MAVIS Newsletter edition 109
Published 25 January 2019
1. News
1.1 MAVIS is changing
What you need to know
This is our last MAVIS as a newsletter. From April the format will be an online MAVIS ‘Hub’ accessible via a quick link on our GOV.UK homepage.
What the ‘Hub’ will contain
The ‘Hub’ will contain regularly featured items as well as other useful links to frequently used areas of our website. The featured information will be refreshed each quarter, based on the current publication cycle and will include a ‘News Summary’ of items published over the previous quarter and grouped by subject.
Why we are doing this
We are doing this because the VMD’s 2018 Customer Satisfaction Survey results indicated that it was time for us to review the way we share information, to make it more accessible, relevant and searchable.
1.2 Non-Executive Director Appointment
David Catlow was successful in the recent exercise to appoint a non-executive Director to the VMD’s Management Board and Audit and Risk Assurance Committee. David took up his appointment with effect from 1 December 2018.
2. Licensing
2.1 Marketing Authorisations
Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
You can see lists on GOV.UK of:
2.2 Quarterly reporting against VMD Published Standards for 2018/19 licensing work
This report is published on a monthly basis under Our Statistics on GOV.UK.
2.3 Top ten imported veterinary medicines - Quarterly report 01 October to 31 December 2018
We provide a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Substance | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,532 |
| Isofluran CP 1ml/ml | Isoflurane | 533 |
| Aquamid | Polyacrylamide | 358 |
| Isofluran USP 250ml | Isoflurane | 323 |
| IsoFlo Vet 100% | Isoflurane | 293 |
| Spectrum Hyposensitisation Vaccine – Injectable Solution | Allergens | 223 |
| Vet-Goid | Allergens | 180 |
| Oncept (Canine Melanoma Vaccine) | Canine Melanoma DNA | 106 |
| Antepsin 1g Tablets Sucrafalto | Sucralfate | 101 |
| Greer Allergenic Extract Patient Prescription | Allergens | 97 |
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
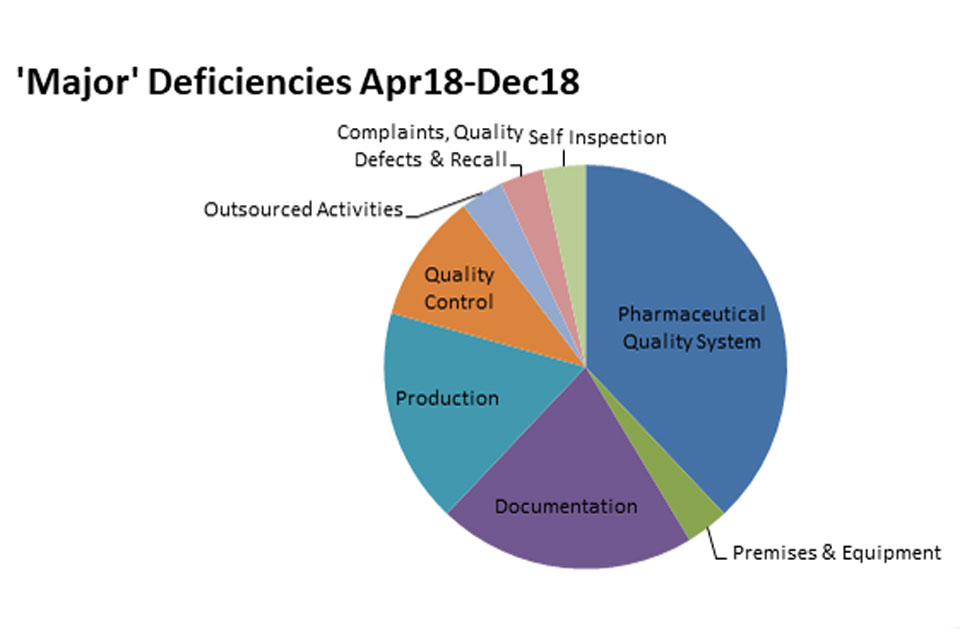
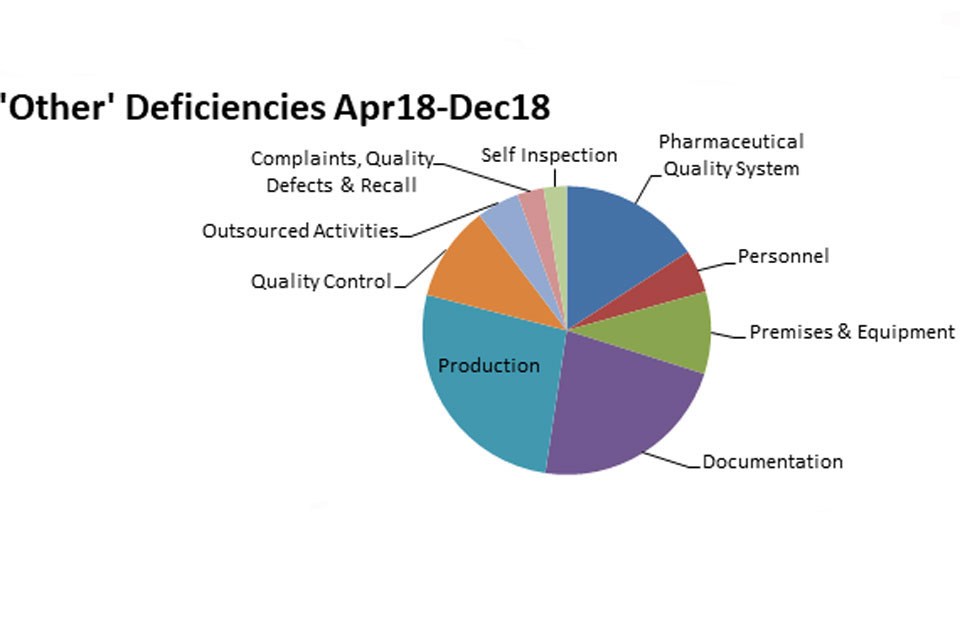
3.2 GMP inspection deficiency findings April 2018 to December 2018
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
‘Other’ Deficiencies Apr 18 - Dec18
GMP inspection major deficiency findings April 2018 to December 2018
‘Major’ Deficiencies Apr 18 - Dec18
GMP Inspection other deficiency findings April 2018 to December 2018
4. Pharmacovigilance reports
4.1 Quarterly report
During the period September 2018 to November 2018, the VMD received 1,959 animal suspected adverse event reports. Of the 3,005[footnote 1] products involved in these reports, 2,916 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,698 | Prescription Only Medicine Veterinarian (POM V) |
