NHS Cervical Screening Programme – Good practice guidance for sample takers
Published 31 July 2023
1. Introduction
1.1 Aims of this guidance
This guidance is designed to:
- describe the sample taker’s responsibilities in the NHS Cervical Screening Programme (NHSCSP)
- promote good practice that is consistent with national policy and guidance
- outline the training requirements for sample takers in the NHSCSP
- set out the existing roles and responsibilities of GP practices involved in the NHSCSP
- outline the audit and documentation requirements for sample takers in the NHSCSP
- advise on some of the issues that may arise during a consultation.
1.2 The NHS Cervical Screening Programme
The NHS Cervical Screening Programme (NHSCSP) aims to reduce the number of people who develop invasive cervical cancer and the number of people who die from it. It does this by regularly screening all people at risk so that conditions which might otherwise develop into invasive cancer can be identified and treated.
Many people are involved in providing the NHSCSP. They include the doctors and nurses who take the samples in primary care, sexual health and community clinics, colposcopy services, laboratory staff who process and screen the samples and the call and recall service who manages the invitations into the programme. The administrative tasks associated with the call and recall service include:
- ensuring all eligible women aged 24.5-64 years are included in the screening programme
- inviting all eligible women to attend for screening
- notifying the participant of their test result
- ensuring appropriate follow up and recall
Further information on call and recall can be found in national call and recall guidance.
1.3 High-risk human papillomavirus testing
Four large European randomised controlled trials have considered the use of high-risk Human Papilloma Virus (hrHPV) testing as a primary screening test. Compared to cytology, hrHPV testing has been shown to reduce the risk of developing cervical cancer through increased sensitivity for underlying disease. Evidence suggests at least 10 years elapses between acquiring hrHPV and developing cervical cancer. The high negative predictive value of hrHPV testing and lower false negative rate means screening intervals can be lengthened in women who test negative for hrHPV. In addition, detailed modelling studies based on the ARTISTIC trial have since shown primary hrHPV screening to be cost effective.
This evidence provides the rationale for moving to primary testing with hrHPV, reserving cytology for women testing hrHPV positive.
In 2013, English pilots of primary hrHPV screening began and in 2015 the first report confirmed the feasibility of use and improved performance of primary HPV screening within the NHSCSP. Following an evidence review and public consultation the UK National Screening Committee (UK NSC) recommended the implementation of primary hrHPV testing to replace primary cytology and on 4 July 2016, the Public Health Minister announced the implementation across England.
A national programme of primary hrHPV screening was fully implemented in England in December 2019.
The hrHPV Primary Screening Pathway Flowchart is available.
More information and frequently asked questions about hrHPV and hrHPV testing can be found below on the:
2. Cervical cancer and cervical screening
2.1 The impact of cervical screening
Although cervical screening cannot be 100% effective in detecting cancer, cervical screening programmes have been shown to reduce the incidence of cervical cancer. The NHS Cervical Screening Programme was established in 1988; over the next decade the incidence of cervical cancer across England and Wales fell by more than 40 per cent, reflecting screening’s impact on a generation of previously unscreened people.
In England cervical screening currently prevents 70% of cervical cancer deaths. If everyone attended screening regularly, 83% could be prevented.
2.2 Cervical cancer: incidence, survival rates and mortality
Information regarding the incidence, survival rates and mortality of cervical cancer is available on the Cancer Research UK website.
2.3 Risk factors for cervical cancer
Risk factors in the development of cervical cancer are:
- Persistent presence of high-risk human papillomavirus
- Smoking – (this weakens the immune system making the body less able to get rid of hrHPV)
- Age (cervical cancer is most common in women and people (with a cervix) under the age of 45)
- Weakened immune system the body is less able to get rid of hrHPV – human immunodeficiency virus (HIV) is the only infection for which NHSCSP recommends annual cervical screening
- Long term use of oral contraceptives
- Multiple sexual partners and sexual debut at a young age
- Non-attendance for cervical screening.
3. Cervical Screening Coverage
3.1 Coverage and links to local data
Coverage is defined as the percentage of people eligible for screening at a given point in time who were screened adequately within a specified period (within 3.5 years for people aged 25 to 49, and within 5.5 years for people aged 50 to 64).
The cervical screening population is women or people with a cervix aged 24.5 to 64, who are registered on the NHS call/recall IT system for cervical screening.
The eligible population is everyone in the screening population excluding those without a cervix, usually due to total hysterectomy.
An adequate screening test is one which does not need to be repeated because the hrHPV component of the test had an unavailable result, or because the cytology component of the test had an inadequate result.
Calculating coverage
In a GP Practice:
- there are 1250 registered female patients aged 25 – 49; of these 14 are ceased from call/recall due to total hysterectomy (no cervix). Of the remaining people, 922 have had an adequate screening test in the 42-month period ending on 31st March.
- there are 950 registered female patients aged 50 – 64; of these 73 are ceased from call/recall due to total hysterectomy (no cervix). Of the remaining people, 776 have had an adequate screening test in the 66-month period ending on 31st March.
Coverage for people aged 25-49 is calculated:
Number of patients: 1250
Number of ineligible patients (no cervix): 14
Number of eligible patients: 1250 – 14 = 1236 (denominator of the percentage calculation)
Number of screened eligible patients: 922 (numerator of the percentage calculation)
At 31st March, 3.5-year coverage: 922/1236 x 100% = 74.6%
Coverage for people aged 50-64 is calculated:
Number of patients: 950
Number of ineligible patients (no cervix): 73
Number of eligible patients: 950 – 73 = 877 (denominator of the percentage calculation)
Number of screened eligible patients: 776 (numerator of the percentage calculation)
At 31st March, 5.5-year coverage: 776/877 x 100% = 88.5%
The acceptable performance threshold for coverage is ≥ 80%
For coverage at GP practice, clinical commissioning group and/or local authority levels see:
Population screening programmes – cervical screening – detailed information available on gov.uk
Cervical screening data and research collections available on gov.uk
Cervical screening coverage and data available on gov.uk
Cervical screening annual statistics
Coverage can be confused with the related measure of ‘uptake’. Screening uptake is a measure of response to invitation. It is the percentage of people invited for screening who are tested within 6 months of their invitation date. Uptake figures for a specific period will therefore only include people who are invited in that period. They will not include people who are not due at that time, or those who have been screened opportunistically if they are overdue for their test. Historically, uptake data has not been reported/published for cervical screening, however, new reports are now being defined and data will be reported in due course.
In general practice, sample takers may also be focussed on achievements under the Quality and Outcome Framework (QOF) where there are points relating to cervical screening in the Public Health domain. QOF points are measured in a different way from Coverage and Uptake as they allow for Personalised Care Adjustments (formerly exception reporting). It is important that practices review their coverage data to ensure that they have the fullest picture of the numbers and groups of eligible patients that are attending or potentially missing their cervical screening.
3.2 Initiatives to improve screening coverage
Call and recall run a process to identify those people who have a Next Test Due Date (NTDD) within the following 10 weeks. A prior notification list (PNL) of patients due to be invited is provided to GP practices. This allows the GP practices to manage any people who do not want or need to be screened. For example, people who need their screening deferred due to undergoing medical treatment. GP practices are therefore in a unique position to know their patients and can help ensure that the PNL is correct and checked for changes of address and contact details.
For non-attenders, the GP practice can attach a message to their record to ensure that screening is raised at the next appropriate visit and that the benefits of regular screening are discussed and any barriers to attending identified and addressed.
GP reception staff should be appropriately trained, so they are fully informed of any changes to the screening programme.
