Vaccine update: issue 319, April 2021, COVID-19 phase 2 special edition
Published 30 April 2021
COVID-19 vaccination programme phase 2
The Joint Committee on Vaccination and Immunisation (JCVI) is an independent expert advisory committee which advises the UK health departments on vaccination.
In December 2020, JCVI advised the vaccination of 9 key priority groups against COVID-19, covering all adults aged 50 years and over, and younger adults with underlying health conditions that put them at specific risk from coronavirus (COVID-19). This part of the programme, termed phase 1, began rollout in the UK from 8 December 2020. Phase 1 aims to reduce mortality from COVID-19, along with the protection of UK health and social care systems.
The programme has been a success in terms of delivery, with over 30 million people having received their first vaccine dose so far. The COVID-19 vaccines in use in the UK are highly effective and have been shown to substantially reduce the risk of infection and severe disease from COVID-19. It has been estimated by Public Health England (PHE) that over 10,000 deaths have been averted by the COVID-19 immunisation in the first 3 months of the programme.
Exceptional efforts deliver
The successful delivery of the phase 1 programme can be attributed to the exceptional efforts of the NHS, volunteers and community organisations and the operational simplicity of the programme. Programmes that are less complicated to organise are more readily delivered at speed and are more likely to achieve high vaccine coverage.
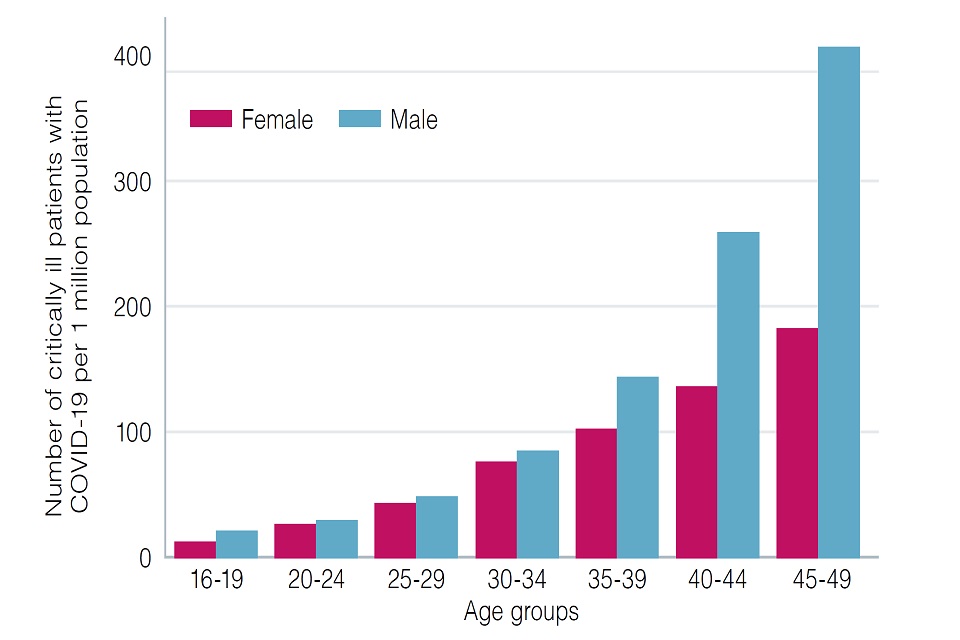
Data on hospitalisations due to COVID-19 indicate that a substantial number of admissions occur in people under the age of 50 years who would not have been vaccinated in the first phase of the vaccination programme (1-5).
JCVI has been asked by the Department for Health and Social Care (DHSC) to formulate advice on the optimal strategy to further reduce mortality, morbidity and hospitalisations from COVID-19 disease in the next phase of the programme.
Options considered for the next phase (phase 2) of the programme include:
- direct protection of those at higher risk of serious disease and hospitalisation, including groups associated with an increased risk
- targeted vaccination to reduce transmission of COVID-19 in the population
- vaccination of occupational groups at higher risk of exposure
These options are not mutually exclusive.
