MAVIS Hub Edition 120
Published 3 November 2021
1. Product news
Ketofen 10% Solution for Injection - Product defect recall alert
Lydaxx 100 mg/ml solution for injection for cattle, pigs and sheep - Product defect recall alert
2. Enforcement
Animal medicine seizure notice: Product stopped at Heathrow Airport August 2021
Animal medicine seizure notice: Parcel addressed to Paddington, London
Prosecution of Donna Kavanagh, Hertfordshire
Animal medicine seizure notice: Donna Kavanagh, K9 Fertility Clinic, Hertfordshire
Animal Medicine Improvement notice: Vale Wildlife Hospital & Rehabilitation Centre
[Animal medicine seizure notice: Parcels addressed to a freight company based in Slough, Berkshire] (https://www.gov.uk/government/news/animal-medicine-seizure-notice-parcels-addressed-to-a-freight-company-based-in-slough-berkshire)
Animal medicine seizure notice: Parcel addressed to premises in Tandragee, County Armagh
3. AMR (Antimicrobial Resistance)
3.1 Policy and Communications
Priorities for the remainder of 2021 are to continue to deliver the animal health-specific aspects goals outlined in the cross-government UK AMR National Action Plan (2019-2024). This includes the joint publication with the FAO, of a case study highlighting the success of the UK’s multisectoral voluntary approach to antibiotic stewardship, showcasing the behaviour change which has resulted in the reduction of antibiotic consumption in the UK’s livestock industry.
3.2 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met virtually on 22 September 2021 to discuss the recent trends in antimicrobial resistance (AMR) in bacteria of importance to animal and public health. The meeting included updates from the Animal and Plant Health Agency, Agri-Food Biosciences Institute and Scotland’s Rural College of Veterinary Services. Additionally, guest speaker Genever Morgan from the University of Liverpool provided a presentation on AMR in raw pet food. Minutes from previous meetings are available here.
4. Inspections
During the period from 01/07/21 to 30/09/21 the Distribution and Supply Chain Inspection Section carried out a total of 250 inspections of retail and feed business premises to assess their compliance with the Veterinary Medicines Regulations 2013. We are carrying out some on-site inspections, however, due to the ongoing pandemic we are still carrying out some remote assessments where we believe they are appropriate.
4.1 Retail Premises
We inspected 212 retail premises (veterinary practice premises and SQP retailers) and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 93 | 43.9% |
| 4 | 94 | 44.3% |
| 3 | 23 | 10.8% |
| 2 | 1 | 0.5% |
| 1 | 1 | 0.5% |
The most common deficiencies that required corrective action which were all observed in veterinary practice premises were:
- Controlled Drug (CD) Registers not maintained as required
- Broach dates exceeded or not recorded
- Cascade labels either missing or missing required information
The requirements for retailers are published on GOV.UK
4.2 Feed Business Operator premises
We inspected 38 Feed Business Operator (FeBO) premises and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 15 | 39.5% |
| 4 | 18 | 47.4% |
| 3 | 2 | 5.3% |
| 2 | 2 | 5.3% |
| 1 | 1 | 2.6% |
*(total is 100.1% due to rounding)
The most common deficiencies that required corrective action were:
- HACCP plan not documented/implemented/maintained as required
- Homogeneity testing issues
- Hygiene concerns
The requirements for Feed Business Operator (FeBO) premises are published on GOV.UK
5. Guidance Updates
Exemption from authorisation for medicines for small pet animals
Application and authorisation information hub explainer
Product Literature Standard (PLS) for veterinary medicines
Fees applied to animal medicine authorisation applications
Report a product defect: veterinary medicine
Timetables for national applications for MAs, ATCs and VHRs
Import a medicine for veterinary use into the UK
Veterinary medicine wholesale dealer’s authorisation (WDA)
Export drugs and medicines: special rules
Extension to submission slots requirement
Veterinary medicines unfettered access from Northern Ireland
Application fees for animal medicines
Manufacturing and distribution of veterinary medicines information hub explainer
Apply for batch release of an immunological veterinary medicine
Marketing Authorisations for Parallel Import of veterinary medicines
VMD export certificates: e-issuing service
Veterinary medicine wholesale dealer’s authorisation (WDA)
Controlled drugs: recording, using, storing and disposal
6. Research and statistics
VMD Published Standards 2021 to 2022: Monitoring performance
Top ten imported veterinary medicines - Quarterly report 1 July 2021 to 30 September 2021
Residues of veterinary medicines in food: 2021
7. stakeholder engagement
VMD delivers its first Wholesale Qualified Persons training event
Joint VMD and VPC open information day - 29 November 2021
Veterinary Products Committee is looking to appoint seven new members
8. Corporate News
Extramural Studies (EMS) Placements at the VMD July 2022
New chief executive appointed for VMD
VMD and FDA Announce Mutual Recognition Agreement
8.1 Organogram as at 31 October 2021
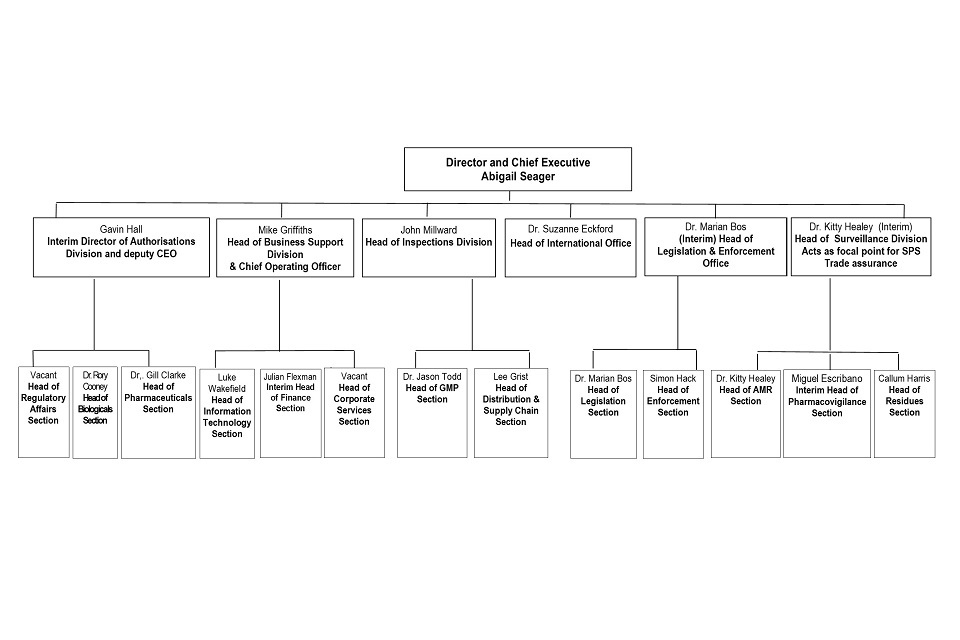
VMD Organogram
