Vaccine update: issue 322, June 2021, COVID-19 phase 2 special edition
Published 17 June 2021
COVID-19 vaccination programme phase 2
On Wednesday 8 June it was 6 months since the COVID-19 vaccination programme started across the UK. Since 8 December 2020, when Margaret Keenan became the first person in the world to receive her first vaccine there have been more than 65 million vaccinations at more than 2,000 sites.
As of 10 June 69,743,980 doses of vaccine have been given, of which 40,886,878 were first doses and 28,857,102 were second doses. Now COVID-19 vaccinations are being offered to people aged 25 to 30 years of age. We really want to encourage anyone eligible who has not yet come forward to have their vaccine to make an appointment. This issue of vaccine update focuses on all the new and revised resources published and the importance of the uptake of these resources to make sure everyone has their vaccination consent information, in a format that meets their needs.
Thank you to everyone who has worked tirelessly around the clock to bring us this far. To all the vaccinators, vaccinees and all of the teams working so hard to give the COVID-19 vaccinations – everyone is a great step forward.
COVID-19 vaccine surveillance
39,700 hospitalisations and 13,200 deaths prevented
Public Health England (PHE) estimates that 13,200 deaths have now been prevented in people aged 60 years or older in England up to 13 May 2021 (11,200 deaths in individuals aged 80 years and older, 1,700 in individuals aged 70 to 79 and 300 in individuals aged 60 to 69 years).
Estimates also indicate that the vaccination programme has prevented around 39,700 hospitalisations in those aged 65 years and over in England (approximately 4,900 admissions in those aged 65 to 74, 15,600 in those aged 75 to 84 and 19,200 in those aged 85 and over).
The method for analysing the approximate number of deaths and hospitalisations prevented by the vaccine programme now takes into account the impact of both first and second doses, due to more data being available.
However, it does not include the impact of vaccination on transmission, therefore the true impact of the vaccination programme is likely to be even greater.
An updated analysis including nearly 3,000 symptomatic cases of B.1.617.2 provides further confidence that 2 doses of either vaccine are highly effective against the Delta variant (first identified in India).
Dr Jamie Lopez Bernal, Consultant Epidemiologist at PHE, said:
This analysis gives further confidence that 2 doses of the vaccine provide vital protection against the variants in circulation in the UK, so it is important to book your second jab when invited, to gain maximum protection. The vaccines are very safe and very effective, and they will protect you and those around you from becoming seriously ill.
JCVI advice on COVID-19 variant
The Joint Committee on Vaccination and Immunisation (JCVI) has issued advice to the government on the use of COVID-19 vaccines to mitigate the impact of the B1.617.2 variant of concern.
Professor Wei Shen Lim, COVID-19 Chair for JCVI, said:
Due to the rapid rise in cases of the B1.617.2 Variant of Concern and notable transmission in parts of the country, the JCVI advises that every effort is made to promote vaccine uptake in those who remain unvaccinated in priority cohorts 1 to 9 – these people remain at highest risk of severe outcomes from COVID-19.
The full list of variants of concerns and variants under investigation are published on GOV.UK.
500,000 COVID-19 jabs booked in ‘Glastonbury rush’ in the morning of Wednesday 8 June
The NHS saw a welcome surge in demand for vaccines on Wednesday morning as 500,000 life-saving jabs were booked in a rush likened to the annual scramble for Glastonbury tickets.
The NHS reported:
By 12pm today, just 5 hours after eligibility widened to those aged between 25 and 29, the National Booking Service had seen 2.5 times the number of total appointments made yesterday, with 493,000 slots reserved, around 100,000 an hour on average and more than 1,600 per minute.
The rollout opened to those aged 25 to 29 today and the huge appetite saw people take to social media to compare it to the festival, with people posting about setting their alarms and being as excited as when they were booking for the music festival.
Text messages began to be sent out to people in the newly-eligible age bracket this morning, with more receiving the invite throughout the day and this week.
Rare syndrome related to the AstraZeneca vaccine
Since March 2021 there have been reports from the UK and internationally of a very rare condition of thrombosis (blood clots) and thrombocytopenia (low platelets) following the first dose of the AstraZeneca (AZ) vaccine.
Whilst the cases to date have primarily had venous blood clots, arterial clots have also been reported. The underlying risk factors have not yet been fully established for this condition and a detailed review of suspected cases of this condition following COVID-19 vaccination is ongoing by the Medicines and Healthcare products Regulatory Agency (MHRA), supported by PHE and other professional groups. This will help us to understand the risk factors for developing this condition.
