Cervical screening: guidance for call and recall administration best practice
Updated 27 September 2024
1. Introduction
1.1 Background
This document is a comprehensive revision of the NHS Cervical Screening Programme (NHS CSP) Good Practice Guide No.18, ‘Cervical screening call and recall: a guide to administrative good practice’ and replaces the version dated 2017. It must be read in conjunction with the NHS cervical screening programme service specification.
All documents relating to the management of cervical screening in England are on the GOV.UK website.
1.2 Screening target population
Cervical screening is for women and people with a cervix. The programme sends screening invitations to people with a cervix who are registered as ‘female’ or ‘indeterminate’ at the following ages and intervals.
Age 24.5: first invitation (invitations to first screen are issued at 24.5 years).
Ages 25 to 49: 3-yearly screening.
Ages 50 to 64: 5-yearly screening.
Ages 65 plus: screening of those who have not been screened since age 50, or those who have not yet met the criteria to be ceased from the programme.
1.3 Target audience for this document
This document is intended for those involved in the commissioning, management, governance, delivery and quality assurance of cervical screening call and recall services. This includes:
- call and recall service providers including managers and staff
- local screening and immunisation teams (SITs), including screening and immunisation leads (SILs)
- NHS primary care commissioners
- NHS public health commissioners
- Public Health England (PHE) Screening Quality Assurance Service (SQAS) teams
- software teams developing IT systems integrating call and recall
2. Legal basis for cervical screening
The Secretary of State for Health and Social Care (SSFH) has a responsibility to protect the health of the public by providing population screening programmes. This responsibility is defined under Section 2 of the National Health Service Act 2006 as amended by Part 1 Section 11 of the Health and Social Care Act 2012. The SSFH delegates the responsibility for the coordination and oversight of the programmes to PHE through the annual remit letter. The SSFH also delegates the responsibility to commission the population screening services to NHS England through Section 7 of the NHS Act 2006 using a set of standard service specifications. Service Specification 25 covers cervical screening.
3. Programme governance
3.1 Public Health England, NHS England and NHS Improvement responsibilities
The Immunisation and Screening National Delivery Framework and Local Operating Model defines the governance structures for screening programmes in England. This includes the roles and responsibilities of the various national and local bodies involved in the commissioning, delivery and oversight of local programmes. A diagram showing high level programme governance is available. Under these arrangements, PHE holds responsibility for the national co-ordination and oversight of the screening programmes, and NHS England and NHS Improvement commission and performance manage operational services. NHS England and NHS Improvement will have effective governance mechanisms in place to monitor operational delivery.
3.2 Provider responsibilities
Providers of all operational aspects of call and recall should have a comprehensive quality management system (QMS) in place including standard operating procedures and detailed work instructions that are maintained in line with national guidance. They must contain clear instructions regarding the operational processes in place to enable the safe delivery of the service in line with the agreed standards as set out within the contract with NHS England and NHS Improvement. The QMS should include a programme of audit and non-conformance monitoring.
3.3 Communication and engagement
The call and recall service is a main component of the patient pathway. Local programme boards should ensure all parties are aware of both national and local developments. Attendance at these programme boards is a requirement of the service. A further requirement is linking with education providers for sample taker training. The call and recall service should provide resources such as presentation slides or staff to deliver call and recall aspects of sample-taker training and engage with training providers to deliver this training as required.
3.4 Managing screening incidents
All providers contributing to a screening pathway have joint accountability to ensure safe and coherent screening for the population screened in accordance with national service specifications.
Each provider is accountable for the safe and coherent delivery of their part of the screening pathway and has joint accountability when working with another provider. Both the NHS England Serious Incident Framework and PHE’s guidance on managing safety incidents in screening programmes apply to the cervical screening programme. Providers of services have a responsibility to operate in accordance with this guidance. Provider incident policies should reference both sets of guidance.
4. Information governance principles
4.1 Records management and retention of records
Records management must comply with the latest version of the Records management code of practice for health and social care published by NHS Digital. This sets out responsibilities and high-level document retention schedules.
The electronic records in the call and recall IT systems constitute the primary or master electronic record for the NHS CSP. They are fundamental to the efficient operation of the service, to the assurance of quality and safety, and for the longer-term evaluation and development of the programme. NHS CSP policy is that these records are retained intact in perpetuity.
This does not rule out a separation of data for operational use from that retained for audit, quality assurance and evaluation purposes.
Where the NHS CSP has requirements to retain specific types of document, these are set out in the record retention schedule accompanying this guidance. The schedule includes the business reasons for the period of retention.
4.2 Processing data for call and recall (Section 251)
The NHS CSP processes demographic data from GP registration IT systems. This data underpins the operational work of the programme including:
- initial invitations for screening
- recall invitations at set periods
- sending results
- invitations for follow up investigations where necessary
- quality assuring the safety and effectiveness of the programme
- long-term evaluation of the safety and effectiveness of the programme
Contracts must be in place and agreed between organisations running call and recall services and those that maintain national registration functions. Regular operational review meetings must take place to ensure adherence to contractual agreements.
