,675 | ||
| International | 559 | 523 | ||
| Other income | ||||
| Government: Devolved Administrations (1) | 70 | 77 | ||
| Government: EU grant (1) | 56 | 15 | ||
| AMR Reference Centre (1) | 378 | 121 | ||
| Recoveries in respect of outward secondments | 331 | 215 | ||
| Other recovery of costs (2) | 18 | 20 | ||
| Total operating income | 11,216 | 10,629 |
-
(1) Income for work undertaken for Government comprise of contributions from the devolved administrations, receipt of EU grant to support the AMR surveillance programme and income from the Fleming Fund to support the development of the AMR reference centre.
-
(2) Other recovery of costs relates to income for advisory services, dossier copying and training delivered by VMD.
Transaction price to remaining performance obligations
The transaction price is the amount of consideration VMD expects to be entitled to in exchange for transferring promised goods or services in the future, excluding amounts collected on behalf of third parties. VMD considers the terms of the contract and its business practices in determining the transaction price as well as the impact of any variable consideration within a contract including any significant financing component and any non-cash consideration. The transaction price is allocated to each performance obligation identified and, therefore, represents the amount of revenue recognised as those performance obligations are satisfied.
The following table includes revenue expected to be recognised in the future related to performance obligations that are (partially) unsatisfied at the reporting date:
| Business Area £’000 | 2022/23 | 2023/24 | Total |
|---|---|---|---|
| Authorisations | 760 | 141 | 901 |
| Inspections | 141 | - | 141 |
| International | - | - | - |
| Total | 901 | 141 | 1,042 |
Contract balances
As at 31 March 2022, the aggregate amount of the transaction price allocated to the performance obligation that are unsatisfied (or partially unsatisfied) is £0.790 million (31 March 2021: £1.337 million). VMD will recognise this revenue as contracts are progressed to completion, which is expected to occur over the next 12 months.
The contract asset £0.983 million (31 March 2021: £0.962 million) represents the amount of consideration recognised as at 31 March 2022 when a performance obligation is satisfied however, the payment is still conditional on future performance.
| £’000 | 2021/22 | 2020/21 |
|---|---|---|
| Contract Asset | 983 | 962 |
| Contract Liabilities | 1,042 | 1,337 |
Revenue recognised in the period from amounts recognised in the contract liability at the beginning of the period.
| Business Area £’000 | 2021/22 | 2020/21 |
|---|---|---|
| Authorisations | 689 | 554 |
| Inspections | 144 | 147 |
| International | 377 | 121 |
The VMD’s major type of income streams from contracts with customers are detailed in the table below:
| Contract Type | Categories of performance obligation | Basis of income recognition | Amount £’000 |
|---|---|---|---|
| Application for a Marketing Authorisation | Assessment of application to market a veterinary medicines product. A contractual obligation is completed when a new Market Authorisation is granted, or an existing Market Authorisation is varied. | An invoice is issued on validation of an application. For each application there is an estimated time required to complete, based on the average time taken from historic record of applications of the same type. Income is deferred and recognised based on the percentage time completed, compared to the average time for the application type. | 2,559 |
| Graded annual and fixed fees for Marketing Authorisation and Inspection | Provision of services as the competent authority, including post authorisation surveillance/pharmacovigilance | Charge based on cost recovery for the financial year. Invoiced in last quarter of each financial year | 2,502 |
| Inspections | Inspection of manufacturers, wholesaler dealers, retailers of veterinary medicines and feed businesses | Invoiced upon completion of the inspection report | 535 |