The risk of this very rare side effect is higher in younger age groups and is 1 in 50,000 for adults aged 18 to 39 years and 1 in 100,000 for adults aged 40 or older. This compares with the risks of severe outcomes following COVID-19 infection being higher in older adults and people with serious health problems.
It is important to note that this is currently only being observed following the first dose with no confirmed reports post second dose. Those who have received their first dose of AZ vaccine and have not suffered any serious side effects should continue to be offered the second dose to complete the course.
The Expert Haematology Panel advise that there is no evidence that individuals with a prior history of thrombosis or known risk factors for thrombosis are more at risk of developing this condition after the AZ vaccine. Furthermore, for the majority of individuals, the risk of recurrent thrombosis due to COVID-19 infection is far greater than the risk of this syndrome.
The current advice from the JCVI is:
- adults aged 39 or younger who do not have serious underlying medical conditions should be preferentially offered an alternative to the AZ vaccine, where possible, and only where no substantial delay or barrier in access to vaccination would arise
- healthy individuals aged 40 years or older should continue to be offered any of the available vaccines
- those who have received their first dose of AZ vaccine without suffering this rare side effect should continue to be offered the second dose to complete the course. This includes individuals who are aged 39 years or younger
Dr Mary Ramsay, Head of Immunisation at PHE, said:
We know that 2 doses of the COVID-19 vaccines are required for the best protection and that side effects from AstraZeneca are generally very mild after the second dose. The evidence shows that mixing vaccine brands is more likely to cause more common side effects. So even if you felt unwell after your first dose of AstraZeneca vaccine, you are best to complete with this vaccine for your second dose. Every effort should be made to give people the same vaccine, but in exceptional circumstances where this is not possible it is better to give a second dose of another vaccine than not at all.
Reporting suspected cases
It is very important that all suspected cases are reported to both the MHRA on the COVID-19 Yellow Card scheme and to PHE’s clinical reporting scheme.
In order to minimise the burden on reporters, for cases reported on the PHE clinical reporting scheme first, the last page of the survey allows all the inputted answers to be copied, and relevant information can then be directly pasted into the COVID-19 Yellow Card form.
A range of resources to support healthcare professionals are available including:
Blood clotting information factsheet has been developed for healthcare professionals.
The COVID-19 green book, chapter 14a has been updated with the latest guidance and evidence.
Guidance has been developed for secondary care, primary care and Emergency Departments and Acute Medical Units.
Further information and patient leaflets can be found on GOV.UK.
Reports of menstrual disorders and unexpected vaginal bleeding following COVID-19 vaccination
The MHRA has reviewed reports of menstrual disorders and unexpected vaginal bleeding received following vaccination with the three COVID-19 vaccines currently being used in the UK: Pfizer/BioNTech, AstraZeneca and Moderna.
These reports have also been reviewed by independent experts of the Commission on Human Medicines’ COVID-19 Vaccines Benefit Risk Expert Working Group and members of Medicines for Women’s Health Expert Advisory Group.
The number of reports of menstrual disorders and vaginal bleeding is low in relation to both the number of females who have received COVID-19 vaccines to date and the background rate of menstrual disorders generally. The current evidence does not suggest an increased risk of either menstrual disorders or unexpected vaginal bleeding following vaccination with the vaccines. The MHRA will continue to closely monitor reports of menstrual disorders and vaginal bleeding with COVID-19 vaccines.
Healthcare professionals are advised that anyone presenting with menstrual disorders and/or unexpected vaginal bleeding following COVID-19 vaccination should be treated according to clinical guidelines for these conditions as usual.
Myocarditis
Worldwide, there have also been recent, rare cases of inflammation of the heart called myocarditis or pericarditis reported after COVID-19 vaccines, although it is not yet clear that these are caused by the vaccine. This has been seen mostly in younger men within several days of vaccination. Most of these people recovered and felt better following rest and simple treatments.
You should seek medical advice urgently if you experience:
- chest pain
- shortness of breath
- feelings of having a fast-beating, fluttering or pounding heart
COVID-19 vaccination coverage
The National Coverage team are working hard to produce vaccine uptake data to support wider public health action and surveillance work.
As of 23 May 2021, the National Programme in England has vaccinated over 65% of adults (18 and over) with dose 1 of the COVID-19 vaccine and nearly 40% with dose 2. We have a regular feature in the weekly national flu and COVID-19 surveillance reports showcasing the roll out of the programme by week, age and sex; as well as ethnicity, with additional data in the backing tables that accompany the report.