| 130 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 59 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 29 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 89 products were:
| Amount | Type |
|---|---|
| 39 | Authorised human medicines |
| 3 | Medicine used in a trial under an Animal Test Certificate |
| 7 | Veterinary products without any medicinal claim |
| 9 | Authorised medicines imported from other countries |
| 7 | Medicines sold under the exemption for small pet animals |
| 15 | Extemporaneous veterinary medicines |
| 9 | Incompletely identified products |
During this period 52 reports of human suspected adverse reactions were received. Of the 56[footnote 1] products involved in these reports; one was a pesticide, one was aan authorised medicine imported from another country and the other 54 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 42 | Prescription Only Medicine Veterinarian (POM V) |
| 6 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 3 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 3 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
One Environmental Incident report was received during this period involving an incompletely identified product.
5. Enforcement
We publish a list of prosecutions and notices involving illegal activity with veterinary medicines in the last year.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 27 November 2018 to discuss the recent trends in antibiotic resistance (AMR) of importance to people and animals. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined the recently published reports with contributions from the Veterinary Medicines Directorate, Public Health England, European Medicines Agency, University of Glasgow and Animal and Plant Health Agency. Summary minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
6.2 Sales Data and Antibiotic Resistance Surveillance Report
The 2017 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report has been published. The report is a collation of UK antibiotic sales data, antibiotic usage data from ten livestock sectors and antibiotic resistance data from the VMD’s surveillance programmes.
- In 2017, the sales of veterinary antibiotics for use in food-producing animals were 37 mg/kg which was a 40% decrease since the implementation of the UK AMR Strategy in 2013.
- HP-CIAs saw a drop in sales of 2.35 tonnes (52%) between 2013 and 2017.
- The levels of resistance in healthy pigs at slaughter have remained relatively stable between 2015 and 2017 for most antibiotics tested; however, a decline has started to be observed in E. coli coinciding with a reduction in antibiotic use in pigs.