| Inspections annual fees | Licencing and maintenance of the register of manufacturers, wholesaler dealers, retailers of veterinary medicines and feed businesses | Income recognised over a year. Accrued for any non-invoiced element or deferred proportionate to the number of months before the next renewal date | 418 |
| Food Industry fees | Provision of the Statutory Residues Surveillance Programme | Charge based on cost recovery for the financial year. Invoiced quarterly or bi-annually or accrued for any non-invoiced elements | 3,790 |
| International Projects | Set out in individual contracts for services and/or provision of training | At agreed milestones, or if, as is generally the case, contract stipulates that money spent up to a specific date can be recovered from the customer prior to completion of the project | 559 |
20.20 3. Staff costs
| Staff costs consist of the following: | 2021/22 | 2020/21 | |||
|---|---|---|---|---|---|
| Permanently employed staff | Temporary staff | Total | Total | ||
| £’000 | £’000 | £’000 | £’000 | ||
| Wages and salaries | 7,055 | 710 | 7,765 | 8,022 | |
| Social security costs | 796 | - | 796 | 783 | |
| Other pension costs | 1,898 | - | 1,898 | 1,896 | |
| Gross total staff costs | 9,749 | 710 | 10,459 | 10,701 | |
| Less amounts charged to capital projects | (1) | - | (1) | (72) | |
| Sub-total as reported in the Statement | |||||
| of Comprehensive Net Expenditure | 9,748 | 710 | 10,458 | 10,629 | |
| Less recoveries in respect of outward secondments | (331) | - | (331) | (215) | |
| 9,417 | 710 | 10,127 | 10,414 |
Included in the permanently employed staff costs is an accrual for untaken annual leave of £302,000, (20120/21: £393,000).
20.21 4. Other non-staff operating expenditure
| 2021/22 | 2020/21 | |||
|---|---|---|---|---|
| Purchase of services | Note | £’000 | £’000 | |
| Statutory Residues Surveillance | 3,458 | 3,356 | ||
| Research and Development Programme | 1,364 | 1,140 | ||
| Government Secure Freight | 874 | 1,484 | ||
| Distance Learning Portal | 11 | 101 | ||
| Antimicrobial Resistance Programme and Surveillance | 521 | 541 | ||
| Antimicrobial Resistance Reference Centre | 760 | 1,060 | ||
| Other direct sub-contracted services | 31 | 55 | ||
| Sub-total purchase of services | 7,019 | 7,737 | ||
| Non-cash items | ||||
| Depreciation of property, plant and equipment | 5 | 365 | 354 | |
| Amortisation of intangible assets | 6 | 669 | 452 | |
| Impairment of intangible assets | 6 | 277 | - | |
| Losses on disposal of non-current assets | 5&6 | - | - | |
| Defra service recharges: | ||||
| DDTS | - | - | ||
| Estates maintenance | 332 | 350 | ||
| Human resources | 176 | 101 | ||
| Defra Investigation Services | 176 | 118 | ||
| Legal services | 83 | 76 | ||
| Auditors’ remuneration | 48 | 46 | ||
| Sub-total non-cash items | 2,126 | 1,497 | ||
| Other operational expenditure | ||||
| Professional programme and technical services | 2,591 | 3,464 | ||
| IT systems maintenance | 940 | 958 | ||
| Travel and subsistence | 35 | (38) | ||
| Training | 44 | 87 | ||
| Staff related costs | 98 | 65 | ||
| Communications | 68 | 89 | ||
| Office related goods and services | 105 | 128 | ||
| Operating leases | 36 | 30 | ||
| Internal Audit | 39 | 36 | ||
| Stationery and publications | 31 | 33 | ||
| Independent expert committees | 11 | 13 | ||
| Customer relations and publicity | 9 | (5) | ||
| Movement on expected credit loss | - | (57) | ||
| IR35 – Contractor Tax and NI (1) | 3,877 | - | ||
| Other costs (1) | 155 | 9 | ||
| Sub-total other operating expenditure | 8,039 | 4,812 | ||
| Total non-staff operating expenditure | 17,184 | 14,046 |
- (1) Following an enquiry launched by HMRC into Defra’s compliance with the off-payroll working (IR35) rules in relation to contingent labour, VMD reassessed the status of all contractors in light of these enquiry. The tax and national insurance lost between 2018-19 and 2021-22 for the contractors is £3.88m plus interest charges £0.15m. The final payment was paid to HMRC in July 2022.