Jo’s Cervical Cancer Trust hold a best practice Cervical Screening Awards to improve awareness of cervical screening with the aim of improving uptake of screening. Case studies from award winners since 2010 demonstrate the many ways local initiatives can improve screening coverage.
When considering any initiatives to improve coverage, it should be remembered that taking part in screening is always an individual choice. Screening initiatives should be linked with local screening programme boards to ensure all services are aware so they can consider the wider impacts of these initiatives and are prepared for an increase in demand for screening.
There is a range of resources available to provide information about cervical screening to support informed choice, including:
Cervical screening leaflet - ‘Helping you decide’
Cervical screening – an easy guide
Cervical screening guidance for lesbian and bi-sexual women
Cervical screening blog – reducing inequalities for trans people
Providers of screening should be aware that every individual’s circumstances are different when accessing screening services. Resources have been made to support people who have previously experienced trauma or sexual abuse in participating in the NHS Cervical Screening Programme. Anyone who is affected by resultant anxiety or finds it difficult to attend cervical screening can access information about available support and how to plan their screening appointment online here. This also includes provision of a support checklist to take along to their appointment and links to advice provided by external organisations such as Jo’s Cervical Cancer Trust and Samaritans.
Ensuring that staff are well informed of the barriers to cervical screening and how to support anyone accessing the NHS Cervical Screening Programme who has suffered sexual assault or abuse is vital. Section 5 of the cervical sample taker training covers the barriers to cervical screening.
4. Ensuring Quality
4.1 Sample taker PIN code
All trained sample takers must have a pin or code number. Each laboratory has local arrangements for this. The allocated PIN code is unique to an individual sample taker (including trainees) and they are accountable for its use. The sample taker must use only their own PIN code during clinical practice. They must not use another sample takers PIN code, share their own PIN code or allow other sample takers to use it (including locum staff for example).
A sample taker may have different PIN codes to reflect roles in multiple organisations. It is important to always use the PIN code relevant to the service in which the sample was taken.
Samples submitted without a valid sample taker identification code will be delayed and reported as result unavailable if not abnormal (see Guidance for acceptance of cervical screening samples in laboratories and pathways, roles and responsibilities - GOV.UK (www.gov.uk)).
When moving employers, sample takers should provide their sample taker training evidence to the cervical screening lead (often the practice manager) and ensure they are appropriately registered on the local sample taker database before any samples are taken.
4.2 What to do if there’s a suspected cervical screening incident
What is a screening incident?
Screening safety incidents are defined as:
- any unintended or unexpected incident(s), acts of commission or acts of omission that occur in the delivery of an NHS screening programme that could have or did lead to harm to one or more people participating in the screening programme, or to staff working in the screening programme
- harm or a risk of harm because one or more people eligible for screening are not offered screening
Serious incidents are defined as:
An incident where the consequences or risks are so significant to people, carers and families; organisations and staff, populations, or represent significant potential learning for the NHS that a heightened response is required.
Dealing with incidents
Incidents frequently affect primary care services, either because they arise in primary care or have an impact on people registered at GP practices.
Any unexpected event that could affect people screened by or registered at a primary care provider should be reported as a potential screening incident promptly. All NHSCSP staff should familiarise themselves with their site’s local policies and procedures for managing incidents, suspected incidents and near misses.
The confidentiality of NHSCSP patients and staff must be protected in accordance with policy and legal requirements.
All incidents and suspected incidents should be reported in the first instance to a senior staff member within your service who will then report it to your NHS England S7a Public Health Commissioning Team who will advise and support you further.
If you are unsure whether an incident has occurred, please speak to your NHS England S7a Public Health Commissioning Team for advice.
The NHS England S7a Public Health Commissioning Teams will notify the Screening Quality Assurance Service (SQAS). SQAS ensures national screening programmes are safe and effective by ensuring that national standards are met and that the screening programme is delivered in accordance with national guidance. SQAS provide advice on incidents and support investigations.
If a problem is confirmed, the provider will be asked to complete a Screening Incident Assessment Form (SIAF) with full details to initiate an investigation. SQAS and the commissioning team will consider the information provided, provide advice and request any further actions.
Incident management in the NHSCSP may involve other agencies within or outside the NHS. Cooperation and collaboration with other agencies are therefore key to understanding what went wrong and learning how the risk of similar incidents occurring in the future can be reduced.
Guidance on dealing with incidents in the NHS Cervical Screening Programme is available.
5. Screening intervals
The programme sends screening invitations to people with a cervix who are registered with their GP practice as ‘female’ or ‘indeterminate’ at the following ages and intervals.
- For those aged 25 to 49 screening is every 3 years with a first invitation issued at 24.5 years to ensure screening starts promptly at 25.
- For those aged 50 to 64 screening is every 5 years,
- Those over age 65 are only screened where they have not been screened since age 50 or have had recent abnormal tests or have never been screened.
The high negative predictive value of hrHPV testing and lower false negative rate means screening intervals can be lengthened in women who test negative for hrHPV. The UK NSC has recommended to NHS England that cervical screening intervals can be extended. The extended screening intervals pathway is currently under review. The date of implementation has not yet been finalised.
5.1 People aged under 24.5
The programme does not invite people under the age of 24.5 for cervical screening because:
- cervical cancer is very rare in people under 25
- infection with high risk human papillomavirus (hrHPV) is very common in people under 25 and may cause abnormal cell changes of the cervix (for most people these cervical abnormalities will regress as the immune system clears the HPV infection)
- Screening people under 25 can lead to over-treatment and could lead to an increased risk of early (premature) birth if they were to get pregnant in the future
- the International Agency for Research on Cancer (IARC) recommends that people should not start cervical screening before the age of 25
- in 2012, the UK NSC advised the NHSCSP that screening under 25 does more harm than good and recommended a consistent screening age across the whole of the UK (from June 2016 all 4 nations screen from age 25)
5.2 Clinical practice guidance for the assessment of young women aged 20 to 24 with abnormal vaginal bleeding
Cervical screening is not a diagnostic test. It is not appropriate to take a cervical screening sample from people experiencing abnormal vaginal bleeding. A clinical pathway is available on the appropriate assessment of young women with abnormal vaginal bleeding, including the need for a speculum examination to enable a clear view of the cervix.
5.3 People aged 65 and over
Routine screening stops at age 64. People will continue to be invited after their 65th birthday only if they are eligible for non-routine screening to complete a period of follow up after a previous abnormality.
Anyone who did not respond to their final screening invitation which was sent on or after their 60th birthday can change their mind and request a screening test at any time even if they have been ceased from recall due to age.
The programme does not invite people over the age of 64 for routine cervical screening because:
- the natural history and progression of cervical cancer means that it is highly unlikely that such people will go on to develop the disease; those aged 65 and over who have had 3 consecutive negative tests are taken out of the call and recall system
- People aged over the age of 65 who are hrHPV positive at their last screen and/or have abnormal screening results will continue to be screened as described in the screening pathway
- people aged 65 and over who have never been screened or have an incomplete screening record are entitled to a test if they request one
Cervical samples taken in primary care that fall outside of the cervical screening programme will be rejected by the laboratory under the ‘guidance for acceptance of cervical screening samples in laboratories and pathways, roles and responsibilities’, for more information refer to section 6.3 of the guidance document.
6. Ceasing and withdrawing from the programme
People who choose to withdraw themselves from the screening programme, and those who are no longer eligible, are removed (ceased) from call and recall.