We continue to work with other organisations such as NHS England and NHS Improvement (NHSEI) and Office for National Statistics (ONS) to produce more statistics that can be used to inform important local public health actions. Please let us know what additional data would be helpful in the pandemic response by emailing us at COVIDvacc@phe.gov.uk.
From 6 January 2021 (week 1 2021), the JCVI advises initially prioritising delivery of the first vaccine dose to maximise the public health impact in the short term and reduce the number of preventable deaths from COVID-19.
The UK Coronavirus Dashboard presents daily counts of people vaccinated aged 18 and over, split by dose, for local authorities in England and Scotland, and regions in England. Cumulative percentage uptake is also presented for these areas (using National Immunisation Management System (NIMS) populations as denominators for English areas, and National Records of Scotland (NRS) mid-year estimates for Scottish local authorities). Uptake by 5 year age groups, initially for English regions and local authorities, now available.
Figure 1: Cumulative weekly COVID-19 vaccine uptake by age in England for dose 1
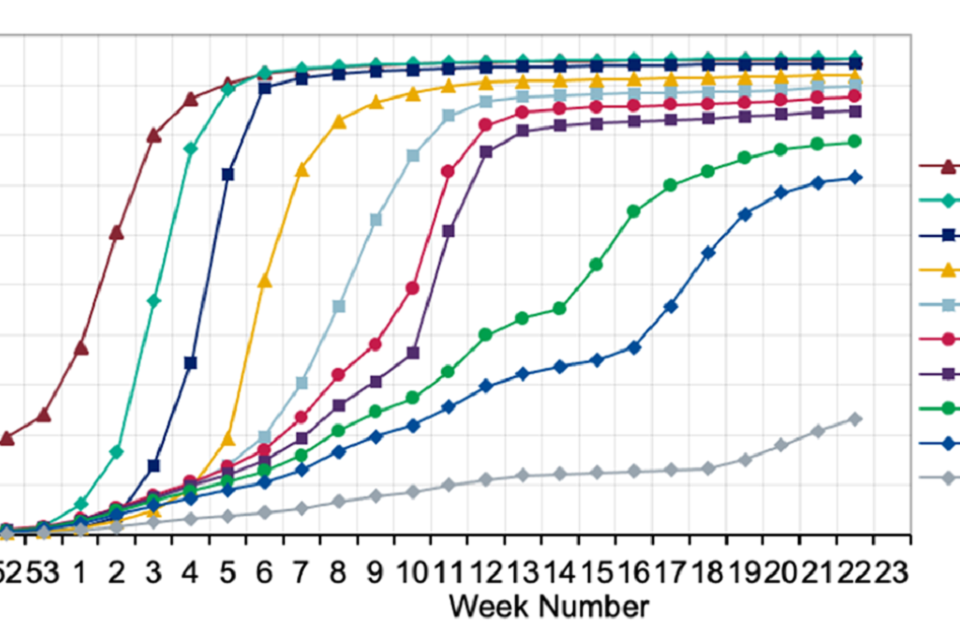
Graph showing the increase of vaccine uptake by week and age group for dose 1. Recent weeks show an uptake of over 90% for those aged 65 years and older.
Figure 2: Cumulative weekly COVID-19 vaccine uptake by age in England for dose 2
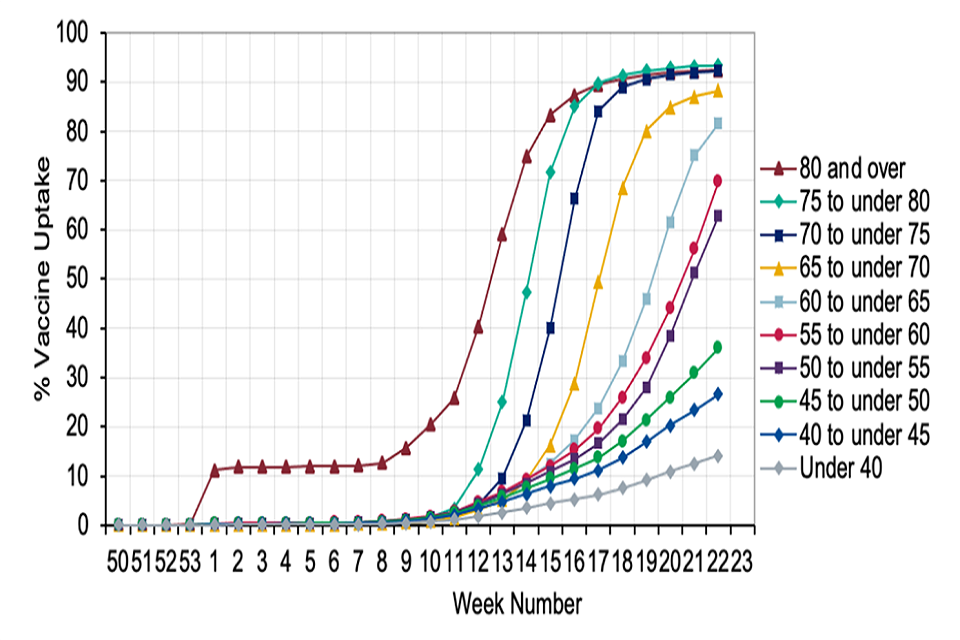
Graph showing the increase of vaccine uptake by week and age group for dose 1. Recent weeks show an uptake of over 90% for those aged 70 years and older.