Screening programmes use data held in GP records to identify people who are eligible for screening. As with all medical records, this information is held in confidence by the GP services. For the call and recall service to access this data the duty of confidentiality must be set aside. This is permitted by using a specific law commonly referred to as Section 251. This law allows the health service to process information without the express consent of the patient, to deliver appropriate medical services.
Section 251 (S251) of the National Health Service Act 2006 allows the SSFH to permit the processing of personal data without consent where there is an overriding public interest to do so, and where gaining explicit consent is not practical.
The S251 approval process is overseen by the Health Research Authority’s (HRA) Confidentiality Advisory Group (CAG) which carries out an annual review of the agreement to ensure it is being applied fairly and lawfully.
4.3 Maintenance of the Section 251 agreement
To maintain S251 approval, PHE must be able to demonstrate to the CAG that appropriate information governance safeguards are in place for all organisations that provide cancer screening services. Many of these safeguards are standard controls mandated by the NHS and enforced through the commissioning process and standard contracts for providers.
PHE complies with the CAG’s required controls through its adherence to the Data Security and Protection Toolkit (DSPT) standards. Organisations providing elements of screening programmes must also adhere to the DSPT standards to ensure they are practising good data security and that personal information is handled correctly.
All organisations that access NHS patient data and systems must use the DSPT to ensure they are practising good data security and that personal information is handled correctly. This is achieved in screening by ensuring that all staff who access screening data:
- have documented information governance training
- have access to established policies to keep data secure
- can identify and report breaches when they occur
5. Informed choice and consent
5.1 Approach to consent
The cervical screening programme aims to maximise coverage and uptake among the eligible population. Individuals are supported to make an informed choice about whether to participate. Comprehensive information material is issued with (or signposted in) every screening invitation and for any follow-up procedure.
Individuals who choose to attend for screening are considered to have provided consent to be screened and to have consented to the data processing necessary to provide a safe and effective service. Consent to receive further screening invitations is assumed unless an individual makes an informed choice not to participate in the screening programme and tells the programme through their GP practice. The call and recall service must include the national information leaflet for the programme with all initial invitations to participate. Reminder letters include a link to the online version of the leaflet.
Colposcopy units must gain additional consent before they perform any procedure. This process is covered in the latest version of the colposcopy and programme management guidelines.
An information leaflet is provided to all people who are referred to colposcopy.
5.2 Opting out of screening
Most individuals who choose not to participate in the programme will do so by not making an appointment to have a sample taken rather than by making a request to be ceased. Where individuals do not respond to a screening invitation, they are designated as ‘non-responders’ after 32 weeks and may receive additional reminder letters from their GP practice. Individuals who remain eligible for screening are recalled at intervals according to current protocols.
An individual can make an informed choice to be permanently ceased from call and recall. The request for permanent withdrawal from cervical screening should be submitted in writing where possible. This is to ensure that there is no misunderstanding and that they are not ceased from call and recall in error. Further details on the ceasing procedure are available in Cervical screening: removing women from routine invitations.
5.3 Objecting to data processing
People have a right to request that their data is no longer processed in the screening programme, however not all data can be removed (see section 17 of the General Data Protection Regulations). Processing personal information is necessary to deliver a safe and effective screening service and is required even if a person has opted out of screening. Historical data held by NHS organisations may need to be retained (for example in case there is an incident and an affected individual needs to be told). Requests to cease data processing must be sent to PCSE Patient Registrations Department to implement. Once received, the relevant details are notified to the call and recall service to enable the screening history to be deleted.
6. Eligibility and inclusion criteria
6.1 Eligibility for NHS care
Individuals must be eligible for NHS care to also be eligible for cervical screening. Further information can be found on the NHS.UK website.
6.2 Residency
To be eligible for screening under the English programme, individuals must have their primary residence in England. This is consistent with policy in Wales and Scotland which is to screen by resident population (often determined by postcode) along the borders. For practical purposes, the address used for GP registration is used as the primary address. However, for individuals not currently registered with a GP practice, the last available address must be used unless it is known that they have moved outside England. For more information about people moving in and out of the English programme, see section 15.
6.3 Age
The NHS CSP sends the first invitation for cervical screening when individuals reach the age of 24.5. Individuals are recalled every 3 years until they turn 50, when the recall interval changes to every 5 years. Automatic recall stops when the next test due date (NTDD) is on or after their 65th birthday.
6.4 Clinical eligibility and ceasing
Ceasing guidance describes the clinical circumstances under which individuals may not be eligible for screening. Such individuals should be ceased from screening.
All ceasing notifications received by the call and recall service should be processed and have written confirmation sent to the GP and the individual concerned within 10 working days of receipt. People ceased due to radiotherapy do not receive a letter of confirmation.