Most ceasing requests are communicated to call and recall directly from a person’s GP practice, either by ad hoc request or via the PNL process. Occasionally ceasing requests are made directly to call and recall by the individual or by other stakeholders using the online CSAS contact form.
Someone who is ceased from call and recall will not receive any further invitations or reminder letters from the screening programme.
Someone for whom a screening invitation is temporarily inappropriate, should be deferred from call and recall by the GP practice. Deferral is achieved by postponing their screening invitation for a specified period, usually when responding to the Prior Notification List (PNL) sent by the call and recall system. The call and recall system will re-invite the person for screening after the period of postponement, if they remain eligible.
6.1 Special circumstances for consideration
Special circumstances which require individual consideration are listed below. Please see the national ceasing guidance for further information about each individual circumstance.
If a sample taker is unable to take a cervical screening sample a further opinion should be sought in the primary care or sexual health setting, if a sample can still not be achieved a referral to colposcopy should be made, this should be done before (if appropriate) ceasing the individual.
In most instances a person should only be ceased from call and recall if they agree to this. They are managed in the same way as people who choose to withdraw from screening. The possible reasons for ceasing are:
-
Female genital mutilation (FGM)
-
Vaginismus
-
Cervical stenosis
-
Physical conditions and disabilities
-
Terminal illness
-
Mental capacity
6.2 Ceasing due to radiotherapy
It is difficult to accurately report samples from women who have undergone radiotherapy for cervical, bladder, rectal and other pelvic cancers. This group should be ceased from the programme and provided with gynaecological follow-up.
6.3 Ceasing due to non-eligibility
The following people are eligible to receive a screening invitation while under the age of 25:
- women or people with a cervix (and registered as female or indeterminate with their GP), who are within 6 months of their 25th birthday and who have been identified as due for their first test
- people who have been previously screened elsewhere in the UK and who are eligible for routine or non-routine recall as a result of their previous test
- people who have been previously screened privately and who require a follow up non-routine recall as a result of their previous test where the screening programme has been notified of the result of a previous test
People should not be ceased from call and recall due to age while under the age of 25, however, other clinical reasons for ceasing under 25 may apply.
6.4 Automatic ceasing at age 60 and over
A person who attends for routine cervical screening on or after their 60th birthday will be automatically ceased from recall if their last test result is normal with routine recall and they have had no recent abnormal results. This is because their next routine test would be due after their 65th birthday.
People who have previously had abnormal cervical cells are only automatically ceased from recall when they have completed the relevant follow up. Those who have not completed relevant follow up continue to be invited for non-routine cervical screening after the age of 65 if necessary.
All people who are ceased automatically due to age after attendance are sent a result letter which includes an explanation that they will not be invited for cervical screening because of their age.
People are also automatically ceased from recall if they fail to respond to a routine invitation which was sent on or after their 60th birthday. This is because their next routine invitation would be due after their 65th birthday. People who have been ceased automatically due to age after non-attendance can request a final routine test at any time regardless of age. If the result of the final routine test is normal, the person is ceased from recall again automatically. If the result of the final routine test is abnormal the person is returned to recall until all necessary follow-up tests have been completed.
6.5 Ceasing at age 65 and over
People aged 65 or over who have had a previous cervical abnormality remain in recall until they have completed follow up, even if they have not responded to their most recent screening invitations. This may include people who have had a positive hrHPV test but no abnormal cytology.
People aged 65 or over who remain eligible for non-routine screening are not ceased automatically due to age.
GPs can request ceasing of persistent non-attenders and/or low-risk people who have passed their 65th birthday if they feel that further invitations would be of no benefit to the person; for example, because they are highly unlikely ever to attend. Requests for ceasing due to age may be made using the template ceasing form available from CSAS, in writing or by email to call and recall, or via the Prior Notification List (PNL) process.
GPs should not request ceasing due to age if a woman is over 65 but is awaiting a recall invitation where her last test recommended early recall or colposcopy attendance.
People who have not attended for a test since their 60th birthday may request their final test at any time, even after they have been ceased due to age. They are returned to recall after this test only if the result is abnormal, and they will remain in recall only until they have completed any relevant follow up or are re-ceased for a new reason.
6.6 Ceasing due to absence of cervix
People who do not have a cervix are not eligible for cervical screening and should be ceased from recall permanently.
Ceasing can be arranged via the electronic PNL or the template ceasing form available from CSAS.
All people ceased from call and recall due to absence of cervix are informed by letter that they will receive no further invitations for screening. Where the person is registered with a GP practice, call and recall must notify the practice that the ceasing process has been completed.
People assigned female at birth who were born without a cervix do not require screening and should be ceased from recall.
6.7 Trachelectomy
People who have undergone a radical trachelectomy (removal of the uterine cervix) for cervical cancer no longer require screening and should be ceased from recall. Clinical follow up may be appropriate and will be managed by the relevant clinician outside of the screening programme.
6.8 Hysterectomy
People who have undergone a total hysterectomy no longer require cervical screening and should be ceased. People with a subtotal hysterectomy still have a cervix and should remain in the NHSCSP as they continue to be at risk of cervical cancer. If their clinical history is unclear, an examination is advised to establish the presence of a cervix.
6.9 Voluntary withdrawal
People can choose to withdraw from the cervical screening programme at any time, and do not have to give a reason for their decision.
Anyone who wishes to withdraw permanently from the screening programme must be:
- provided with (or signposted to) sufficient information, in an appropriate and accessible format, to support an informed decision
- given the opportunity to discuss their decision with a GP or other suitably qualified health professional (but they do not have to take up this opportunity)
- informed that withdrawing from the programme means they will not receive any future invitations or reminder letters about cervical screening from the programme
- asked to put their withdrawal request in writing, ideally using the standard template ceasing form available from the NHS Cervical Screening Administration Service (CSAS); if this is not possible, a record should be made at the time of the request (call and recall must receive and retain a copy)
- advised that they can return to the programme at any time providing they are still eligible for screening (by requesting an appointment at their GP practice or other screening provider)
- notified in writing when the ceasing has been completed (their name is removed from call and recall)
Ceasing documentation
A person should put their request for permanent withdrawal from cervical screening in writing if possible (to their GP practice or screening provider). This is to ensure there is no misunderstanding and that they are not ceased from call and recall in error. The template ceasing form available from CSAS should be used. This contains the information needed to support a withdrawal request and is the preferred method of communication. The GP practice should arrange for the form to be provided to the individual. A clear and unambiguous written request signed by the individual is also acceptable.
If a person will not or cannot provide a written request, the GP or a suitably qualified healthcare professional should document the request in writing, noting the time, date and content of the conversation.
The withdrawal request is an important part of the person’s screening record. The GP practice must retain a copy of the document within the person’s medical record.
6.10 Return to recall after voluntary withdrawal
A person who has been ceased from call and recall will not receive screening invitations but can be screened on request if they are eligible but overdue for a test. This includes anyone whose last routine test was more than 3 or 5 years ago (depending on their age), and people who have never had a test.
As soon the call and recall system receives a new test result, the system returns the person to recall automatically. A ‘next test due date’ (NTDD) is set according to the result of their last test, and an invitation is generated at the appropriate time.
If a person is not due or overdue for a test but wishes to return to recall so that they will receive an invitation when due, they can ask their GP to contact call and recall to cancel their ceasing. A signed form is not required to cancel a ceasing due to informed choice but can be used if preferred.