Figure 3: Cumulative weekly COVID-19 vaccine uptake by ethnicity in England in those aged 50 and over
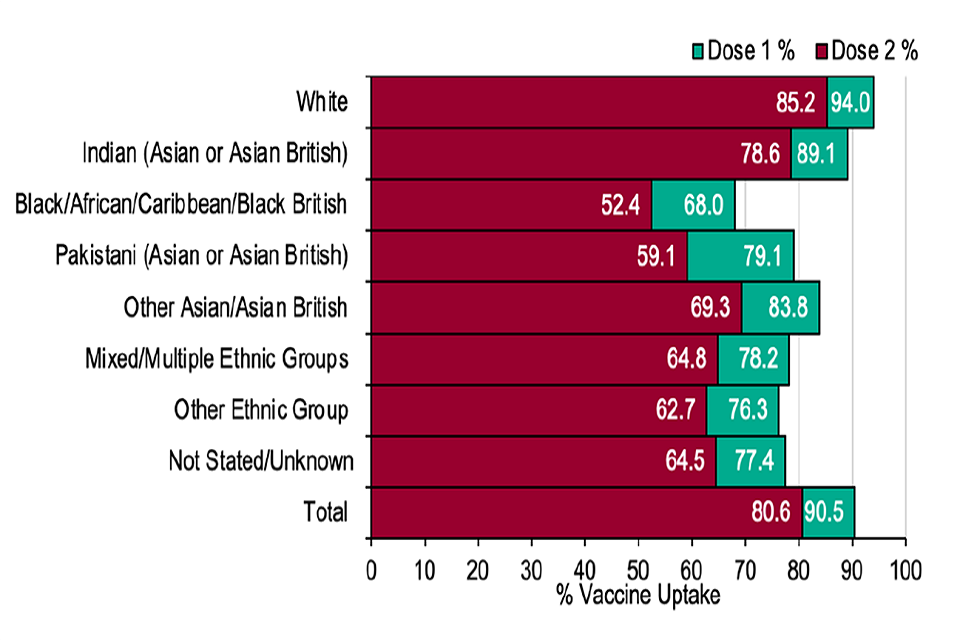
Graph showing the number of doses received by ethnicity.
Figure 4: Age and sex pyramid for COVID-19 vaccine uptake by age in England for dose 1

Graph showing the sex and age group of those have have received dose 1.Proportion of gender is similar in older age groups.