Previous reports are also available.
6.3 UK AMR strategy High Level Steering Group meeting
The most recent HLSG was held on 16 October 2018. HLSG members discussed progress on the drafting of the next UK AMR Strategy and acknowledge the need to take into consideration the recommendations from the Health and Social Care Committee inquiry report. Attendees were supportive of the proposed 20-year vision and 5-year National Action Plan. The ministerial clearance process started early November. The meeting is chaired by the Chief Medical Officer and is represented by cross-government department leaders, including devolved administrations.
6.4 Communications
European Antibiotic Awareness Day (EAAD) and World Antibiotic Awareness Week (WAAW) took place between 12-18 November 2018 and VMD supported the campaigns by celebrating 10 years of EAAD and releasing infographical lists of ten AMR tips, facts, truths and educational resources via VMD’s twitter feed @vmdgovuk.
Professor Dame Sally Davies, UK Chief Medical Officer, supported the campaign: “Antibiotic resistance is a One Health issue that impacts upon humans, animals, food and the environment. We must all #BeAntibioticAware and play our part to #KeepAntibioticsWorking. Remember to #TrustYourDoctor and #TrustYourVet too”. VMD antibiotic awareness messaging was also supported by Christine Middlemiss; the UK’s Chief Veterinary Officer, Lord Gardiner; the Parliamentary under Secretary of State for Rural Affairs and Biosecurity and Professor Peter Borriello; Chief Executive of the VMD.
In November, VMD colleagues attended the National Pet Show and the London Vet Show to answer questions on veterinary medicines and demonstrate GOV.UK’s online services such as the product information database and the accredited internet retailers scheme (AIRS).
VMD colleagues were honoured and presented with an award from the British Poultry Council (BPC) in December 2018 at the BPC annual reception and awards. The award commends VMD for the work done to promote antibiotic stewardship across the sector.
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in September 2018. Summary minutes of the meetings held since October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
The VPC held its Open meeting on 28 September. A presentation given by one of its members, Professor Jason Weeks, is available.
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2018 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
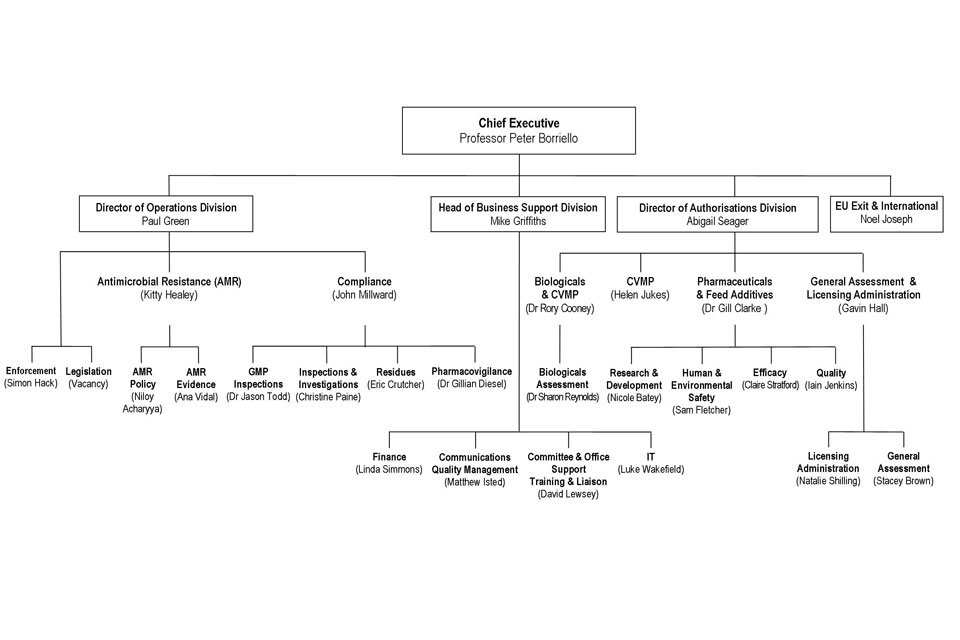
9. Organogram as at 01 January 2019
VMD Organogram as at January 2019