6.5 Registration with a GP
Individuals are invited by the call and recall service to take part in the programme using demographic data on GP practice registration. The call and recall service uses this data to manage their progress through the screening pathway.
6.6 Screening samples taken in other NHS settings
Individuals do not have to attend their GP practice to have their sample taken. It may be taken in other settings (such as community and sexual health (CaSH) clinics). When this happens the sample taker must make sure they:
- confirm the individual’s eligibility for screening in line with national sample acceptance guidance
- record accurate information about the individual’s identity
- record accurate contact information (for provision of results)
This will ensure that the individual can be followed up if necessary.
Individuals screened through this route must be informed by the sample taker that their contact details, including registered address, will be kept on record by the call and recall services and used to contact them for future screening invitations as well as to provide test results. The programme does not support anonymous screening.
6.7 Transgender and non-binary individuals
Eligibility
Every person who has a cervix and is within the screening age range is eligible for NHS cervical screening regardless of their gender identity. However, current cervical screening IT systems do not have the facility to include individuals registered with the NHS as ‘male’, and current registration systems are unable to record the gender category of ‘non-binary’. For eligible people not registered as ‘female’ or ‘indeterminate’, screening should be offered by the person’s GP practice or, where appropriate, a gender clinic healthcare team.
Ineligible people
People registered as female (or indeterminate) but who do not have a cervix should be ceased from screening call and recall as soon as possible, stating the reason ‘absence of cervix’.
Screening records
When a registration record containing cervical screening history is closed due to an individual’s gender changing from female to male, manual action is required to ensure that their cervical screening records are not lost.
The registration department at PCSE must notify the call and recall service of the change so that a copy of the person’s screening history can be produced. The call and recall service must forward this information either by secure email, digital transfer, registered post or courier service, to the patient’s GP practice. If the preferred method is via post, then a risk-assessed standard operating procedure (SOP) should be in place covering this process. All screening records must be in a sealed and marked envelope, in line with the Guidance for the Classification Marking of NHS Information.
If an individual asks their GP to delete their cervical screening history, GPs should review relevant guidance from the General Medical Council (GMC) or NHS England and NHS Improvement (NHSE/I) as the matter goes beyond the screening programme. GPs may wish to seek further advice from their medical defence organisations regarding their liability. See section 22 of the Gender Recognition Act 2004 for more details.
Trans men
A trans man still registered as a female (or indeterminate) who has a cervix will automatically be included in the screening programme. He will be invited for screening at appropriate intervals unless he chooses to opt out of the programme in accordance with national guidance.
A trans man registered as a male who has a cervix cannot be invited for screening by the national programme. Screening invitations should be made either by his GP practice or the healthcare team managing his gender reassignment. Alternatively he can request screening every 3 or 5 years (depending on his age).
All GP practice staff need to understand the screening eligibility criteria described in this document and ensure that they offer appointments to eligible trans men and treat all trans individuals with respect and sensitivity.
As the screening programme cannot automatically include people registered as male, the GP practice must:
- communicate with their screening laboratory to ensure that samples are processed appropriately and that results are returned to the practice rather than the call and recall service
- make arrangements with the individual concerned regarding providing test results and make referrals for colposcopy where required
- ensure that local failsafe systems include all individuals who require further investigations, treatment or follow-up, regardless of gender
PHE has produced a blog on reducing cervical screening inequalities for trans people which may be helpful.
In all cases where a trans man is no longer eligible for cervical screening, the GP practice must ensure that any outstanding treatment or follow up is continued in accordance with national colposcopy guidance.
Trans women
A trans woman is ineligible for screening as she has no cervix. The GP practice should ensure that the individual is ceased from the screening programme for the correct reason. This can be done as soon as her registration gender is changed (or a new registration is created under the new gender), or when the woman appears on a screening prior notification list (PNL).
Any individual who is ceased due to absence of cervix will automatically receive a letter from the screening programme to explain that they will not receive any further invitation letters. This includes trans women who have not previously received screening invitations.
Non-binary individuals
Current registration systems are unable to record the gender category of ‘non-binary’. For this reason, the GP practice must invite non-binary individuals who are eligible for cervical screening.
An information leaflet on screening for trans and non-binary people is available.
6.8 Health and justice residents
Offenders in the English residential prison estate are eligible for screening by NHS CSP if they meet the criteria described elsewhere within this guidance.
There are practical difficulties with identifying and contacting offenders as there is currently no index of offenders and institutions available to the screening programmes. However, many prisons have procedures for screening their eligible populations.
When women offenders are received into prison their screening history should be checked. Where possible they should be offered screening in line with their NTDD. The relevant prison health service should be recorded on the HMR 101 sample request form and used as a correspondence address for results and follow-up for the screening episode.
6.9 Defence Medical Service (DMS)
Individuals registered under the Defence Medical Service (DMS) are eligible for screening by the NHS CSP if they meet the criteria described elsewhere within this guidance. The management of call and recall for this population requires additional security arrangements that must be agreed with DMS and NHS England. The protocol agreed for the routing of call and recall information to the DMS population is that the DMS primary care practice is used as the point of contact. A separate user guide exists to support these processes.