Figure 5: Age and sex pyramid for COVID-19 vaccine uptake by age in England for dose 2
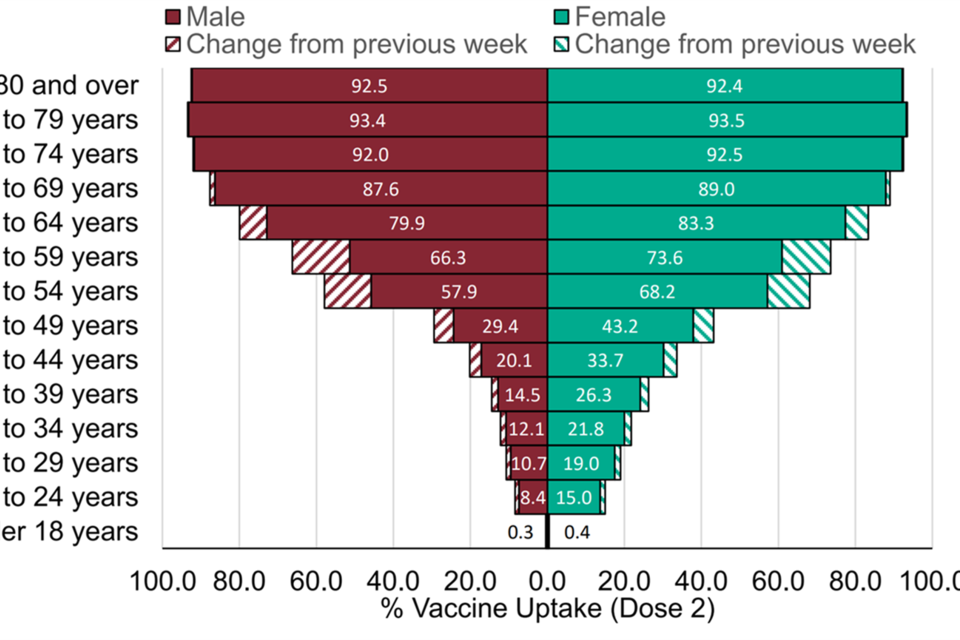
Graph showing the sex and age group of those have have received dose 2.Proportion of gender is similar in older age groups.
COVID-19 testing
| With symptoms | Without symptoms | |||
|---|---|---|---|---|
| Test type | PCR tests. | Rapid lateral flow tests or rapid COVID-19 tests. | ||
| When to take the test | If you have COVID-19 symptoms. To confirm your lateral flow tests result. | If you do not have symptoms of COVID-19. As part of routine testing twice a week. | ||
| How long it takes | These tests are processed in labs. Up to 72 hours, most results the next day | Result processed by test device, around 30 minutes. | ||
| Get a test | Order online, at a test site or call 119. | Order online, at a test site, call 119 or at participating pharmacies and workplaces. |
For further information visit NHS.UK or call 119.
COVID-19 vaccination programme publications available to order now
Only the record cards and Patient Information Leaflet (PIL) from the vaccine manufacturers are supplied with the vaccines:
People being offered a COVID-19 vaccination should be offered the adult phase 2 version 5 leaflet in a format or language of their choice (English, translated Easy Read, British Sign Language (BSL), braille or large print). Women should be given the COVID-19 vaccination guide for women (version 4).
Anyone being offered a first or second dose of AZ vaccine should be given the COVID-19 guide to blood clotting and vaccination (version 2).
At vaccination, patients should be given the What to expect after your COVID-19 vaccination (version 5) leaflet as well as the PIL and record card and they should be offered a COVID-19 vaccination sticker. It is so important to make sure that everyone is offered these essential consent resources to have the best vaccination experience.
These leaflets are available to download or order in various formats and languages.
COVID-19 Vaccination guide to phase 2 version 5
BSL, Braille and English large print.
English, Albanian, Arabic, Bengali, Brazilian Portuguese, Bulgarian, Chinese, Estonian, Farsi, Greek, Gujarati, Hindi, Latvian, Lithuanian, Panjabi, Polish, Romanian, Russian, Somali, Spanish, Turkish, Twi, Ukrainian and Urdu.
COVID-19 Easy Read vaccination guide for adults
English, Albanian, Arabic, Bengali, Bulgarian, Chinese, French, Gujarati, Hindi, Lithuanian, Panjabi, Pashto, Polish, Portuguese, Romanian, Russian, Somali, Tagalog, Ukrainian, Urdu and Vietnamese.
COVID-19 vaccination and blood clotting guide version 2
BSL, Braille and English large print.
English, Albanian, Arabic, Bengali, Brazilian Portuguese, Bulgarian, Chinese, Estonian, Farsi, Greek, Gujarati, Hindi, Latvian, Lithuanian, Panjabi, Polish, Romanian, Russian, Somali, Spanish, Turkish, Twi, Ukrainian and Urdu.
What to expect after your COVID-19 vaccination version 4
Paper copies, alternate versions and translations of version 5 will be available soon.
BSL, Braille and English large print.
Albanian, Amharic, Arabic, Bengali, Bulgarian, Chinese, Estonian, Greek, Gujarati, Hindi, Kurdish, Latvian, Lithuanian, Panjabi, Polish, Portuguese Brazilian, Russian, Twi, Ukrainian and Urdu
Easy-read leaflet on what to expect after COVID-19 vaccination
English, Albanian, Arabic, Bengali, Bulgarian, Chinese, French, Gujarati, Hindi, Lithuanian, Panjabi, Pashto, Polish, Portuguese, Romanian, Russian, Somali, Tagalog, Ukrainian, Urdu and Vietnamese.