Mathematical modelling of vaccination strategies for phase 2 indicates that rapid vaccine deployment is the most important means to maximise public health benefits against severe outcomes from COVID-19 (6,7).
A strategy aimed primarily at reducing transmission of infection would take longer to achieve reductions in hospitalisations and would require very high vaccine uptake in the target populations. There is evidence that some occupations have an increased risk of morbidity due to COVID-19 and that males aged 40 to 49 years are more likely to be employed in these occupations (8-9).
However, a mass vaccination strategy centred specifically on occupational groups would be more complex to deliver and may require new vaccine deployment structures which would slow down vaccine delivery to the population as a whole, leaving some individuals unvaccinated for longer. Operationally, simple and easy-to-deliver programmes are critical for rapid deployment and high vaccine uptake.
Advice
This advice refers to COVID-19 vaccines authorised for use in the UK. Progress into phase 2 of the programme should be accompanied by continued efforts to extend coverage among those prioritised in phase 1 but who remain unvaccinated, and to complete delivery of second doses to all those given first doses in phase 1.
There is good evidence that the risks of hospitalisation and critical care admission from COVID-19 increase with age. In occupations where the risk of exposure to SARS-CoV-2 is potentially higher, persons of older age are also those at highest risk of severe outcomes from COVID-19.
JCVI therefore advises that the offer of vaccination during phase 2 is age-based, starting with the oldest adults first and proceeding in the following order:
- All those aged 40 to 49 years.
- All those aged 30 to 39 years.
- All those aged 18 to 29 years.
In individuals aged 18 to 49 years there is an increased risk of hospitalisation in males, those from certain ethnic minority backgrounds, those with a body-mass index (BMI) of 30 or more (obese or morbidly obese), and those experiencing socio-economic deprivation (1-5, 10)
JCVI strongly advises that individuals in these groups promptly take up the offer of vaccination when they are offered, and that deployment teams should utilise their understanding of local health systems and demographics, combined with clear communications and outreach activity to promote vaccination in these groups.
Unvaccinated individuals who are at increased risk of severe outcomes from COVID-19 on account of their occupation, male sex, obesity or ethnic background are likely to be vaccinated most rapidly by an operationally simple vaccine strategy.
Reactogenicity
Typical side effects from vaccination include injection site pain, headache or mild fever. Such side events are relatively common and are usually mild to moderate in intensity and short-lived. The frequency of these events is associated with age, with the effects seen most commonly in younger adults. Events typically resolve within a few days of vaccination.
Given the frequency of mild to moderate reactions in younger adults, JCVI advises that those attending for vaccination should be informed of the frequency of reactions and that paracetamol can be used in improving symptoms.
Special considerations
There have been reports of an extremely rare adverse event of concurrent thrombosis (blood clots) and thrombocytopenia (low platelet count) following vaccination with the first dose of AstraZeneca ChAdOx1 nCoV-19 vaccine (AZD1222). There has been no signal for thrombosis with thrombocytopenia following receipt of other COVID-19 vaccines approved for use in the UK (Pfizer/BioNTech and Moderna).
Given the very low numbers of events reported overall, there is currently a high level of uncertainty in estimates of the incidence of this extremely rare adverse event by age group. The available data suggests there may be a trend for increasing incidence of this adverse event at younger ages. In contrast, the risk of severe disease associated with COVID-19 increases steeply with age, with the youngest adults at lowest risk. There are currently no known risk factors for this extremely rare condition, which appears to be an idiosyncratic reaction on first exposure to the AstraZeneca COVID-19 vaccine.
Alternatives to the AstraZeneca COVID-19 vaccine currently approved for use in the UK include the Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 vaccines. JCVI has weighed the relative balance of benefits and risks, and advises that the benefits of prompt vaccination with the AstraZeneca COVID-19 vaccine far outweigh the risk of adverse events for individuals aged 30 years and over or those at any age who have underlying health conditions which put them at higher risk of severe COVID-19 disease.