6.10 Private tests, non-NHS and self sampling
Private and non-NHS tests
Non-NHS tests are those taken outside the NHS screening programme. They include samples from private healthcare services, workplace schemes, overseas and charitable organisations as well as purchased HPV home testing kits.
The results of non-NHS tests will not necessarily use standard NHS reporting categories for HPV infection or cytology classification, or follow standard NHS screening protocols. This means that non-standard results or non-protocol code combinations may arise which cannot be accommodated by the call and recall system.
The results of a non-NHS screening test must not be recorded in an individual’s NHS screening record. Historic non-NHS test results may appear in a screening record if they were provided to the call and recall service and recorded prior to 2020.
Individuals who have a non-NHS test remain eligible for NHS screening at the appropriate programme screening intervals. Where an individual responds to an NHS screening invitation, the sample taker should ensure that there has been an interval of at least 3 months since any previous cytology sample was taken.
If an individual has an abnormal non-NHS test that necessitates a follow-up test or colposcopy referral, this may be offered privately or by the NHS following a primary care consultation and referral.
Individuals requiring surveillance or follow-up tests after non-NHS screening or treatment who are referred back to the NHS remain eligible for NHS recalls in accordance with the NHS management protocol. NHS screening records may therefore appear incomplete if the screening laboratory or colposcopy service advises one or more non-routine recalls following unrecorded non-NHS activity.
Self sampling
Self sampling involves test kits for personal use which allow individuals to submit a sample for HPV testing only.
Self sampling may be offered in some areas as part of an approved trial, or as a pilot or formal project within the NHS screening programme. The results of self-sampled tests which were taken under or by arrangement with NHS services must be recorded in a person’s screening record using the appropriate codes for HPV infection and next action provided by the screening laboratory, in accordance with the relevant screening protocol. The call and recall IT system supports the code combinations required for each approved scenario.
Private screening services, including self-purchased home-testing kits, are not part of the NHS screening programme and results from these must not be recorded in an individual’s NHS screening record.
Self-referral into the programme
Individuals not registered with an NHS GP practice do not receive an invitation for cervical screening automatically. They can choose to self-refer for screening at the routine intervals if they satisfy the age and residency requirements. Self-referrals will usually attend a CaSH clinic. Sample takers must ensure that accurate contact information is recorded and that age, residency and any other requirements are validated.
Individuals who have made an informed choice to be ceased from the programme remain eligible for screening. They may self-refer at any time after their last test would have become due. If they do choose to self-refer, their name is returned to the screening list. They are then sent invitations and reminders in line with current protocols.
Individuals aged 65 and over who have not had an adequate screening test reported since age 50 can be screened on request.
Opportunistic sampling
Opportunistic sampling is defined as any sample taken from an individual after they have reached non-responder status following a screening invitation (see information on non-responders below). All opportunistic screening is for individuals whose attendance is overdue.
Opportunistic screening is not appropriate for individuals who present with symptoms. For such individuals, appropriate gynaecological referral pathways should be followed.
Supporting information
PHE provides invitation and result letter texts for use by the English call and recall service (the Cervical Screening Administration Service (CSAS)). It also provides information leaflets to support informed choice.
The invitation leaflet for cervical screening is available on GOV.UK in 10 languages and also in HTML format. HTML leaflets can be read online or printed in large print. They can also be used with screen-reader technology for an audio version.
PHE can provide other accessible forms of information leaflets on request (including braille and audio disks) if an individual is unable to access existing resources.
Further information, an online video and support regarding cervical screening is available on NHS.UK.
7. Call and recall process
7.1 Summary
The call and recall process uses demographic information from NHS systems (currently NHAIS). When individuals reach the age of 24.5, their details are automatically included on the call and recall system and an initial next test due date (NTDD) is calculated. This means they can be invited for screening. The NTDD is a target date for a person to have a cervical sample taken.
Call and recall runs a process to identify those individuals who have a NTDD within the following 10 weeks. A prior notification list (PNL) of these individuals is sent to GP practices (see section 8). This allows the GP practices to manage any individuals who do not want or need to be screened. For example, individuals who need their screening deferred due to pregnancy.
After 4 weeks, irrespective of whether or not all PNLs have been reviewed, invitation letters are sent to the identified individuals minus those removed from the current round by their GP (see section 8).
Individuals who accept their invitation arrange an appointment to have a sample taken, usually at their GP practice, but sometimes via other routes such as a sexual health clinic. The GP practice sends the sample to a cervical screening laboratory (see section 10) for analysis and reporting (see section 11).
The result of the laboratory analysis is returned to the call and recall service. Call and recall then notifies the individual of the test result by letter (see section 12), and the GP also receives a copy. Sending this result notification also triggers the calculation of the NTDD.