7. Failsafe responsibilities
Responsibility for failsafe lies with all providers of the cervical screening programme i.e. GPs, sample takers, cervical screening laboratories, colposcopy clinics and commissioners. It is important to ensure appropriate action is taken to make sure that the right people are invited and that those with abnormal test results are followed up correctly. Providers should have local protocols to ensure that all processes close within an appropriate timescale. Find out more about GP and sample takers’ failsafe responsibilities.
7.1 Summary of call and recall failsafe responsibilities
Invitation letters
All eligible people will receive a written invitation to attend for screening together with the national information leaflet (or a link to the information online) to enable them to make an informed choice about attending. The Cervical Screening Administration Service issues screening invitations up to 6 weeks before a person’s Next Test Due Date (NTDD).
1st reminder letter
Where call and recall has not received a test result from the relevant cytology laboratory within 126 days (18 weeks) of an invitation letter being created, the person becomes ‘overdue’ for screening and a reminder letter is created and sent. Reminder letters do not include the national information leaflet; they include a link in the letter text to the leaflet online. All invitation and reminder letters use national letter texts provided by NHS England.
2nd reminder
GP practices will be advised on the call and recall system of non-responders. It is best practice for a second reminder to be sent by the GP practice, this may be in the form of a letter, postcard, phone call, text message or other.
Refer to the call and recall timetable.
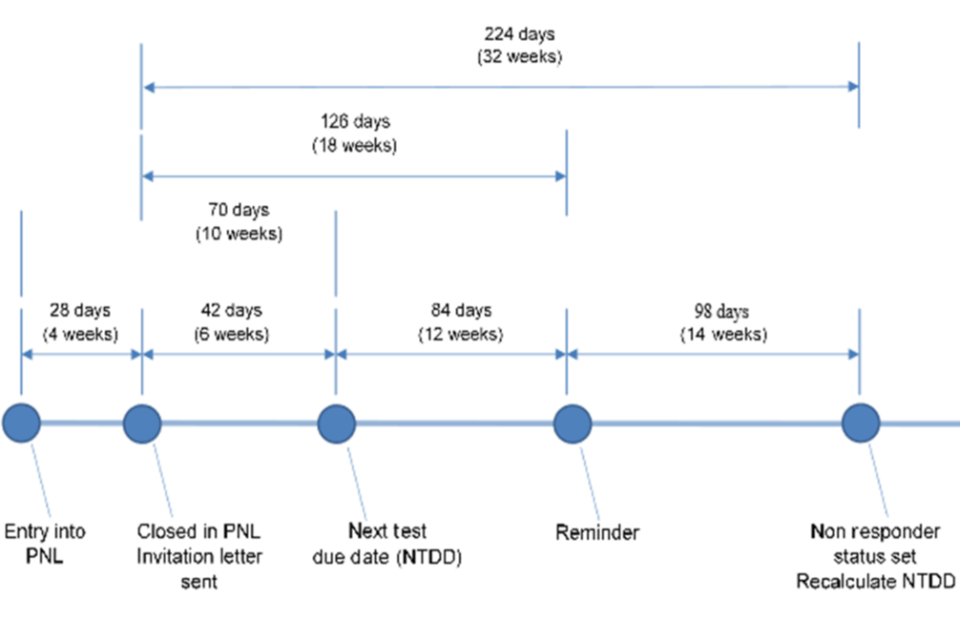
7.2 Summary of sample taker failsafe responsibilities
Sample takers responsibilities include:
Taking the sample -
- make sure the person has been provided with or signposted to the national information leaflet to support them in making an informed choice about attending screening
- Take the cervical sample and complete the sample request in line with the cervical screening sample taker guidance.
- Ensure the sample and documentation are correctly labeled in line with the sample acceptance guidance, it is essential that the request documentation and sample vial are correctly labeled with matching patient identifiers for the correct individual – sample takers are advised to do that while the patient is still present and to check identifiers with the patient -There are 3 minimum identifiers – full name (at least first name & surname), date of birth and either NHS Number (preferably) or full address. Samples without the 3 minimum identifiers will be rejected by the laboratory.
- Results and failsafe – maintain a register of tests taken and check that a test result has been received from the laboratory for every sample taken, (the practice must have a procedure in place to investigate any sample result not returned)
- make sure the person is informed of their test result
-
make sure the test result is followed up
- communicate with the person if their sample is rejected and reason why (and reflecting on this) - the sample taker also needs to arrange for another sample to be taken in 3 months’ time. Guidance on rejection codes is available.
- Make sure referrals take place for people who require further investigation and treatment
-
co-operate with failsafe enquiries in a timely manner in conjunction with reviewing own internal sample take audit. Example of a sample taker audit template can be found here.
- Make sure reasonable adjustments are offered for people who need additional support
- Make sure adverse events and incidents are reported and discussed within the service, reported to the SIT and actions taken as stipulated on the completed screening incident investigation form (SIAF)
7.3 Summary of GP practice failsafe responsibility
The GP practice is responsible for:
- making sure that the person is provided with the necessary information and advice to assist them in making an informed choice about whether to participate
- completion of Prior Notification Lists (PNLs) in line with guidance on call and recall services, Cervical Screening Administration Service CSAS website and gov.uk guidance.
- for intersex people with a cervix, female to male transgender (trans) men, and for people who identify as male but require cervical screening, the GP should take responsibility for and arrange the screening process, as the Cervical Screening Administration Service is not commissioned to send invitations or results to people registered as male – the GP should also notify the laboratory that the results should be returned to the practice directly
- arranging for a person to be informed of their test result *
- ensuring that arrangements are made for people who fall outside the call and recall system to receive their test results
- discussing the test result in person with the person if required for cases of high-grade dyskaryosis/ ?invasive squamous carcinoma or ?glandular neoplasia of endocervical type
- giving a person their test result in person where an urgent referral is required for? glandular neoplasia (non-cervical)
- referring a person for further investigation and treatment where necessary (for example, a person needing colposcopy who has moved from another area, or a person who has been discharged following a previous non-attendance at colposcopy)**
- acting on the non-responder notification from the colposcopy clinic for people who have not attended for colposcopy
- cooperating promptly with failsafe enquiries from the laboratory about a person who requires further investigation and treatment***
- ceasing and deferral of people in line with the cervical screening ceasing guidance. Practices should submit cease and deferral requests via the Cervical Screening Administration Service before the cut off shown on the Prior Notification List (PNL) to avoid inappropriate invitations.
- appropriate follow up of non-responders with particular reference to those who are not on routine recall, and notification of relevant changes to the Cervical Screening Administration Service – see section 9.5 of the cervical screening call and recall guidance.
- sending a reminder to people who have not responded to their call and recall invitation and reminder letters (sending a third invitation)
- making sure that people no longer eligible for screening (due to absence of cervix or pelvic radiotherapy) are notified to the Cervical Screening Administration Service promptly
- ensure that all employed cervical sample takers have completed the initial training and subsequent 3 yearly national update requirements as described in the cervical screening sample taker training guidance.
- managing non-attendees at colposcopy/responding to failsafe enquiries, see section 5 of cervical screening failsafe guidance.
- ensure transport and other links are set up with the local screening laboratory to which samples will be sent and adhere to their guidance around sample taker codes and requesting samples (electronic or otherwise).
-
identifying and managing safety screening incidents
- Although it remains the responsibility of the GP to make sure women are informed of their test result, this function is provided automatically by the Cervical Screening Administration Service once a valid result is received
** All laboratories operate a direct referral system for colposcopy in conjunction with their local colposcopy clinics. In areas using this system, the GP still has a responsibility to make sure that colposcopy has taken place.
*** It should be noted that failure by providers to respond to failsafe enquiries should be considered as a clinical governance issue.