COVID-19 vaccination guide for childbearing, pregnant or breastfeeding women version 4
BSL, Braille and English large print.
Albanian, Arabic, Bengali, Bulgarian, Chinese, Estonian, Farsi, Greek, Gujarati, Hindi, Latvian, Lithuanian, Panjabi, Polish, Portuguese Brazilian, Romanian, Russian, Somali, Spanish, Turkish, Twi, Ukrainian and Urdu.
Easy-read leaflet on COVID-19 vaccination for women who might get pregnant, who are pregnant or are breastfeeding their baby.
English, Albanian, Arabic, Bengali, Bulgarian, Chinese, French, Gujarati, Hindi, Lithuanian, Panjabi, Pashto, Polish, Portuguese, Romanian, Russian, Somali, Tagalog, Ukrainian, Urdu and Vietnamese.
Which COVID-19 vaccine poster – new version D includes Janssen COVID-19 vaccine.
The guidance for Pfizer BioNTech vaccine Routine storage section, previously ‘Thawed and undiluted. 5 days (120 hours) at +2°C to +8°C’ has been changed to ‘Thawed and undiluted: up to 1 month (31 days) at +2°C to +8°C’ and this has been updated on posters A, B and C.
The set of posters contain information on the presentation, doses and storage of COVID-19 vaccines. Suitable for all healthcare and vaccination settings:
Poster A is available to download and copies are available to order.
Poster B includes the new AstraZeneca vial, which could be supplied with a green cap and the blue syringes that accompany it for a short period of time. Poster B is available for download only.
Poster C includes the Moderna vaccine is available to download and copies are available to order.
Poster D includes the Janssen vaccine and will be available soon to order using product code COV2020520D.
Guidance and training
The training advice and guidance is subject to continual revision. Here is a summary of the latest changes and revised editions with the links.
Pregnancy section updated and new contraindications for AZ vaccine added. Advice about which vaccines to give those vaccinated abroad added 28 April 2021.
Added information about the exceptional circumstances in which a different second vaccine to the first can be given added 11 May 2021.
Updated vaccine schedule section and added section about administering second dose beyond recommended interval added 20 May 2021.
Pfizer BioNTech vaccine storage conditions updated from 5 days to 31 days added 9 June 2021.
COVID-19: the green book, chapter 14a.
COVID-19 vaccination: information for healthcare practitioners.
COVID-19 vaccination training slide set.
COVID-19 vaccination e-learning programme.
COVID-19: vaccinator training recommendations.
COVID-19: vaccinator competency assessment tool.
Vaccines for the national COVID-19 programme supplied by PHE
The vaccines currently available to order by the pre-agreed providers are in the table below. Latest information for each vaccine can be found on ImmForm.
| Manufacturer | Vaccine name | Presentation | Storage |
|---|---|---|---|
| Pfizer/BioNTech | BNT162b2 | Each pack of vaccine contains 195 vials with 6 doses per vial (1170) doses per pack) | This vaccine requires ultra-low temperature storage (-80°C to -60°C) |
| AstraZeneca | ChAdOx1-S | Each pack of vaccine contains 10 vials with 8 doses per vial (80 doses per pack) | 2°C to 8°C |
| Moderna | Moderna COVID-19 vaccine | Each pack of vaccine contains 10 vials with 10 doses per vial (100 doses per pack) | This vaccine requires freezer (-25°C to -15°C) |
The delivery schedule for ordering Moderna vaccine via ImmForm changed from next day delivery to 48 hour delivery. The existing next day delivery schedule for AZ and Pfizer vaccines remains unchanged: orders placed before 11:55 AM each day, will be delivered next day.
If you have a query in respect of access to COVID-19 vaccines, contact your System Vaccination Operations Centre (SVOC) and Regional Vaccination Operations Centre (RVOC) teams.
If you have any queries about ImmForm ordering, email Helpdesk@immform.org.uk
If you have any queries about deliveries, call 01234 587199 or email NHS.VaccineSupport@movianto.com
If you have any queries about the vaccine or associated products, email COVID19PHEsupplies@phe.gov.uk
Colleagues from Devolved Administrations, refer to guidance from your respective health departments for arrangements in Scotland, Wales and Northern Ireland.
Further information on other COVID-19 vaccines, including vaccine availability, associated products, vaccine ordering and delivery schedule, will be communicated as soon as it is available.