JCVI currently advises that in phase 2, it’s preferable for adults aged 18 to 29 years without underlying health conditions that put them at higher risk of severe COVID-19 disease, to be offered an alternative to the AstraZeneca COVID-19 vaccine, if available. People may make an informed choice to receive the AstraZeneca COVID-19 vaccine to receive earlier protection.
Further details on contra-indications, precautions and scheduling will be outlined in the Green Book: immunisation against infectious disease.
Male sex
Evidence on hospitalisations indicates an increased risk of hospitalisation in males, particularly those aged 40 to 49 years.
This risk may be associated with male sex itself, exposure to infection, occupation, and, or other factors. JCVI strongly advises that males promptly take up the offer of vaccination.
Profile of critical care admissions with COVID-19, 1 August 2020 to 31 January 2021
Vaccine priority group 10, admitted 1 August 2020 to 31 January 2021
Persons from ethnic minority backgrounds
People from certain ethnic minority backgrounds are at higher risk of hospitalisation from COVID-19 (1-5, 10). This follows a similar pattern to data on mortality reviewed in considerations relating to phase 1 of the programme. There is no strong evidence that ethnicity by itself (or genetic characteristics) is the sole explanation for observed differences in rates of severe illness and deaths.
Between waves 1 and 2 of the pandemic, changes in the risk of infection and mortality in people from ethnic minority backgrounds, and within ethnic minorities, have been observed (11). Such large rapid changes are unlikely to be due to biological factors and are more likely to be related to environmental and behavioural changes.
Discussions with ethnic minority communities and opinion leaders highlight the importance of building trust and the avoidance of stigmatisation or discrimination when planning the delivery of vaccination programmes (12-13). Thus far, in phase 1 of the vaccination programme, slower coverage has been noted in persons from certain ethnic minority backgrounds.
Well-recognised factors that impact on vaccine uptake are:
- access to vaccination
- vaccine confidence (safety and efficacy)
- perception of risk from disease
The reasons for slower coverage in people from certain ethnic minority backgrounds are unclear at present, but there is evidence that addressing structural issues related to access and involving community influencers can have a positive impact.
JCVI strongly advises that priority is given to the deployment of vaccination in the most appropriate manner to promote vaccine uptake in persons from ethnic minority backgrounds who have not yet been vaccinated.
This may include planning to enable easy access to vaccination sites, supported engagement with local ethnic minority community and opinion leaders, and tailored communication with local and national coverage. As appropriate, these efforts should consider a longer-term view beyond the current COVID-19 mass vaccination programme and seek to address inequalities that already exist across the wider immunisation programme.
JCVI strongly advises persons from ethnic minority backgrounds who have not yet been vaccinated to promptly take up the offer of vaccination.
Underlying health conditions
Evidence indicates that individuals aged 18 to 49 years who have a BMI over 30 (obese or morbidly obese) (1-5) are at higher risk of hospitalisation compared with age-matched peers. Only very modest increased risks for hospitalisation were identified in persons with certain other underlying health conditions not already covered in phase 1 of the programme. As the risk is also age-associated, JCVI continues to advise that an age-based programme is the best way of delivering vaccine to these populations.
Post-acute COVID-19 syndrome
Prolonged symptoms after acute infection with SARS-CoV-2 have been described in people of all ages, including healthy young adults. At present, there is limited data on the aetiology, extent, duration and severity of these symptoms. Studies are ongoing to better define the nature of post-acute COVID-19 syndrome and to identify any factors which could predispose individuals to it. Existing evidence is inadequate to structure a vaccination policy specifically targeting populations at risk of post-acute COVID-19 syndrome.
As COVID-19 vaccination reduces the risk of acquiring SARS-CoV-2 infection, it will reduce the risk of vaccinated individuals developing post-acute COVID-19 syndrome.
COVID-19 vaccination during pregnancy – JCVI letter
Statement 16 April 2021
The advice, published in PHE’s Green Book, a clinical professional guide for vaccinators in the UK, still advises that pregnant women should discuss the risks and benefits of vaccination with their clinician, including the latest evidence on safety and which vaccines they should receive.