Colposcopy services arrange appointments for individuals with an abnormal cervical screening result.
Any invited individual who has not received a result letter 32 weeks after their initial invitation is classified as a non-responder and a new NTDD is set.
The diagram below illustrates the timeline of the call and recall pathway.
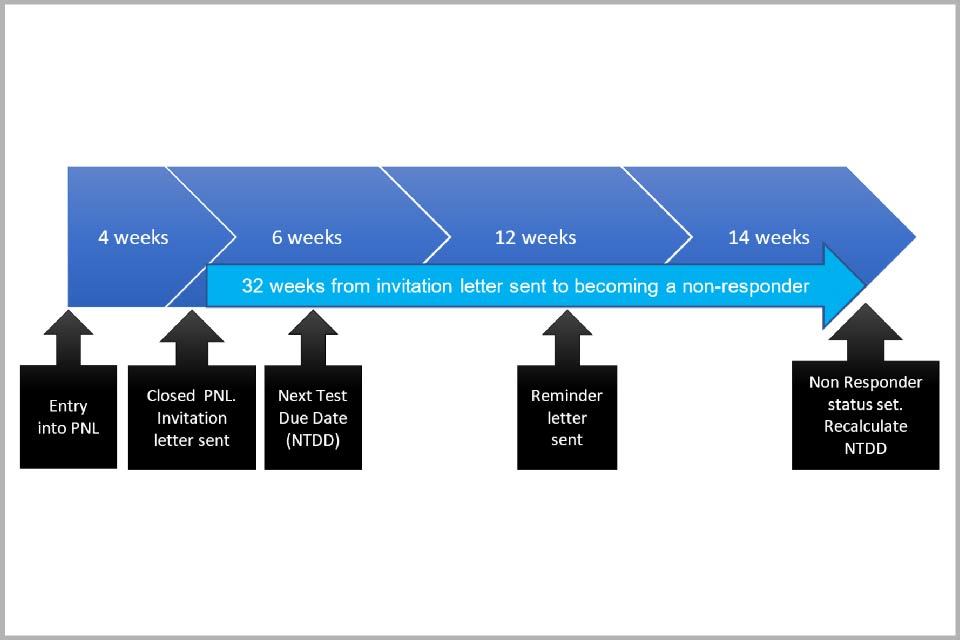
Diagram showing the 32 week pathway from an invitation letter being sent to a person becoming a non-responder
A more detailed summary of the call and recall timetable for a standard screening round accompanies this guidance.
7.2 Calculating next test due date (NTDD)
Individuals are set a NTDD in preparation for their initial screening invitation. Subsequent NTDDs are calculated from the result and action code of their NHS screening tests.
7.3 Routine recall intervals
Routine recall intervals are determined by PHE. Current policy is outlined below.
Age under 24.5: no invitation.
Age 24.5: eligible for screening, and first invitation issued (to ensure screening test can be completed by their 25th birthday).
Age 25 to 49: recall every 3 years (with invitations issued 34.5 months after previous test).
Age 50 to 64: recall every 5 years (with invitations issued 58.5 months after previous test).
Age 65-plus: invitation as required for individuals who have had recent abnormal tests (individuals 65 and over that are undergoing further investigation or treatment will remain in the programme until their treatment is complete).
7.4 Colposcopy referral and discharge
The process for referring individuals into colposcopy and discharging them back to call and recall is covered in the Colposcopy and programme management guidelines.
Colposcopy departments must use the colposcopy discharge notification template form (or an equivalent electronic output) to notify the call and recall service of individuals discharged, and to specify their NTDD. The call and recall service processes the colposcopy discharge data within 5 working days of receipt. During the processing of the data the service ensures that the colposcopy discharge lists are either in sequential order or concurrent date order. If any data is suspected of being missed, the service contacts the colposcopy service. Any colposcopy discharge data that cannot be updated is notified to the colposcopy service so that any further action needed can be taken.
8. Prior notification lists
8.1 Overview
GP practices have a responsibility to provide assurance that the right individuals are being offered screening. This is managed through the prior notification list (PNL) process. The PNL is a list of individuals from the GP practice who are due to be called or recalled for screening. This provides an opportunity for practice staff to consider deferral or ceasing of individuals if appropriate.
Call and recall must send all PNL lists to GP practices 70 days prior to the individuals’ NTDDs. The programme recommends these are sent electronically to the practices. When the PNLs are returned, the call and recall service should process any requests for update within 5 working days.
The GP practice must specify how long any deferral is for. A period up to 18 months is acceptable. GP practices are responsible for making sure that requests to defer are appropriate. When a deferral ends, the individual is returned to the PNL. It is acceptable for subsequent deferrals to be created, but any multiple deferrals must be identified and reported to the SIT to be audited, and for them to take action by contacting GP practices directly. Audits should not include patient identifiable data. Repeated deferrals leave individuals at risk of undetected cervical cancer.