No remuneration was paid to the external auditors (National Audit Office) in respect of non-audit work.
20.22 5. Property, plant and equipment
| Information | Furniture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Land | Buildings | Technology | & Fittings | Total | ||||||
| £’000 | £’000 | £’000 | £’000 | £’000 | ||||||
| Cost or Valuation: | ||||||||||
| At 1 April 2021 | 316 | 5,362 | 692 | 323 | 6,693 | |||||
| Additions | - | - | 43 | - | 43 | |||||
| Disposals | - | - | - | (17) | (17) | |||||
| Revaluation | 24 | 527 | (8) | 9 | 552 | |||||
| At 31 March 2022 | 340 | 5,889 | 727 | 315 | 7,271 | |||||
| Depreciation: | ||||||||||
| At 1 April 2021 | - | - | (581) | (221) | (802) | |||||
| Charged in year | - | (320) | (35) | (10) | (365) | |||||
| Disposals | - | - | - | 17 | 17 | |||||
| Revaluation | - | 320 | 7 | (6) | 321 | |||||
| At 31 March 2022 | - | - | (609) | (220) | (829) | |||||
| Carrying Value | ||||||||||
| At 31 March 2022 | 340 | 5,889 | 118 | 95 | 6,442 | |||||
| Cost or Valuation: | ||||||||||
| At 1 April 2020 | 316 | 5,613 | 675 | 335 | 6,939 | |||||
| Additions | - | - | - | - | _ | |||||
| Disposals | - | - | - | (15) | (15) | |||||
| Revaluation | - | (251) | 17 | 3 | (231) | |||||
| At 31 March 2021 | 316 | 5,362 | 692 | 323 | 6,693 | |||||
| Depreciation: | ||||||||||
| At 1 April 2020 | - | - | (536) | (223) | (759) | |||||
| Charged in year | - | (312) | (31) | (11) | (354) | |||||
| Disposals | - | - | - | 15 | 15 | |||||
| Revaluation | - | 312 | (14) | (2) | 296 | |||||
| At 31 March 2021 | - | - | (581) | (221) | (802) | |||||
| Carrying Value | ||||||||||
| At 31 March 2021 | 316 | 5,362 | 111 | 102 | 5,891 | |||||
| At 31 March 2020 | 316 | 5,613 | 139 | 112 | 6,180 |
An interim desktop valuation was undertaken based on the existing use of the property by an independent valuer (Montague Evans), in accordance with the guidance issued by the Royal Institution of Chartered Surveyors. This resulted in Land and Buildings being revalued as of 31 March 2022 at £6.229m, a net increase of £0.551m from the valuation at 31 March 2021.
In arriving at the value, the valuers have undertaken a Depreciated Replacement Cost (DRC) method of valuation and assessed the cost to provide the building on a modern equivalent asset basis. It was carried out based on information provided by the Defra group to the valuers with regard to the RICS Build Cost Information Service (BCIS) cost indices which is relevant at the date of valuation for the site. The revaluation relied on the BCIS location weightings. The RICS BCIS costs is applied according to the use categorisation. This review also considers the remaining economic life of the buildings. All of VMD’s assets are owned and none are held under finance leases.