Professor Wei Shen Lim, COVID-19 Chair for JCVI, said:
We encourage pregnant women to discuss the risks and benefits with their clinician – those at increased risk of severe outcomes from COVID-19 are encouraged to promptly take up the offer of vaccination when offered. There have been no specific safety concerns from any brand of COVID-19 vaccines in relation to pregnancy.
There is more real-world safety data from the US in relation to the Pfizer-BioNTech and Moderna vaccines in women who are pregnant – therefore, we advise a preference for these to be offered to pregnant women.
All vaccines being used in the UK have undergone robust clinical trials and have met the Medicines and Healthcare products Regulatory Agency (MHRA)’s strict standards of safety, effectiveness and quality.
Dr Mary Ramsay, Head of Immunisation at PHE said:
The available data on the Pfizer-BioNTech and Moderna vaccines provides confidence that they can be offered safely to pregnant women. The COVID-19 vaccines continue to save thousands of lives and it is important that we encourage as many people as possible to take up the offer when it is their turn.
COVID-19 vaccination programme leaflets and posters
New version 4 COVID-19 vaccination leaflets
These have been published and are available to order here:
- guide for childbearing, pregnant or breastfeeding women v4
- guide for adults – phase 2 COVID-19 vaccination programme v4
- guide on what to expect after your COVID-19 vaccination v4
Which COVID-19 vaccine to use? poster
The Which COVID-19 vaccine? poster has been updated to include the Moderna vaccine and is available to order.
Ramadan
Ramadan COVID-19 and vaccination posters and graphics
As Ramadan started earlier this month and lockdown begins easing, the Muslim community will want to start to reunite to celebrate this holy month. These posters are a reminder to continue to practise social distancing, to wear a mask and avoid crowded gatherings so people can continue to protect themselves and their families from COVID-19. It also explains that for most Muslims, it’s permitted to have their COVID-19 vaccinations if they are called to do so during the fasting period.
We hope that they will be ordered by screening and immunisation teams, local councils and widely used in communities, retail, food outlets, religious settings, high street locations, hairdresser, barber and beauty shops, vaccination centres and transport settings such as train stations and cab offices.
We have published a set of 3 posters and paper copies are available to order from Health Publications.
The 3 posters are:
- COVID-19 vaccination and Ramadan collage poster A
- COVID-19 vaccination and Ramadan poster B
- COVID-19 vaccination and Ramadan poster C
They are also available in Arabic, Bengali and Urdu.
Ramadan COVID-19 social media assets
Download the Ramadan COVID-19 GIFs and graphics using product code: COV2021006. You can share them using the Muslim Council of Britain hashtag #SafeRamadan.
Ramadan COVID-19 resources
You can download the complete Ramadan COVID-19 resources folder, which contains 15 files in total, including:
- 3 poster designs (print for local commercial printing, web-ready version and 3 JPGs for convenience)
- 3 infographics designs
- 4 GIF designs
Please promote the availability of these posters and resources widely in all your local networks. Paper copies can be ordered free of charge, especially for local councils, as well as all settings mentioned above.
We hope that you find these resources useful. We’re always interested in any feedback about them from your local communities.
National protocols for COVID-19 vaccines
These are updated in line with changes to the programme. An updated protocol for the AstraZeneca COVID-19 vaccine programme, which includes cautions relating to reports of blood clots, has been published on 27 April 2021.
Look after your vaccines
Please take a few minutes over the next week to check that all the staff involved with monitoring the vaccine fridge temperatures in the setting in which you give vaccines know how to accurately record the current, minimum and maximum temperatures and know how to RE-SET the fridge thermometer after each reading.
The PHE national immunisation team has been involved in advising on several cold chain incidents already this year and one of the main issues has been the absence of re-setting. Pages of temperature recordings for some of these incidents have been provided, all with identical minimum or maximum readings, often out of range for days or weeks with no action taken.
Readings of current, minimum or maximum temperature should ideally be taken and recorded at the beginning and end of each day and immediate action should be taken if any of these are found to be outside the recommended +2°C to +8°C temperature range.
