MAVIS Hub Edition 118
Published 13 May 2021
1. Product news
Otodex Skin Cream - Product defect recall alert
Availability of Lactating Cow Intramammary Antibiotics
Known supply problems with animal medicines
2. Enforcement
Prosecution of Daniel Rendell, Cheshire
Animal medicine seizure notice: Product seized at the Port of Dover
Animal medicine seizure notice: Parcel addressed to Stoke-on-Trent, Staffordshire
Animal medicine seizure notice: Canine Fertility Centre, Jarrow, Durham
Animal medicine seizure notice: Border Force, Gatwick Airport
3. AMR (Antimicrobial Resistance)
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met virtually on 4 March 2021 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to animal and public health. The meeting included an update on AMR in the UK’s G7 presidency and discussions regarding the formation of a One Health Integrated Surveillance DARC sub-group. Minutes from previous meetings.
3.2 FAO Reference Centre
The VMD provided virtual training and consultancy on a range of regulatory aspects of veterinary medicines, including pharmacovigilance and authorisations; surveillance and policy around AMR; and on engagement with stakeholders to support the Fleming Fund’s fellows in Nigeria.
3.3 Communications
During this quarter, VMD ran a mini social media campaign in February to support Agriculture and Horticulture Development Board’s (AHDB) #ColostrumIsGold campaign. Tweets included tips on colostrum management to promote neonatal health and reduce the need for use of antibiotics, and links to AHDB’s resources.
VMD will also be supporting the Farm Vet Champions promotional campaign, led by Royal College of Veterinary Surgeons Knowledge. This project promotes the appointment of Farm Vet Champions within veterinary practices to unite and empower UK farm animal practitioners as they establish good antimicrobial stewardship in practices and on farms.
4. Inspections
During the period from 01/01/21 to 31/03/21 the Distribution and Supply Chain Inspection Section carried out a total of 216 inspections of retail and feed business premises to assess their compliance with the Veterinary Medicines Regulations 2013. Due to the current pandemic, the majority of these inspections were conducted as remote assessments.
4.1 a) Retail Premises
We inspected 167 retail premises (veterinary practice premises and SQP retailers) and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 68 | 40.7 |
| 4 | 85 | 50.9 |
| 3 | 11 | 6.6 |
| 2 | 2 | 1.2 |
| 1 | 1 | 0.6 |
The most common issues that required corrective action were:
- Controlled Drug (CD) Registers not maintained as required
- Broach dates not recorded or exceeded
- Insufficient information recorded at receipt/supply
The requirements for retailers are published on Gov.UK.
4.2 b) Feed Business Operator premises
We inspected 49 Feed Business Operator (FeBO) premises and the compliance ratings for these inspections were as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage |
|---|---|---|
| 5 | 20 | 40.8% |
| 4 | 26 | 53.1% |
| 3 | 3 6 | 1% |
| 2 | 0 | - |
| 1 | 0 | - |
The most common issues that required corrective action were:
- Supply against Medicated Feedingstuffs Prescriptions that were missing required information/invalid prescriptions
- HACCP plan not documented/implemented/maintained as required
- Feed labelling issues
The requirements for Feed Business Operator (FeBO) premises are published on Gov.UK.
5. Guidance Updates
Pharmacovigilance information hub explainer
Veterinary medicine wholesale dealer’s authorisation (WDA)
Manufacturing and distribution of veterinary medicines information hub explainer
Product Literature Standard (PLS) for veterinary medicines
Summary of Product Characteristics and product literature for veterinary medicines
Joint labelling for veterinary medicines for use in the UK and Ireland
Application and authorisation information hub explainer
Authorisations to manufacture veterinary medicines
Marketing authorisations for veterinary medicines
Apply for a Marketing Authorisation in the UK for a veterinary medicine or expiry
Controlled drugs: recording, using, storing and disposal
Veterinary medicine wholesale dealer’s authorisation (WDA)
Important Information for applicants of marketing authorisations: New Applications
6. Surveillance
Veterinary Medicines Pharmacovigilance Annual Review 2019: Summary
Residues of veterinary medicines in food: 2021
7. Statistics
VMD Published Standards 2020 to 2021: Monitoring performance Updated 9 April 2021
Top ten imported veterinary medicines - Quarterly report 1 January 2021 to 31 March 2021
8. Corporate News
Job Advert for a Pharmaceutical Assessor
Job Advert for 2 Biologicals Assessors
Veterinary Medicines Directorate: privacy notices
9. Organogram as at 30 April 2021
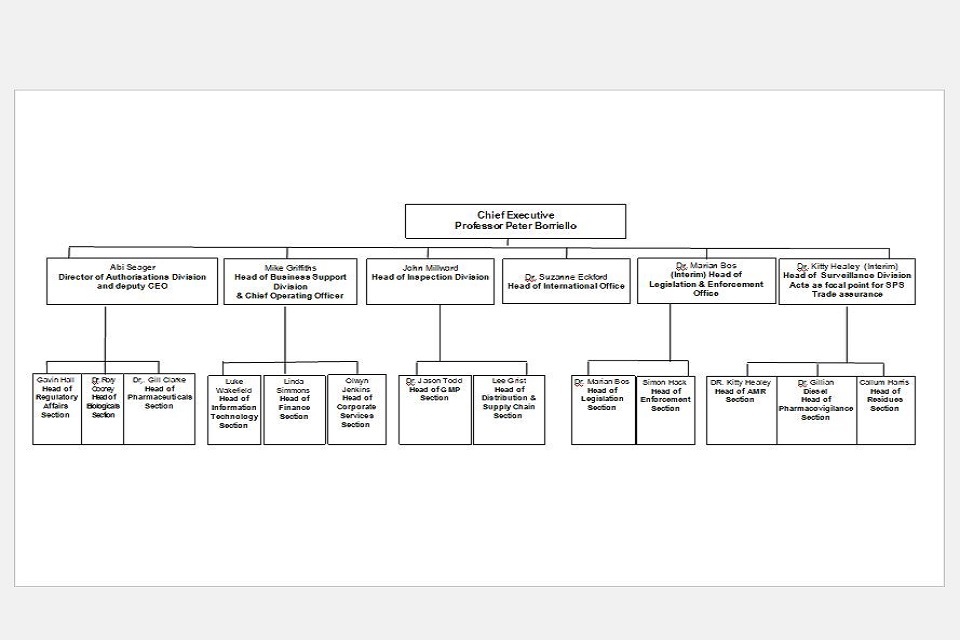
VMD Organogram