The programme recommends that call and recall undertake a regular audit to check that practices are returning their PNL list with appropriate updates. Call and recall should inform the SIT of any practices not completing and returning their PNL. Call and recall should notify the practice, and the SIT should request an action plan from the practice to resolve the issue.
8.2 Deferring screening
GPs may defer an individual’s screening invitation for a limited number of reasons through the PNL process. Any deferral must specify a reason. The identified reason is used to recalculate the NTDD based on the length of the deferral. Section 5 of the guidance Cervical screening: removing women from routine invitations describes the valid reasons for deferral in detail.
9. Invitations, reminders and non responders
9.1 Invitation and reminder letters
All eligible individuals must receive a written invitation to attend for screening together with the national information leaflet (or a link to the information online) to enable them to make an informed choice about attending. Call and recall issues screening invitations up to 6 weeks before a person’s NTDD.
A delay of several months may occur between a person receiving their invitation and booking their screening test. Sending invitations well before a NTDD reduces the chance that someone will go beyond their NTDD before being screened.
Where call and recall has not received a test result from the relevant cytology lab within 126 days (18 weeks) of an invitation letter being created, the individual becomes ‘overdue’ for screening and a reminder letter is created and sent. Reminder letters must not include the national information leaflet; they include a link in the letter text to the leaflet online. All invitation and reminder letters must use the national letter texts provided by PHE.
Call and recall services must have appropriate audit trails in place to ensure that letters are produced and supplied to the contracted print provider, and that the print provider has dispatched the letters within agreed timescales.
9.2 Failsafe process for newly registered individuals
If the individual’s screening history has not been received by call and recall within 21 days of registering in England, a ‘failsafe’ NTDD is set. This is calculated within the current systems to be 91 days (13 weeks) from the date of registration.
If the screening history is received after this failsafe NTDD is set and before invitation letters are created, the correct NTDD should be calculated and set in the system and the GP practice notified.
If the individual’s screening history is received after the invitation letter has been sent, any sample taken should be processed. The NTDD calculated from their full screening history must be recorded in their screening history record.
9.3 Local content in letters
National invitation letter templates allow up to 5 lines of additional text to be added to provide locally relevant information. Each line can be up to 76 characters per line including spaces and punctuation. Additional text must be clear, factual and of direct relevance to the screening programme. It should also match the existing overall letter style.
Standard letters can include an additional paragraph of free text specific to a GP practice. Any GP requiring practice-level text should request this from their local SIT. The SIT will verify the content to ensure that the local text does not contradict the overall message, and supply the text to call and recall for inclusion into the letter text for the practice.
Call and recall, in conjunction with local SITs, should undertake an annual audit of localised text to ensure it still meets the needs of the relevant service.
9.4 Returned mail
Letters that are returned undelivered are notified to call and recall. CSAS raises a request with PCSE to check the address with the individual’s GP practice. This process must not stop further letters being sent to the individual’s last known address. Following investigation by PCSE, either a change of address will be notified, or the original address will be confirmed as still valid.
Where a change of address is notified, call and recall must recreate the most recent correspondence and send it to the new address. Where the original address is validated, the most recent correspondence must be sent to this address again. If an address is not validated, the individual is removed from the GPs practice list after 6 months.
9.5 Non responders
Individuals become a ‘non-responder’ if they have been sent an invitation and a reminder but have not attended for a test. Non-responder notifications are issued to GP practices (currently via Open Exeter). GP practices should check the details and report any changes that may be relevant to the individual’s screening record back to CSAS for updating. Non-responder notifications are available online for 8 weeks, after which they are archived.
Individuals who become a non-responder have their NTDD recalculated in line with the appropriate recall interval which are for:
- call and routine recall non-responders, 36 or 60 months from their last NTDD (depending on their age)
- early repeat non-responders, 12 months from their last NTDD
- suspended non-responders, 12 months from the last NTDD
10. Sample requests
10.1 HMR101 request forms
All screening samples must be submitted to the laboratory together with a suitable test request form which has been completed legibly and in full. The HMR101 is the national standard request form for cervical screening. It includes all essential data fields necessary to support patient identification and reporting. Sample takers may use locally-produced versions if these include all standard HMR101 data fields as a minimum.
The HMR101 (2009 version) available from the Open Exeter system is pre-printed with each person’s demographic details and screening history. This system should always be used in preference to hand-completed forms, to ensure that laboratories are provided with all relevant information already recorded on the master index. It is essential that all pre-printed information is checked with the individual at their screening appointment in case there are any errors or updates.
Where GP practices have electronic ordering communications or ‘order-comms’ systems available, it is acceptable for these to be used to transmit data to laboratories to support the sample request instead of an HMR101 form. Reliable technology must be in place to link the sample to the order-comms data, such as bar coding conforming to the relevant NHS Information Standard.
11. Sample processing and reporting
11.1 Cervical screening laboratories
Cervical screening laboratories are responsible for analysing samples and for assigning standard results codes that determine follow-up actions. This includes both HPV and cytology results.