Please help prevent vaccine wastage and maintain vaccine potency and patient confidence by ensuring everyone involved has access to your local cold chain protocol, that all know how to find and use the RE-SET button appropriately and that all staff know what action they need to take if any of the current, minimum or maximum temperatures are outside of the recommended range.
To help staff prevent wastage, please order a free keep your vaccine healthy cold chain fridge magnet and keep your vaccines healthy poster.
Vaccines for the national COVID-19 programme supplied by PHE
The vaccines currently available to order by the pre-agreed providers is in the table below:
| Manufacturer | Vaccine name | Presentation | Storage |
|---|---|---|---|
| Pfizer/BioNTech | BNT162b2 | Each pack of vaccine contains 195 vials with 5 doses per vial (975 doses per pack) | This vaccine requires ultra-low temperature storage (-80°C to -60°C) |
| AstraZeneca | ChAdOx1-S | Each pack of vaccine contains 10 vials with 10 doses per vial (100 doses per pack) | 2°C to 8°C |
| AstraZeneca | ChAdOx1-S | Each pack of vaccine contains 10 vials with 8 doses per vial (80 doses per pack) | 2°C to 8°C |
| Moderna[footnote 1] | Moderna COVID-19 vaccine | Each pack of vaccine contains 10 vials with 10 doses per vial (100 doses per pack) | This vaccine requires freezer (-25°C to -15°C) |
If you have a query in respect of access to COVID-19 vaccines, please contact your System Vaccination Operations Centre (SVOC) and Regional Vaccination Operations Centre (RVOC) teams. Colleagues from devolved administrations should refer to guidance from their respective health departments for arrangements in Scotland, Wales and Northern Ireland.
Moderna COVID-19 vaccine
Access to product is for pre-authorised sites only.
The vaccine is available as a 100 dose pack (10 vials, each with 10 doses). Each pack of vaccine ordered will be automatically linked to 3 associated products which will arrive with the vaccine pack:
- PHE-issued vaccination record cards (pack of 100)
- package leaflet: information for the user (pad of 100)
- a sheet of stickers with vaccine name and batch number on them
Combined needles and syringes for administration of vaccine, including to those morbidly obese, are available to order separately. Please note the combined needles and syringes available to order for administration of the Moderna vaccine are the same products being issued for use with the AstraZeneca vaccine:
- standard vaccine administration – BD Flu+ Combined Needle and Syringe 23G x 25mm (1 inch) with 1ml syringe with graduations at 0.25ml
- vaccine administration to morbidly obese patients – Prosum Combined Needle and Syringe 23G x 38mm (1.5 inch) with 1ml syringe with graduations at 0.1ml and 0.25ml
Extra vaccination record cards can be ordered should you require. These are available in packs of 50. Please only order what you need.
The vaccine will be delivered to your site according to your delivery schedule. The vaccine will be packed in validated packaging to maintain the vaccine storage conditions between -25°C and -15°C during transportation. The vaccine packaging will be dependent on the size of your order.
Unlike with deliveries of Pfizer and AstraZeneca COVID-19 vaccines, a mixed delivery model is utilised for Moderna COVID-19 vaccine using isothermic boxes and Tower cold chain solutions containers.
The single use isothermic boxes (validated for up to 84 hours) will contain a quantity of ice packs and will be used for delivery of small orders of up to and including 12 packs of vaccine. Upon delivery, these boxes will be left at the site and should be disposed of in line with local protocols. Dry ice is not included with the product.
The reusable cold chain containers (validated for up to 120 hours) will be used for delivery of vaccine orders of between 13 to 459 vaccine packs. Upon delivery, the Movianto driver will move containers from the vehicle to the unloading area where the vaccine will be unpacked by the customer and transferred to a freezer. Dry ice is not included with the product. Containers will not be left at the delivery location and the drivers are aware it will take a few minutes to unload if there are significant quantities.
When placing your orders, take care to ensure that there are staff within the department trained to be able to handle vaccine transfer to the freezer on the expected delivery date.