20.23 6. Intangible assets
| Internally Generated Software | IGS - Assets Under Construction | IT Software and licences | Total | |||||
|---|---|---|---|---|---|---|---|---|
| £’000 | £’000 | £’000 | £’000 | |||||
| Cost or valuation: | ||||||||
| At 1 April 2021 | 6,295 | 575 | 622 | 7,492 | ||||
| Additions | - | 1,131 | 358 | 1,489 | ||||
| Reclassification | - | - | - | - | ||||
| Impairments | - | (277) | - | (277) | ||||
| Disposals | - | - | - | - | ||||
| At 31 March 2022 | 6,295 | 1,429 | 980 | 8,704 | ||||
| Amortisation: | ||||||||
| At 1 April 2021 | (654) | - | (428) | (1,082) | ||||
| Charged in year | (629) | - | (40) | (669) | ||||
| Disposals | - | - | - | - | ||||
| At 31 March 2022 | (1,283) | - | (468) | (1,751) | ||||
| Carrying Value | ||||||||
| At 31 March 2022 | 5,012 | 1,429 | 512 | 6,953 | ||||
| Cost or valuation: | ||||||||
| At 1 April 2020 | 2,366 | 2,634 | 841 | 5,841 | ||||
| Additions | 1,572 | 298 | 57 | 1,927 | ||||
| Transfers in | 2,357 | (2,357) | - | - | ||||
| Disposals | - | - | (276) | (276) | ||||
| At 31 March 2021 | 6,295 | 575 | 622 | 7,492 | ||||
| Amortisation: | ||||||||
| At 1 April 2020 | (237) | - | (669) | (906) | ||||
| Charged in year | (417) | - | (35) | (452) | ||||
| Disposals | - | - | 276 | 276 | ||||
| At 31 March 2021 | (654) | - | (428) | (1,082) | ||||
| Carrying Value | ||||||||
| At 31 March 2021 | 5,641 | 575 | 194 | 6,410 | ||||
| At 31 March 2020 | 2,129 | 2,634 | 172 | 4,935 |
The net book value for internally generated software includes IT solutions developed to replace EU systems that have ceased to be available upon leaving the EU. These include Registration and Login £0.539m; Licencing £1.102m; Adverse Event Reporting £1.931m; Service Hub £0.574m; Secure Messaging £0.347m; Cloud Infrastructure £0.519m.
The Adverse Event Reporting system was brought into use in April 2019 with additions in March and April 2020. It has a remaining amortisation period of seven years. The other systems were brought into use in September 2020 they have a remaining amortisation period of eight years.
The net book value for Assets under construction includes Identity & Access Management £0.341m added in March 2022 and Special Imports System £1.088m of which £0.790m was added in March 2022. An impairment review carried out during the year identified that the initial Special Imports System development (£0.277m) added in 2020 is impaired because of a strategic decision to focus on licensing as the primary service line for the VMD digital service post Brexit. This made it incompatible with the future development.
The net book value for IT Software and licences includes development costs for expenses@work system £0.100m, Pharmacovigilance software for adverse event report processing and analysis £0.341m which was added and brought into use in Mar 2022 and other purchased software £0.070m.
Cash additions (adjusted for capital accruals) shown in the SoCF amount to £1,489,000 (2020/21 £1,927,000).
20.24 7. Trade receivables and other current assets
| Amounts falling due within one year | 31-Mar-22 | 31-Mar-21 | |
|---|---|---|---|
| £’000 | £’000 | ||
| Amounts falling due within one year: | |||
| Trade receivables | 849 | 1,953 | |
| Other receivables | 12 | 17 | |
| VAT recoverable | 226 | 431 | |
| Prepayments | 185 | 342 | |
| Contract Assets | 983 | 962 | |
| Total trade receivables and other current assets | 2,255 | 3,705 |
Trade receivables are shown net of a provision of £39,000 (2020/21: £39,000) for expected credit loss. The provision is calculated according to the age and status of the debt and recent sector-specific debt-recovery information.
20.25 8. Cash and cash equivalents
| 2021/22 | 2020/21 | ||
|---|---|---|---|
| £’000 | £’000 | ||
| Balance at 1 April 2021 | 1,893 | 3,891 | |
| Net change in cash and cash equivalents | 1,845 | (1,998) | |
| At 31 March 2022 | 3,738 | 1,893 |
All balances were held in accounts administered by Government Banking Services.
20.26 9. Trade payables and other current liabilities
| 31-Mar-22 | 31-Mar-21 | ||
|---|---|---|---|
| £’000 | £’000 | ||
| Amounts falling due within one year: | |||
| Trade payables | 143 | 2 | |
| Other taxation and social security (1) | 4,264 | 211 | |
| Accruals | 3,896 | 3,988 | |
| Contract liabilities | 901 | 1,202 | |
| Total trade payables and other current liabilities | 9,204 | 5,403 | |
| Amounts falling due after more than one year | |||
| Contract liabilities | 141 | 135 | |
| Total trade payables and other liabilities | 9,345 | 5,538 |
8 (1) Other taxation and social security includes £3.88m Tax and NI payable to HMRC for underpayment of taxation for the year 2018-19 to 2021-22 for off-payroll working rules.