8. Access to patient screening records on the call/recall IT system
Open Exeter is a web-enabled viewer which allows limited access to patient records on the national call/recall IT system ‘NHAIS’ operated by the NHS Cervical Screening Administration Service (CSAS). Open Exeter can only be used by authorised users in organisations which have secure access to the N3 NHS network.
Within the cervical screening programme, Open Exeter enables
- practices to view the screening records of their registered patients – particularly useful when new patients join a practice
- practices to check cervical screening Prior Notification Lists and submit returns on-line
- practices to receive non-responder notifications and submit a response to the screening agency if appropriate
- a sample taker to generate and print a pre-populated HMR101 sample request form (where the receiving laboratory has agreed that this is the preferred method for submitting forms)
- laboratories to give the correct recall advice in the light of the persons screening history
- the screening service to advise the practice when patients are ceased from recall
- the screening service to notify the practice of any newly registered individuals who are on ‘early follow-up’ owing to an abnormal test result
Information about Open Exeter and how to gain access to the system is available in the Cervical Screening Administration Service Frequently Asked Questions document.
9. HPV vaccination
The routine HPV vaccination programme began in September 2008 for girls aged 12-13 years in school year 8. A catch-up programme also started in September 2008 and offered the vaccine to older girls up to the age of 18:
Young women aged 17 - 18 years (school year 13) - born between 1 September 1990 to 31 August 1991 inclusive - were offered the vaccine in the 2008/09 school year.
Young women aged 14-18 years (school years 10, 11, 12 and 13 if in education) - born between 1 September 1991 and 31 August 1995 inclusive - were offered the vaccine from autumn 2009.
In most areas the catch-up programme ended in August 2010.
In September 2019 the adolescent HPV vaccination programme became universal with 12 to 13-year-old males becoming eligible alongside females.
If an individual misses the HPV vaccine offered in Year 8 at school (and still remains eligible), it’s available for free on the NHS up until their 25th birthday for:
- girls born after 1 September 1991
- boys born after 1 September 2006
Guidance on the HPV vaccination programme is available.
More information about the HPV vaccination programme is available:
Human papillomavirus vaccination information – nhs.uk website
9.1 HPV vaccination records
Details of vaccinations administered through the national programme are held primarily on local Child Health Information Systems with additional information held on GP systems, especially relating to vaccines administered in primary care.
Child Health departments are currently required to upload information about vaccines administered to females using functionality available in Open Exeter so that it can be added to their future cervical screening records on NHAIS. This data will be used to support the evaluation of the screening programme and the vaccination programme and will therefore allow evidence-based changes to be made to screening programme protocols in future.
10. Cervical screening programme sample taker training
A cervical screening test is a consultation and clinical examination. A cervical sample taker must have the required level of knowledge and understanding of the cervical screening programme and clinical skill to safeguard the person attending their screening appointment.
The Cervical Screening Programme guidance for sample taker training:
- details the training requirements for all sample takers working in the NHSCSP
- ensures, via completion of the training, that sample takers meet the competency framework standard CHS37 ‘Obtaining cervical cytology samples from women’
The sample taker education pathway sets out :
- which registered health care professionals are eligible to undertake the sample taker role
- the requirements for trainee cervical sample takers
- the required 3 yearly updating for qualified sample takers to maintain competency and meet their clinical and professional responsibilities for continuing professional development and revalidation
10.1 Trainee sample takers
Your NHS England S7a Public Health Commissioning Team may be able to provide a list of training providers offering courses in your area. Check that the training provider holds current accreditation for their theory course.
Training for sample takers includes a theory course, a period of practical training and a final assessment. A trainee must successfully complete all elements of the training described in the guidance on cervical sample taker training.
All training should be completed within a nine-month period from enrolling on the initial course through to their final clinical assessment.
Trainees should make sure they book to visit a local colposcopy clinic early in their training due to the variation in local availability. Trainees must factor in protected time for the visit and any follow up visits if necessary. Trainees must attend for the duration of a clinic.
11. Trained Sample Takers
11.1 Maintaining competency (trained sample takers)
To ensure continued competence in accordance with their professional codes of conduct, (NMC CODE 6.2) (GMC) sample takers should conduct continuous self-evaluation and be proactive in seeking advice should they identify any issues as referenced in Education pathway - Guidance for trained sample takers. They should audit and reflect on their individual rates of abnormal tests and any rejected samples, including those inadequate for cytology as reported by the local cervical screening laboratory (see Guidance for acceptance of cervical screening samples in laboratories)
There is no minimum number of cervical samples that need to be taken to maintain competence. However, all sample takers need to undertake a minimum of one half day’s update training every 3 years and audit and reflect on their abnormal samples and sample rejection rates.
11.2 Professional obligations for CPD and requirements for update training
See ‘Guidance for trained sample takers’, section 3.22 of the Education Pathway.
All UK registered health professionals involved in cervical screening must keep up to date with developments in the programme and meet their professional obligations for continuing professional development.
For cervical screening guidance you can refer to the standards and guidance for healthcare professionals and managers working in the NHSCSP.
Sample takers must undertake a minimum of 3 hours update training every 3 years. This is done by either attending an accredited face to face training session or completing the national eLearning resource for sample takers which meet the programme requirements for update training. On completion of the 3 yearly update training, sample takers must ensure the sample taker database is updated to reflect this.
All sample takers must complete the HPV e-learning at e-learning for health.
11.3 Returning to sample taking after a period of absence
The sample taker should undertake the required level of training needed, based on the length of absence from practice.
See section 3.23 of the Education Pathway - Returning to sample taking after an extended period of absence.
12. Sample requests
12.1 Points to remember when completing sample request and sending samples to laboratory:
- Taking a clinical history - See Topic 8 Section 3.4 ‘Taking and recording a clinical history’.
- Preparation of the sample request form (HMR 101) – See Topic 8 Section 3.7 ‘Preparing the sample request form’. The HMR101 form, with the demographic and GP details, can be downloaded from Open Exeter.
- It is essential that an individual’s details are obtained directly from them – do not fill out a request form prior to an appointment. Forms must be completed in full and must be legible.
- Samples should be dispatched the same day to ensure the person receives their results within two weeks. Request forms and samples which have a major mismatch will result in the sample being rejected by the cervical screening laboratory and a repeat test, carried out in no less than 3 months’ time, will need to be arranged by the sample taker. See Topic 8 section 11 in the sample taker training guidance for information on ‘Sending the sample to the cervical laboratory’. Guidance on the minimum identifying requirement for cervical samples is available.
12.2 Factors affecting whether cervical screening is appropriate
Management of people with cervical cancer symptoms
Individuals presenting with symptoms of cervical cancer (for example postcoital bleeding or persistent vaginal discharge that cannot be explained by infection or other causes) are not suitable candidates for screening.
If the common causes of these symptoms have been excluded in general practice (for example infection or contraception usage), the person must be referred for examination by a gynaecologist experienced in the management of cervical disease (for example a cancer lead gynaecologist). Gynaecologists may refer such people on for symptomatic colposcopic examination outside the cervical screening programme if cancer is suspected.
The Advisory Committee on Cervical Screening developed guidance on the management of young women with gynaecological symptoms such as persistent bleeding on intercourse or between periods. This involves primary care, GUM, gynaecology and cervical screening experts.
An individual must be referred to colposcopy and should be seen within 2 weeks of referral (greater than or equal to 93% of cases) if the appearance of the cervix is suspicious or they have symptoms consistent with cervical cancer.