Further detailed information on each type of delivery is available on ImmForm.
May bank holiday COVID-19 vaccine deliveries
Vaccine and associated products will continue being delivered on a next day delivery schedule.
Customers in Isle of Wight, Welsh GPs and Scottish customers in Shetland, Orkney, Stornoway, Fort William, Inverness and Wick will continue receiving orders per their delivery schedule.
Jersey and Guernsey customers needing delivery during the bank holiday period should contact COVID19PHEsupplies@phe.gov.uk to discuss possible delivery options individually tailored to their locality.
Vaccine supply for the routine vaccination programme
Reporting an ordering discrepancy via ImmForm
You can now report delivery problems and order discrepancies online via your ImmForm account. For more information, please see the ImmForm Help Guide – How To Report An Order Discrepancy, which will be available in due course via the help guides link on the ImmForm home page.
Vaccines for the 2020 to 2021 children’s flu programme supplied by PHE
Fluarix® Tetra remains available to order for children in England.
PHE does not supply any flu vaccines for patients aged 18 years and over.
All batches of Fluenz® Tetra issued for the 2020 to 2021 children’s flu programme have now expired.
Please ensure that all remaining stock is removed from fridges and disposed of in line with local policies. Any disposed stock should be recorded through the stock incident page.
Providing a second dose of flu vaccine after all Fluenz® Tetra has expired
If you still need to give a second dose of flu vaccine 4 weeks after the first dose (for example, for children in clinical risk groups aged 2 to under 9 years who have not received influenza vaccine before), then it is safe and effective to give inactivated vaccine (Fluarix® Tetra) as a second dose.
All influenza vaccines for the 2020 to 2021 season
Information on all influenza vaccines that were available in the UK for the 2020 to 2021 season is available.
May bank holidays order and delivery schedules for routine vaccinations
Due to the May bank holidays, there will be no deliveries or order processing by Movianto UK on Monday 3 May and Monday 31 May 2021. Please see the table below for revised order and delivery dates.
For customers with standard delivery dates of Monday, please be aware that:
- after 26 April, your next available delivery day will be 10 May 2021
- after 24 May, your next available delivery day will be 7 June 2021
You are reminded to be prepared for the break in deliveries and to order accordingly. Please make sure you have sufficient room in your fridge for any additional vaccine you wish to stock over this holiday period.
Early May bank holiday – Monday 3 May 2021
| Delivery date | Order cut-off date | Order cut-off time |
|---|---|---|
| Monday 26 April | Thursday 22 April | 11:55 AM |
| Tuesday 27 April | Friday 23 April | 11:55 AM |
| Wednesday 28 April | Monday 26 April | 11:55 AM |
| Thursday 29 April | Tuesday 27 April | 11:55 AM |
| Friday 30 April | Wednesday 28 April | 11:55 AM |
| Monday 3 May | Closed – no deliveries or order processing | |
| Tuesday 4 May | Thursday 29 April | 11:55 AM |
| Wednesday 5 May | Friday 30 April | 11:55 AM |
| Thursday 6 May | Tuesday 4 May | 11:55 AM |
| Friday 7 May | Wednesday 5 May | 11:55 AM |
| Monday 10 May | Thursday 6 May | 11:55 AM |
Late May bank holiday – Monday 31 May 2021
| Delivery date | Order cut-off date | Order cut-off time |
|---|---|---|
| Monday 24 May | Thursday 20 May | 11:55 AM |
| Tuesday 25 May | Friday 21 May | 11:55 AM |
| Wednesday 26 May | Monday 24 May | 11:55 AM |
| Thursday 27 May | Tuesday 25 May | 11:55 AM |
| Friday 28 May | Wednesday 26 May | 11:55 AM |
| Monday 31 May | Closed – no deliveries or order processing | |
| Tuesday 1 June | Thursday 27 May | 11:55 AM |
| Wednesday 2 June | Friday 28 May | 11:55 AM |
| Thursday 3 June | Tuesday 1 June | 11:55 AM |
| Friday 4 June | Wednesday 2 June | 11:55 AM |
| Monday 7 June | Thursday 3 June | 11:55 AM |
Please be advised that emergency or ‘out of schedule’ deliveries cannot be arranged for failure to place orders in good time.