Laboratories have a contractual responsibility to process samples promptly and send results to the call and recall service to support the 14-day turnaround time. The programme recommends the use of electronic messaging systems to do this.
The standard coding system specified by NHS Digital must be used for all results. Appendix 2 in Cervical screening: cytology reporting failsafe contains a table of valid result code combinations. All results files must be formatted correctly and quality checked before electronic transmission to call and recall.
11.2 Receipt by call and recall services
On receipt of the results file from the laboratory, the call and recall service must validate and process the laboratory link file and issue a confirmation of receipt back to the sending cervical screening laboratory. This must include:
- the number of records received
- details of any results failing validation
Results failing validation include those that:
- do not meet national protocol
- are incomplete records
- have a screening history that indicates requirement for an earlier recall in line with national protocol
The call and recall service should validate the file against previous files received to ensure no data has been missed. If any missing files are suspected they should check with the laboratory the same day.
Results files received before 12.30pm must be processed on the same day. Result files received after 12.30pm must be processed no later than 12.30pm the following working day.
The call and recall service must maintain a record of those samples failing validation. It must notify the laboratory via the system generated reports, to ensure that they are updated by the laboratory and returned to call and recall. This enables timely communication with the individual. The sending laboratory must correct any files failing validation as a matter of urgency and then re-submit them. Results that have not been resubmitted within 2 weeks are notified to the SIL (without including patient identifiable information).
12. Notification of test results
12.1 Notification timescales and standards
In line with the programme standard CSP-S03, individuals should expect to receive their screening results in writing within 14 days from the date of the sample being taken.
So this can be achieved, all result files received from laboratories prior to 12.30pm are processed and result notifications sent to the print provider by 3pm. This enables production of the result letters for dispatch to the individuals the same day.
All normal results should be dispatched by the print provider using second or business class postage. All non- negative results should be dispatched by first class post. The method of postage used for each result type must be reflected in the appropriate system parameters to enable accurate measurement of the standard.
Sometimes screening results will require redirection to another database. In such cases, results should be processed within 24 hours of receipt. The call and recall service should maintain an audit trail to enable verification of processing and dispatch of letters to individuals. The audit trail should be available to NHSE if required and the performance (key performance indicators (KPIs)) of the service reported each month to the relevant boards.
Colposcopy
The call and recall system can specify if the colposcopy service has agreed that it will be responsible for informing individuals for tests taken within their service. This is defined by setting the sender code as a source code 7. If source code 7 is set, annual verification with the local service should be undertaken to confirm that all parties are aware of their responsibilities in the notification of results to the individual. The outcome of the verification process should be submitted to SITs.
12.2 Test result letters
PHE determines the wording and content of result letters. As a minimum, result letters contain the details of the result and provide information on what follow-up actions are recommended.
Local SITs can provide localised text to the call and recall service for incorporation within the result letters for individuals who require referral to colposcopy. This text should provide details of the local arrangements for getting an appointment with the colposcopy service.
Call and recall services must work with the local SIT to audit locally relevant text each year to ensure it still meets the needs of the local service, or when there has been implementation of new screening letters.
12.3 Notification to GP practices
Cervical screening laboratories notify GP practices of the results of all screening tests for individuals on their practice list who take part in the programme. The programme recommends electronic messaging to GP systems where possible. This includes the results of primary screening and the results of any further tests.
12.4 Amendments to results
Occasionally, amendments may need to be made after results have been sent to the GP, call and recall and the individual. Under these circumstances, bespoke letters may be required to clarify the reason for the change. The laboratory should contact the individual’s GP practice to pass on the information regarding the change in recall. Any changes to the previously reported codes must be sent from the laboratory to the call and recall service to update the screening history.
12.5 Addresses for results letters
Results letters must be sent to the address supplied by the laboratory via the laboratory links process. Where an alternative correspondence address is provided, or there is a different address to that on the registrations system, this must only be used for a single screening episode. It must not be used for future call and recall letters or for results messaging for a future screening episode unless a further instruction is received from the relevant sample taker.
Where an individual chooses not to register with a GP, the address details provided by the sample taker must be used for all communications including future call and recall. However, all efforts must be made to ensure the individual’s details are identified in the NHS Spine Personal Demographics Service (PDS). Where a PDS record is identified, the address provided by the sample taker must be treated as a correspondence address and future call and recall letters must be sent using the address from PDS. Where call and recall services cannot identify an up to date address for an individual (for example if mail is returned or the individual has de-registered from their last GP), the sample-taking organisation must be informed and provided with their results.
When the service is notified that individuals have relocated to another home nation, their results information must be forwarded on to the responsible authorities.
12.6 Addresses for result letters if no GP registration
Occasionally results are received from the laboratory where, after searches by call and recall across databases, a registration cannot be identified to enable the result to be recorded. To ensure these individuals can receive their result, their details should be placed onto the local database and recorded against a nationally defined dummy screening registration (ZZZ489). Guidance on how this should be done is available from NHS Digital.