Post-coital bleeding
Evidence for the precise predictive value of post-coital bleeding for cervical cancer is poor. Most cases of post-coital bleeding are not due to malignant disease, and in younger people chlamydial infection or problems with contraception are more likely causes. Guidance on the investigation and management of post coital bleeding is available.
Cervical screening when menstruating
An appointment should be made for when the individual is not bleeding, avoiding the two days before and after bleeding, if possible. This is because if a cytology sample is required the cells may be obscured by blood. This will reduce the risk of an inadequate sample and the need for a retest.
If a person has abnormal/unexplained vaginal bleeding they should seek a consultation and examination from their GP. Contact bleeding at the time of cervical sampling may occur and is not an indication for referral to colposcopy in the absence of other symptoms.
Cervical screening after recent sexual intercourse
The general advice is for individuals to refrain from sexual intercourse for 24 hrs before their cervical screening test. Spermicides, barrier methods of contraception and lubricants contain chemicals that may affect the screening test.
Taking a sample from a menopausal or post-menopausal person
People may suffer discomfort due to vaginal atrophic changes that lead to vaginal dryness. In these situations, use an appropriately sized and well lubricated speculum. Avoid applying lubrication to the tip of the speculum.
If the vagina is atrophic and lubrication is not adequate for insertion of the speculum or visualisation of the cervix, a short course of intravaginal oestrogen may be prescribed if not contraindicated. This helps to restore the vaginal epithelium so that a speculum may be passed and an adequate sample taken. Topical oestrogen should be stopped 48 hours ahead of taking the cervical sample.
Taking a cervical sample following a gynaecological clinical procedure
If any gynaecological clinical procedure (including a previous cervical screening test or fitting of an intra uterine contraceptive device) has taken place which could potentially interfere/remove cells from the cervix there is an increased risk of an inadequate result. Therefore, cervical screening should be postponed for 12 weeks post-procedure in order for the cells to regenerate.
If an intra uterine contraceptive device (IUCD) is fitted you may see the threads from the device when taking a sample. Take care not to tangle the threads or remove the IUCD. If you inadvertently dislodge or remove the IUCD, refer to a clinician on site and provide the person with contraceptive advice
If an IUS/IUD is being removed or inserted, take the cervical sample first.
Cervical screening after termination of pregnancy, miscarriage, post-natal or previous inadequate sample
Eligible people due for cervical screening should wait 12 weeks after a termination of pregnancy, miscarriage, vaginal delivery or previous inadequate sample. This is to allow the cervical epithelium to regenerate and avoids a false result.
HPV positive result following private screening
Individuals who have a private HPV screening test remain eligible for NHS testing at appropriate intervals dependent on their age. If a person has received a positive HPV result from a private screening provider and is due or overdue NHS screening, a cervical screening appointment can be made. The NHS screening result will inform future clinical management (not the private test result).
Note: Private HPV tests are not recorded on the cervical screening call and recall I.T system or acted upon.
For those individuals who are not eligible or not due for screening, the private HPV test will not affect the timing of their next NHS cervical screening invitation.
Cervical screening for individuals who have never been sexually active
Evidence suggests that if an individual has never been sexually active or exposed to the hrHPV virus then their risk of developing cervical cancer is very low, however, this does not mean there is no risk.
Overall, nearly all cases of cervical cancer (99.7%) are caused by hrHPV. HrHPV is transmitted from any kind of skin-to-skin contact of the genital area, not just from penetrative sex.
This includes:
- vaginal, oral or anal sex
- any skin-to-skin contact of the genital area
- sharing sex toys
If an individual has never been sexually active, their request for screening should be considered on a case by case basis with their risk fully explained, in order for them to make an informed decision.
Family history of cervical cancer and frequency of screening
Nearly all cervical cancers are caused by the persistent presence of hrHPV which is passed on through any type of sexual contact with a man or woman. As yet there is no proven link to suggest that cervical cancer runs in families. Individuals whose close relatives have had cervical cancer or abnormal cells removed are not thought to be at higher risk of developing cervical cancer themselves. We therefore do not recommend screening more frequently than the routine intervals.
Screening for individuals with Peutz-Jeghers syndrome
There is no enhanced screening available for individuals diagnosed with Peutz-Jeghers syndrome, and there is no evidence that it would be advantageous for those with this condition. Individuals should have regular screening when invited, and if the sample taker has any concerns then these should be addressed as part of the clinical investigation and follow up.
HPV Self-Sampling home testing kits
The purchase of HPV self-sampling home testing kits is increasing. Results of these private tests will not be acted on by the NHS CSP and cannot be recorded in an individual’s NHS screening record. If a private test result is positive, the person should be advised that having HPV does not mean they have or will get cervical cancer.
Individuals eligible for the NHS CSP remain so, even if they have had a private test. Most hrHPV infections clear themselves without causing problems. If someone has a persistent hrHPV infection, it will be identified when they accept their next NHS CSP invitation. Cervical cancer usually develops slowly over 10 years, in three stages: infection with hrHPV; development of abnormal cells if the immune system does not rid the hrHPV infection; and development of cancer if abnormal cells are not treated.
13. Laboratory terminology and management guidelines
This section explains screening result codes and guidance on management.
Colposcopy and programme management pathways are available, summarising the pathways for:
-
cervical screening
-
colposcopy
-
management of an abnormal colposcopy result
All cervical samples undergo a primary hrHPV test. People who have an hrHPV negative result are returned to routine recall (3 or 5 years depending on age). No further testing is needed for the episode, except if the person had a previous abnormal result. In this case they will follow the cervical screening: colposcopy and programme management pathway.
Samples reported as hrHPV positive are cytology screened. If they are cytology negative the person is recalled in 12 months for a further cervical hrHPV test. If the test shows abnormal cytology (any grade) the person is directly referred to colposcopy.
If a person has a second hrHPV positive cytology negative result, they are again recalled in 12 months’ time. If a person has a third hrHPV positive, cytology negative result, at 24 months they are directly referred to colposcopy. If they are hrHPV negative, cytology negative result, at 24 months they are returned to routine recall.
The cervical sample is used to detect hrHPV (DNA or RNA depending on local laboratory technology) and the same sample is processed to produce a slide for cytology screening if hrHPV positive.
People who have had treatment for CIN or CGIN in colposcopy will have a test of cure (ToC) cervical sample test at 6 months after treatment (routinely taken in community). A primary hrHPV test is performed with cervical cytology if the hrHPV test is positive. Any people who test hrHPV positive should be re-referred to colposcopy regardless of the cytology result.
13.1 Result, and action codes used in primary hrHPV testing with cytology triage are described below.
13.1.1 hrHPV result codes
Ø (zero) hrHPV negative
9 (nine) hrHPV positive
U hrHPV result unavailable
Please note: Code Q (no hrHPV test carried out due to recent positive hrHPV test) is retired although it may be seen in a person’s screening history.
13.1.2 Cytology result codes
X No cytology required
Ø * ?glandular neoplasia (non-cervical)
1 inadequate
2 negative
3 low grade dyskaryosis
4 high grade dyskaryosis (severe)
5 high grade dyskaryosis ?invasive squamous carcinoma
6 ?glandular neoplasia of endocervical type
7 high grade dyskaryosis (moderate)
8 borderline change in squamous cells
9 borderline change in endocervical cells
Please note: * non-cervical neoplasia treated as negative for NHSCSP management.
Dyskaryosis is the technical term used to describe cellular abnormalities identified cytologically. The corresponding term for histological abnormalities is CIN (Cervical Intraepithelial Neoplasia).