Viper antivenom has changed
The viper antivenom product supplied via ImmForm has recently changed from ViperaTAb® to Viperfav®. The products have different active ingredients, formulations and presentations:
| Product | ViperaTAb® | Viperfav® |
|---|---|---|
| Source of immune sera | Ovine | Equine |
| Licensed status | Unlicensed in the UK | Unlicensed in the UK |
| Storage | Store in a refrigerator between 2°C and 8°C | Store in a refrigerator between 2°C and 8°C |
| Presentation | Each pack includes 2 x 4ml vials, containing 100mg Fab fragments each | Each pack includes 1 x 4ml vial containing F(ab’) 2 fragments |
| Initial treatment recommendation | The initial dose of ViperaTAb® is the contents of 2 x 4ml vials (i.e. 1 pack per patient) | The initial dose of Viperfav® is the contents of 1 x 4ml vial (i.e. 1 pack per patient) |
Recommendations for the treatment of common adder bites and the administration of Viperfav® can be found on TOXBASE. To minimise wastage, please use all locally held stocks of in date ViperaTAb® to treat eligible patients, before switching to Viperfav®.
Change to dTaP/IPV vaccine for both the pre-school booster and maternal pertussis dTaP/IPV programmes
Boostrix-IPV® is currently supplied for both the pre-school booster and maternal pertussis dTaP/IPV programmes. This has recently changed from Repevax®.
The 2 vaccines are equivalent. To minimise wastage, please use all your locally held stocks of Repevax® to vaccinate eligible individuals, before switching to Boostrix-IPV®. There is no other change to the pre-school booster or maternal pertussis immunisation programme.
Further details about this programme can be found in chapter 24 of the Green Book.
Update to Bexsero Patient Information Leaflet
Every pack of Bexsero (Meningitis B vaccine, 10 doses) is supplied with a pad of 10 Patient Information Leaflets (PILs), as well as there being a single PIL inside each Bexsero pack. Since September 2020, an updated version of the PIL pad has been distributed with Bexsero orders. Please dispose of the single PIL from inside the pack, as it will be out of date. We will advise further when the PIL supplied in the pack is in line with the PIL pad.
MMR vaccine ordering
To rebalance central supplies of both MMR vaccines please consider ordering M-M-RvaxPRO® as your first choice, which is available without restriction.
Customers in England and Wales who require Priorix®, for example because you serve communities that do not accept vaccines containing porcine gelatine, may order up to 6 packs of Priorix® per ImmForm account per week. For assistance please contact the ImmForm Helpdesk at helpdesk@immform.org.uk
Customers in Scotland should refer to their local ordering restrictions.
The EU Falsified Medicines Directive (FMD) and Delegated Regulation as applicable to PHE supplied vaccines for the national immunisation programme
From 11pm on 31 December 2020, when the UK’s EU exit transition period ended, the ‘safety features’ Delegated Regulation (2016/161) under the EU Falsified Medicines Directive (FMD; 2011/62/EU) no longer applied in Great Britain (England, Wales and Scotland).
This means that in Great Britain, end users of the majority of prescription-only medicines, including the FMD-compliant products supplied by PHE via ImmForm, are no longer required to verify or decommission the unique identifiers on serialised packs. Serialised packs can nonetheless continue to be dispensed for as long as they are still in date.
Registering for a new or updating your existing ImmForm vaccine ordering account
When you register for or update an existing ImmForm account, PHE as a wholesaler of vaccines need to verify the requesting customer.
Please ensure you have your professional regulatory body registration number or Wholesaler Dealer Licence and an organisation code which can be verified when requesting updates or requesting a new vaccine ordering account.
For more information, please see the ImmForm helpsheet – how to register.