If a dummy registration is created to record a test result, it must be retained until the individual is next invited. If the individual becomes a non-responder and the last test was reported as negative routine recall, the registration should be cancelled as O/R (other reason). If the last test was not negative, further checks should be made in an attempt to trace a registration. These individuals should not be removed from the ZZZ489 list until a registration is traced. This will ensure none of these individuals are lost to follow up. Individuals should be retained on the dummy registration and recalled in line with their NTDD.
Annual audits should be undertaken to ensure that individuals on a dummy GP registration have not registered as a live registration. If a live registration is found then the screening record should be recorded on the live record and the dummy registration removed.
12.7 Notification on changing GP practice
Where an individual who has had an abnormal result or is under care or surveillance changes GP practice, the call and recall service must ensure the receiving GP practice is aware of the individual’s status and any requirement for early follow up by sending an electronic notification to the GP practice (currently via Open Exeter). GP practices are expected to check the details and report any changes that may be relevant to the individual’s screening record back to CSAS to be updated. The notifications are available online for 8 weeks, following which they are archived.
13. Protecting data and information
The call and recall system contains several features designed to protect the integrity and accuracy of the information held on the database. They require user action to review, validate and update systems at the intervals described below. Data that is processed and input manually should be quality checked to further assure database accuracy. The call and recall service should have detailed standard operating procedures that describe actions to be taken relating to each of the following subheadings.
13.1 Manual entry
Manual input of data is not ideal. It increases risk of error and should only be used when there is no other option. All data needing manual input requires validation by a second member of the call and recall screening team to confirm accuracy. This process should take place in a timely manner to ensure that correct information is provided to external stakeholders, for example prior to sending to the result letter print provider.
13.2 Network failures
Daily checks should be in place to monitor network transfers to ensure data is not missed.
13.3 Processing information from other NHS services
PCSE and other providers of NHS services may receive information relevant for call and recall services. This information (for example emails and whitemail) must be redirected to call and recall for processing in line with agreed standards and KPIs. Governance of these arrangements should be managed in line with agreed contracts.
13.4 Integrity checkers
The call and recall system integrity checkers must be run (and entries resolved) at least monthly for live records and quarterly for deducted records. These integrity checkers highlight anomalies within the screening records that may prevent individuals proceeding through the screening pathway.
13.5 Exception reports
Various outputs are created which provide data requiring some manual intervention to ensure that records are updated accurately. One example is the transfer of screening records from laboratory link files that have been transferred between databases. These reports must be reviewed and records processed accurately within 5 working days.
13.6 System parameters
A number of parameters are set which ensure the system runs in line with national programme standards. The call and recall service should check these annually by reviewing the audit trail reports available. Documentation should be maintained about any changes to these parameters including when and why the change was made. SITs should approve all changes to parameters. SQAS advice is provided to support assessment of any change. The SIT, with SQAS advice, should approve the return to normal parameters within a suitable timescale. If any temporary change is made, the call and recall service is responsible for making sure the parameters are reverted to the standard settings in line with agreements.
13.7 System security
The key parameter screens should be secured as ‘read only’ and only released as and when changes are required. The screens should be returned to ‘read only’ once the changes have been made. Access to these screens should be strictly controlled and accessed only by senior staff, for example a subject matter expert or key user with a detailed knowledge of implications of changing any of the parameters. This requirement should be described in a SOP.
14. Invasive cancer audit
National guidance describes the process for auditing cases of invasive cervical cancer. The purpose of the cervical cancer audit is to monitor the effectiveness of the screening programme and to identify areas of good practice and where improvements can be made.
Call and recall have a responsibility to:
- supply screening histories to the cervical screening programme lead (CSPL) or SQAS as required
- identify controls and supply screening information to the CSPL or SQAS as required
Any request for invasive cancer audit data should be processed within 5 working days and should be provided in the format described by NHS Digital .
15. Migration in and out of England
15.1 Migration from England
Where individuals move from England to become a resident in Scotland, Wales, Northern Ireland or the Isle of Man, they are no longer eligible for recall in England. Their screening history must be copied to the relevant NHS body for the home nation to inform their call and recall services. The records of individuals who relocate outside England must be retained in IT systems on the assumption that they may return to England and re-join the screening programme.
15.2 Migration into England
Where an individual relocates from Scotland, Wales, Northern Ireland or the Isle of Man to become a resident in England, the programme expects the relevant NHS body to make the individual’s screening history available. This includes the details of individuals who have been ceased, to ensure their wishes continue to be respected.
15.3 Acknowledging receipt of screening history
When a screening history is provided to the call and recall services in England, an acknowledgement of receipt must be provided by the call and recall service to the sending site either electronically or by printed notification. The screening history provided must be entered into the individual’s English screening record and used to calculate their NTDD.