13.1.3 Action codes
A routine recall
R early repeat (in 3, 6, 12 or 36 months)
S suspended from recall
H no action (for example, private tests) *
Please note: * Code H means ‘no action’ (or ‘do not change next-test-due-date’). It must only be used for negative and inadequate tests taken outside the UK cervical screening programmes. Code H is not used for non-NHS tests that are abnormal.
Valid code combinations can be seen in Table 2: Valid cytology result, HPV result and action code combinations.
13.1.4 Histology result codes
Typically, the histological and cytological terms correlate as follows (although people may have a greater or lesser degree of Cervical Intraepithelial Neoplasia (CIN) on biopsy than initially suggested by cytology):
CIN1 = Low grade dyskaryosis
CIN2 = High grade dyskaryosis (moderate)
CIN3 = High grade dyskaryosis (severe)
13.2 13.2 Results and management protocol
| hrHPV primary screening results, recall interval and or action | |
|---|---|
| Primary screening result | Recall interval |
| hrHPV negative | Routine recall |
| hrHPV positive, cytology negative | Repeat test in 12 months (unless 3rd occurrence – refer to colposcopy) |
| hrHPV result unavailable or hrHPV positive, cytology inadequate | Repeat test no sooner than 3 months (if this is a 2nd consecutive of this result– refer to colposcopy) |
| hrHPV positive, cytology low grade (mild dyskaryosis) | Refer to colposcopy |
| hrHPV positive, cytology low grade (borderline change in squamous cells) | Refer to colposcopy |
| hrHPV positive, cytology low grade (borderline change in endocervical cells) | Refer to colposcopy 2 week rule |
| hrHPV positive, cytology high grade (moderate) | Refer to colposcopy 2-week rule |
| hrHPV positive, cytology high grade (severe) | Refer to colposcopy 2-week rule |
| hrHPV positive, cytology high grade (?invasive squamous carcinoma) | Refer to colposcopy 2-week rule |
| hrHPV positive, cytology ?glandular neoplasia of endocervical type | Refer to colposcopy 2-week rule |
| hrHPV positive, cytology ?glandular neoplasia (non-cervical) The presence of a ?non-cervical glandular abnormality may originate from a part of the genital tract other than the cervix and requires urgent gynaecological referral by the sample taker | Sample taker refer to gynaecology 2 week rule |
| Test of cure protocol 6 months after treatment for CIN in colposcopy. All people remain at risk after treatment for CIN and should be followed up in community with ToC sample as shown below | |
| hrHPV negative | Recall in 36 months regardless of age |
| hrHPV positive, cytology negative, inadequate or abnormal | Refer to colposcopy |
| Test of cure protocol 6 months after treatment for CGIN in colposcopy. All people remain at risk after treatment for CGIN and should be followed up as shown below | |
| hrHPV negative (first test after treatment) | Recall in 12 months |
| hrHPV negative (second test after treatment and first result hrHPV negative) | Recall in 36 months regardless of age |
| hrHPV positive | Refer to colposcopy* |
*If cytology normal or inadequate and colposcopy normal, will be discharged for recall in 12 months
If cytology abnormal and colposcopy normal / no further treatment, will be subject to annual recall for 10 years. For an overview, See the screening and colposcopy pathways.
13.3 Colposcopy referral and the GP’s responsibility
GPs that provide cervical screening services in accordance with the GMS contract are responsible for making sure that the test result is known and followed up appropriately and refer a person for further investigation and treatment where necessary (for example, a person needing colposcopy who has moved from another area, or a person who has been discharged following a previous non-attendance at colposcopy). All laboratories operate a direct referral system for colposcopy in conjunction with their local colposcopy clinics. The GP still has a responsibility to make sure that colposcopy has taken place (see Cervical Screening Failsafe Guidance.)).
13.4 Colposcopy referral and the laboratory’s responsibility
The NHS CSP has implemented direct referral to colposcopy. This is defined as referral directly from the cytology laboratory to colposcopy. This process has several advantages: it speeds up the patient journey, enables better management of clinics and so reduces waiting lists.
All laboratories operate a direct referral system for colposcopy in conjunction with their local colposcopy clinics. In areas using this system, the GP still has a responsibility to make sure that colposcopy has taken place.
It is the sample taker’s responsibility to make sure a person has the correct follow up and management as advised in the cervical screening report. The sample taker should respond to any failsafe enquiries from the cervical screening laboratory or colposcopy clinic and document any correspondence or communication with the person regarding follow up.
Colposcopy Programme Management Guidance is available.
13.5 Cervical Screening Reports
13.5.1 hrHPV negative results
Samples that test negative for hrHPV are classified as ‘negative’. People who receive a negative result are returned to routine recall unless on:
- the test of cure (TOC) pathway
- the untreated CIN1 pathway
- follow-up for incompletely excised CGIN/SMILE or cervical cancer
- follow-up for borderline changes in endocervical cells
13.5.2 hrHPV positive results and negative cytology
Negative cytology
People who are hrHPV positive and have negative cytology as part of routine primary hrHPV screening should have the hrHPV test repeated at 12 months. If their hrHPV test is negative at 12 months, they are returned to routine recall.
People who remain hrHPV positive and cytology negative at 12 months should have a repeat hrHPV test in a further 12 months.
People who become hrHPV negative at 24 months are returned to routine recall.
As part of the TOC pathway, people who are hrHPV positive and have negative cytology are referred to colposcopy.
13.5.3 Referral on the basis of consecutive hrHPV positive samples as part of primary hrHPV screening
People who remain hrHPV positive with cytology reported as borderline dyskaryosis or worse at 12 or 24 months are referred to colposcopy.
People who remain hrHPV positive, cytology negative or inadequate at 24 months are referred to colposcopy.
People who are hrHPV positive and non-cervical ?glandular neoplasia is detected require referral to gynaecology. Follow-up of the hrHPV result is managed in the same way as those with hrHPV positive cytology negative results.
13.5.4 hrHPV positive results and abnormal cytology
People who are hrHPV positive and have abnormal cytology are referred to colposcopy.
13.5.5 Benign endometrial cells in cervical samples
Benign endometrial cells are only reported in samples tested as hrHPV positive from people aged 45 or over. Management recommendations made by the programme are based only on the cervical abnormalities.
The significance of cytologically benign endometrial cells in cervical samples varies with the phase of the menstrual cycle, medication, clinical history and age of the person. However, in a population-based cervical screening programme, some, if not most, of the information listed above is often unavailable. This should be reflected in the clinical management advice provided. For example, if the day of the menstrual cycle is not known and the sample is otherwise negative, it should be reported as negative, with a comment such as ‘Endometrial cells are present but menstrual history not stated. If there is any history of abnormal vaginal bleeding, referral for a gynaecological opinion should be considered.’
13.5.6 Inadequate samples
Referral on the basis of consecutive inadequate samples
When the hrHPV test result is unavailable or cytology is inadequate at any screening episode in the pathway, the sample must be repeated in no less than 3 months. At the repeat test, people are referred for colposcopy if they have:
- · inadequate cytology at the repeat test following 2 previous hrHPV positive/cytology negative tests
- · 2 consecutive hrHPV unavailable or inadequate cytology results, in any combination
People referred following 2 consecutive hrHPV unavailable or inadequate cytology results
Following a referral due to 2 consecutive screening tests reported as either hrHPV unavailable or cytology inadequate, people who have a normal and adequate colposcopy examination are followed up in the community at 12 months.
If hrHPV testing is negative at 12 months, people are returned to routine recall.