Vaccine supply for the non-routine programme
Hepatitis A vaccine
Adult
- GSK: Havrix Adult pre-filled syringe (PFS) singles and packs of 10 are available
- Sanofi Pasteur: Avaxim PFS singles and packs of 10 are available
- MSD: VAQTA adult is available
Paediatric
- GSK: Havrix Paediatric PFS singles and packs of 10 are available
- MSD: VAQTA paediatric is available
Hepatitis B vaccine
Adult
- GSK: Engerix B PFS singles and packs of 10 are available
- GSK: Engerix B single vials are discontinued
- GSK: Engerix B vials packs of 10 are discontinued
- GSK: Fendrix is available
- MSD: HBVAXPRO 10 µg is unavailable until late April 2021
- MSD: HBVAXPRO 40 µg is available
Paediatric:
- GSK: limited supplies of Engerix B Paediatric singles are available
- MSD: HBVAXPRO 5µg is available
Combined hepatitis A and B vaccine
- GSK: Twinrix Adult singles and packs of 10 are available
- GSK: Twinrix paediatric is available
- GSK: Ambirix is available
Combined hepatitis A and typhoid vaccine
- Sanofi Pasteur: Viatim is available
Typhoid vaccine
- Sanofi Pasteur: Typhim singles and packs of 10 are available
- Emergent: Vivotif is available
Rabies vaccine
- Valneva: Rabipur is currently available – orders should be placed directly with Valneva or via your preferred wholesaler. Vaccine supply contact details:
Valneva UK
Telephone: 01252 761 007 - Sanofi Pasteur: Rabies BP is currently available without restriction
Pneumococcal polysaccharide vaccine (PPV)
- MSD: supply of Pneumovax 23 (PPV23) PFS is currently limited
Pneumococcal polysaccharide conjugate vaccine (PCV)
- Pfizer: Prevenar 13 is currently available
Varicella Zoster vaccine
- GSK: VARILRIX is available
- MSD: VARIVAX is available
- MSD: ZOSTAVAX is currently available without restrictions
Diphtheria, tetanus and poliomyelitis (inactivated) vaccine
- Sanofi Pasteur: Revaxis is available
Diphtheria, tetanus, pertussis (acellular) and poliomyelitis (inactivated) vaccine
- GSK: Supply of Boostrix-IPV is currently limited
- Sanofi Pasteur: Repevax is available without restrictions
MMR vaccine
- MSD: MMR Vaxpro is currently available without restrictions
- GSK: Priorix is currently available
Meningitis ACWY vaccine
- GSK: Menveo is available
- Pfizer: Nimenrix is currently available
Yellow fever vaccine
- Sanofi Pasteur: Stamaril is available
Human papillomavirus vaccine (HPV)
- MSD: GARDASIL is available but will be discontinued in May 2021
- MSD: Gardasil 9 is currently available
- GSK: Cervarix has been discontinued
Cholera vaccine
- Valneva: Dukoral is available
Japanese encephalytis vaccine
- Valneva: Ixiaro is available
References
- ICNARC data on hospitalisations in those aged under 50 years (unpublished).
- BMJ. Features of 20,133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study.
- ISARIC data on hospitalisation in those aged under 50 years (unpublished).
- National flu and COVID-19 surveillance reports.
- OpenSAFELY analysis on the risk of hospitalisation in those aged under 50 (unpublished).
- Moore S and others Vaccination and non-pharmaceutical interventions: When Can the UK Relax About COVID-19?.
- University of Warwick modelling on options for phase 2 of the COVID-19 programme (unpublished).
- See Annex A for references.
- SAGE-EMG-Transmission Working Group paper – COVID-19 risk by occupation and workplace.
- COVID-19: review of disparities in risks and outcomes.
- Bhaskaran K, OpenSAFELY. Ethnicity and COVID-19 death in the early part of the COVID-19 second wave in England: an analysis of OpenSAFELY data from 1 September to 9 November 2020 (unpublished).
- COVID-19 vaccine and health inequalities: considerations for prioritisation and implementation.
- SPI-B consideration of priorities for phase 2 of the programme.
-
Recently made available. ↩