At the year end the VMD had contract liabilities (£141,000) falling due after more than one year (2020/21: £135,000).
20.27 10. Capital commitments
There were no contracted capital commitments at 31 March 2022 (31 March 2021: nil).
20.28 11. Commitments under operating leases
Total future minimum lease payments under operating leases are given in the table below for each of the following periods:
| Contract hire cars £’000 | 2021/22 | 2020/21 |
|---|---|---|
| Not later than one year | 13 | 32 |
| Later than one year not later than five years | 2 | 15 |
| Total | 15 | 47 |
20.29 12. Other financial commitments
Defra has entered into a contract (which is not a lease or Public Finance Initiative contract) for Estate Maintenance and Facilities Management services associated with buildings that are either leased by Defra or held on the Agency’s Statement of Financial Position. The Agency incurs a charge proportionate to the benefit it receives from this contract. Based on Defra’s estimate, the payments to which the Agency is committed at the year end, analysed by the period during which the commitment expires are as follows:
| Other financial commitments £’000 | 2021/22 | 2020/21 |
|---|---|---|
| Not later than one year | 158 | 206 |
| Later than one year but not later than five years | 158 | 414 |
| Later than five years but not later than ten years | - | - |
| Total | 316 | 620 |
20.30 13. Related party transactions
As the VMD is an Executive Agency of Defra, Defra is regarded as a related party. During the year, the VMD has had significant transactions with Defra and several of its agencies, including the Animal and Plant Health Agency and Centre for Environment, Fisheries & Aquaculture Science.
The VMD has transacted with various other central government bodies. Most of these transactions have been with the Department for Transport, Food Standards Agency, Food Standards Scotland, Medicines and Healthcare products Regulatory Agency, and The Scottish Government.
The former CEO and one of the non-Executive Directors (who’s NED term also came to an end this financial year) were also Trustees of the SMArt (Safe Medicines for Animals through regulatory training) Charity Board. During the 2021/22 financial year, there was no financial transaction between SMArt and VMD. As of 31 March 2022, there are no SMArt Trustees on VMD’s board.
None of the Board members or key managerial staff have undertaken any material transactions with the VMD during the year other than salaries and reimbursement for travel and subsistence in the normal course of business.
20.31 14. Financial instruments
As the cash requirements of the VMD are met from income from industry and funding through the Estimates process, financial instruments play a more limited role in creating and managing risk than would apply to a non-public sector body of a similar size. The majority of financial instruments relate to contracts to buy non-financial items in line with the Agency’s expected purchase and usage requirements and the Agency is therefore exposed to little credit, liquidity or market risk.
20.32 15. Events after the reporting period
The VMD’s financial statements are laid before the House of Parliament by the Secretary of State for Defra. In accordance with the requirements of IAS 10, events after the reporting period are considered up to the date on which the Accounts are authorised for issue. This is interpreted as the date of the Certificate and Report of the Comptroller and Auditor General.
Up to the date of issue, there have been no other events since 31 March 2022 that would have a significant impact on the Annual Report and Accounts. There are potentially two issues that could have an impact on the future performance of the VMD. Firstly, the war in the Ukraine may have currently unforeseen consequential knock-on impacts on the VMD, as a minimum the predicted increase in inflation may put additional pressures on the budget. Secondly, Defra resourcing controls which include recruitment freezes for all but critical posts was put in place in May 2022 and is expected to last for approximately six months. The continued implication could result in missing KPIs and additional pressure on staff due to lack of resources. VMD is collaborating with Defra to recruit critical roles.
Veterinary Medicines Directorate
Woodham Lane
New Haw
Addlestone
Surrey
KT15 3LS
