MAVIS Hub Edition 113
Published 6 February 2020
1. Product news
Veterinary medicines will continue to be available after Brexit: Joint statement from VMD and NOAH
Suspension of Veterinary Medicines containing the excipient Diethanolamine (DEA)
Advice for users of live Porcine Reproductive and Respiratory Syndrome (PRRS) vaccines
Synulox Lactating Cow Intramammary Suspension - Product defect recall alert
Product defect recall alert Clavubactin 50/12.5mg, 250/62.5mg, 500/125mg
2. Enforcement
Animal medicine seizure notice: Meadowbrook Equine Clinic
Animal medicine seizure notice: Pawfection Pets
Animal medicines seizure notice: NPD Ventures Ltd (T/A World of Pets)
Animal medicine seizure notice: SF Equine Vets Ltd
Animal medicine improvement notice: Alex the Vet Updated: 3 December 2019
Animal medicine improvement notice: Johnston Agri Supplies
Animal medicine improvement notice: St Paul’s Veterinary Clinic
Animal medicine seizure notice: Guasco & Associates
3. AMR (Antimicrobial Resistance)
Veterinary Antimicrobial Resistance and Sales Surveillance 2016
New VARSS report confirms that sales of veterinary antibiotics have halved over the past four years.
Veterinary Antimicrobial Resistance and Sales Surveillance 2017
Veterinary Antimicrobial Resistance and Sales Surveillance 2018
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 26 November 2019 to discuss the recent trends in antibiotic resistance (AMR) of importance to people and animals. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined recently published reports with contributions from Public Health England, National Health Services Scotland and the Veterinary Medicines Directorate. Summary minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
3.2 Policy
In December, the VMD and Food Standards Agency represented the UK at the Codex Alimentarius Task Force on AMR (TFAMR) in Pyongchang, Republic of Korea. TFAMR’s mission is to achieve international consensus on a revised Code of Practice to Minimise and Contain Foodborne AMR and develop new Guidelines for Integrated Surveillance. Agreement was reached on the Code of Practice (progressing it from a draft proposal to a draft) with the focus being on the Guidelines in the upcoming year.
3.3 FAO Reference Centre
In 2019 the Reference Centre completed scoping and training visits to Ghana, Bangladesh, Nigeria, Laos, Philippines, and Ethiopia (12 visits in total). Support in establishing quality management systems was identified as a common requirement.
The training visits have delivered coaching in subjects including: establishing AMR and AMU surveillance systems; collection, analysis, and interpretation of AMU data; antimicrobial residue testing; determinative bacteriology from livestock and aquaculture samples; antimicrobial susceptibility testing (AST); and establishing policy frameworks to regulate antimicrobials and other veterinary medicines. In the UK the Reference Centre has welcomed and hosted visitors from several countries, as a host institute for the Fleming Fund Fellowship program, it hosted two Nigerian animal health fellows in 2019. Over 3-4 weeks the fellows obtained coaching in a wide variety of areas, including microbiology techniques and laboratory quality management systems. This learning is now being applied at the fellows’ home laboratories.
2020 hopes to be an exciting year for the FAO Reference Centre (UK) as there is an increased demand for its expertise in AMR, AMU and Residues surveillance. We continue to explore further opportunities with partner countries in Africa and Asia.
4. Inspections
During the period from 01/09/19 to 30/11/19 the Inspections and Investigations Team carried out a total of 238 inspections of retail and feed business premises.
4.1 Retail Premises
There were 199 retail premise visits (vet practice premises and SQP retailers). The compliance ratings for these inspections are as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage | ||
|---|---|---|---|---|
| 5 | 71 | 35.7% | ||
| 4 | 90 | 45.2% | ||
| 3 | 22 | 11.1% | ||
| 2 | 9 | 4.5% | ||
| 1 | 5 | 2.5% | ||
| NICO (no inspection carried out) | 2 | 1.0% |
The most common issues that required corrective action were:
- Insufficient temperature records for ambient and/or cold chain products
- Broach dates not recorded or exceeded
- Controlled Drug (CD) Registers not maintained as required
The requirements for retailers are published on Gov.UK.
4.2 Feed Business Operator premises
There were 39 Feed Business Operator (FeBO) premise inspection visits. The compliance ratings for these inspection visits are as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage* | ||
|---|---|---|---|---|
| 5 | 15 | 38.5% | ||
| 4 | 18 | 46.2% | ||
| 3 | 2 | 5.1% | ||
| 2 | 0 | 0% | ||
| 1 | 3 | 7.7% | ||
| NICO (no inspection carried out) | 1 | 2.6% |
*discrepancy of 0.1% in total due to rounding
The most common issues that required corrective action were:
- HACCP plan not documented/implemented/maintained as required
- Medicated feedingstuffs (MFS) Prescriptions inadequately recorded/managed
- Cleanliness and/or cleaning records not of the required standard
The requirements for animal feed manufacturers are published on GOV.UK.
5. Guidance updates
Launch of national application forms for a marketing authorisation
Guidance on applying for an authorisation to manufacture veterinary medicines
Apply to change a veterinary marketing authorisation or homeopathic remedy
Veterinary medicine wholesale dealer’s authorisation (WDA)
Veterinary medicine wholesale dealer’s authorisation (WDA)
Authorisations to manufacture veterinary medicines
Report a product defect: veterinary medicine
Veterinary Medicines Regulations
6. Research and statistics
VMD Published Standards 2019 to 2020: Monitoring performance
Top ten imported veterinary medicines - Quarterly report 1 October 2019 to 31 December 2019
VMD Published Standards 2019 to 2020: Monitoring performance
Residues of veterinary medicines in food: 2019
7. Stakeholder engagement
National Office of Animal Health: Animal Health – are you ready for Brexit?
VMD email address: the old gsi address will not work from 1 April 2020
New members appointed to serve on the Membership of the Veterinary Products Committee
8. Corporate news
Re-certification to ISO 9001 and ISO 27001 standards following audit.
VMD FOI requests received between 1 January and 30 June 2019
Veterinary Medicines Directorate – our governance
9. Organogram as at 31 January
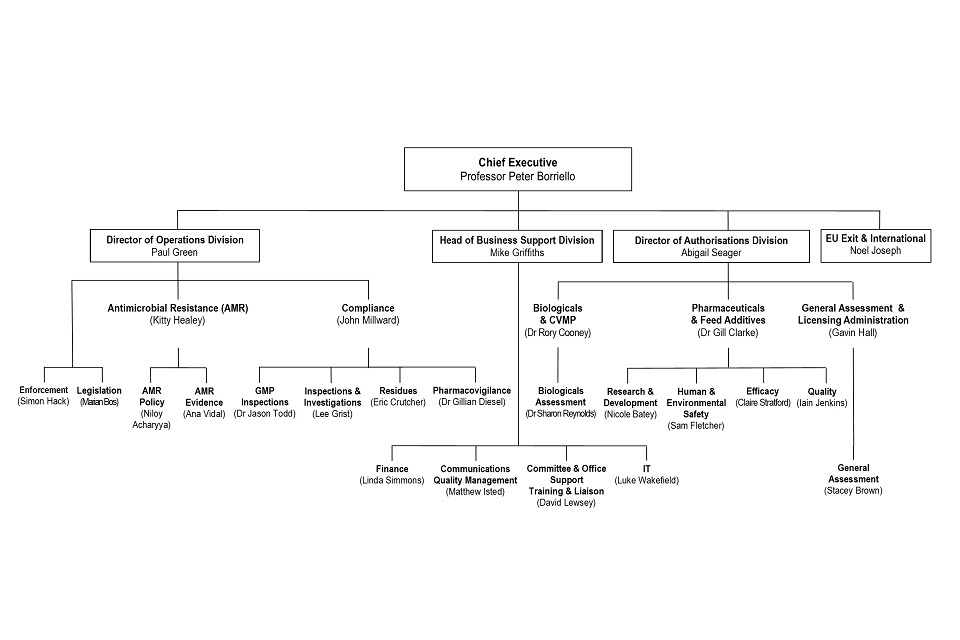
VMD Organogram as at 31 January 2020