When colposcopy is inadequate, people should have a repeat screening test and colposcopy examination in 12 months. If the repeat colposcopy is normal and hrHPV negative, the person is discharged to routine recall. If the colposcopy is abnormal, management is as set out in national protocols.
13.5.7 Treatment and follow-up
Follow-up after treatment for cervical intraepithelial neoplasia (CIN) and test of cure
People who have been treated for CIN are at increased risk of developing cervical cancer. Research published in 2016 and 2017 indicates they should be returned to community-based follow-up, irrespective of their excision margin status. A cervical sample should be taken 6 months after treatment. Following that sample:
- people who are negative for hrHPV are recalled for a repeat cervical sample in 3 years
- people who are positive for hrHPV are referred to colposcopy; a reflex cytology sample will be processed to help inform colposcopy
Follow-up after treatment for cervical glandular intraepithelial neoplasia (CGIN) and test of cure (TOC)
People who undergo excision for CGIN are at risk of recurrence. If the CGIN has been completely excised at the time of first excision or subsequent re-excision, a test of cure (TOC) sample is taken 6 months after treatment.
The location for follow-up TOC (colposcopy or community) should be decided at the multidisciplinary team (MDT) meeting. If negative for hrHPV a second TOC sample is taken 12 months later (18 months after treatment). If this is also negative for hrHPV the person is discharged or returned to recall in 3 years.
If at 6 or 18 months after treatment the TOC sample is positive for hrHPV, the laboratory makes a direct referral to colposcopy. A reflex cytology sample is processed to help inform colposcopy.
If the person fails TOC at 6 months only because of a positive hrHPV test (no abnormality detected at colposcopic examination), the person should have a second TOC sample 12 months later. If this sample is negative for hrHPV the person is discharged to recall in 3 years. Further recall will depend on the result of this test and the age of the person.
If a positive cytology result is reported in either of the 6 or 18-months TOC samples, the laboratory makes a direct referral to colposcopy. If no colposcopic abnormality is present and re-excision is not appropriate, the person reverts to follow up for 10 years of annual hrHPV testing.
People who have incompletely excised CGIN and have declined re-excision are followed up in the colposcopy clinic. hrHPV testing should be performed 6 months after treatment. If the result is negative, the test is repeated 6 months later (12 months after treatment) and then annually for the subsequent 9 years. People who have hrHPV positive results require referral to colposcopy regardless of the outcome of the cervical cytology test result. All CGIN cases must be discussed at the colposcopy MDT.
13.5.8 Guidelines for cytological follow up after hysterectomy
Vault sampling is not part of the routine screening programme. People who have had a hysterectomy with CIN present are potentially at risk of developing vaginal intraepithelial neoplasia (VaIN) and invasive vaginal disease. There is no clear evidence that colposcopy increases the detection of disease on follow up. Responsibility for implementing follow up policies rests with the treating gynaecologist and will be informed by the local lead colposcopist.
Following hysterectomy:
- people on routine recall and with no CIN in their hysterectomy specimen, do not require a further vaginal vault sample
- people who have completely excised CIN should have vaginal vault sample at 6 months following their hysterectomy - if they have a negative hrHPV result they can be discharged
- people who have completely excised CIN and are hrHPV positive cytology negative at 6 months are referred to colposcopy; - if there is no evidence of VaIN at colposcopy they can be discharged
- people who have incompletely excised CIN (or uncertain excision), primary hrHPV screening follow up should be as follows:
- CIN 1: vault sample at 6, 12 and 24 months
- CIN 2/3: vault samples at 6 and 12 months followed by 9 annual vault samples
- follow up for incompletely excised CIN continues to age 65 or until 10 years after surgery (whichever is later)
- any gynaecologist discharging a patient who requires further vault samples should ensure that the GP receives clear written guidance for follow up
- the clinician in charge (gynaecologist or GP) is responsible for failsafe mechanisms for this small group of people
People who undergo subtotal hysterectomy still have their cervix in situ, and so must remain within the cervical screening programme.
In addition, people who have radical trachelectomy as part of conservative management of cervical cancer should remain under the care and guidance of their treating gynaecologist or gynaecological oncologist. Follow up is recommended with colposcopy and hrHPV testing. Owing to the limited information on outcome however, all cases should be subject to local audit at the diagnosing trust. These people are under the individual care of a gynaecologist and are no longer within the cervical screening programme. Therefore, local commissioning arrangements need to be put in place.
14. Equality of access to cervical screening
14.1 Cervical screening for lesbian and bisexual women
Lesbian and bisexual (LB) women are less likely to have been for cervical screening. Figures show that 15% of LB women over 25 have never had a cervical screening test compared to 7% of women over 25 in general. This lower uptake is partly due to the misconception that women who only have sex with women do not require cervical screening tests. More information on potential barriers to screening for LB women is available in the guidance ‘Cervical screening for lesbian and bisexual women’.
Screening providers should encourage the attendance of lesbian and bisexual women. Sample takers should avoid assuming that all women attending are heterosexual (for example when asking about contraception). Information on cervical screening for lesbian and bisexual women is available, and Jo’s Cervical Cancer Trust has a useful blog on ‘smear tests for lesbian, gay and bisexual women’.
For more information see Topic 1 Section 5 ‘Barriers to cervical screening’.
14.2 Trans men and non-binary people
Adapted from https://www.jostrust.org.uk/professionals/health-professionals/nurse-gp/trans-non-binary/barriers
Accessing cervical screening in an environment where a person feels safe and in control can be difficult for many people. This can be exacerbated for trans men and/or non-binary people with a cervix who may face personal and systematic barriers to accessing routine cervical screening, as well as discrimination because of their gender identity.
Impact
It is impossible to understate the importance of the sample taker, and the patient’s relationship with their sample taker, when it comes to a sensitive test like cervical screening. By becoming a model for trans and/or non-binary healthcare within their GP surgery or clinic, sample takers will be supporting someone to have an important health test, as well as making it more likely that they will come back again.
Barriers to cervical screening for trans men and/or non-binary people
Invitations
Only people who are registered as female with a GP surgery are invited for cervical screening via the call and recall system. This means the GP surgery or clinic needs to support trans men and/or non-binary people who are not registered as female but do have a cervix to attend for cervical screening at appropriate intervals.
Disclosure - making something new or previously unknown known.
Points to consider when creating a safe environment for disclosure:
- Avoid making assumptions
- Offer alternative ways to disclose
- Write it down
- Have a trusted person speak on their behalf – in this case, the patient may need to provide written consent
- Be discreet and respectful
- Offer or use the non-verbal methods of disclosure.
- Avoid openly naming the test that the patient is there for, which may unintentionally disclose their sex assigned at birth to others in the room.
- If you are a sample taker and want to check whether someone is there for cervical screening, invite them into a private space like the consultation room.
Confidentiality
If a patient discloses their gender identity, sample takers should have a conversation with them about recording this information. Some trans and/or non-binary patients may be anxious about having this on record, but reassurance can be offered by:
- explaining that medical records are confidential
- explaining why this information is relevant to their overall health
Negative or inappropriate reactions
Although understanding and recognition of trans and/or non-binary people has improved in recent years, prejudice and discrimination against the community is still a reality. As well as overt transphobia, this can include microaggressions, such as commenting on someone’s name or making a joke about their physical appearance.
Avoid negative or inappropriate reactions by:
- Offering appropriate training to clinical and non-clinical staff
- Being explicitly welcoming of trans and/or non-binary people
- Making it easy for trans and/or non-binary to see that they are recognised and welcomed
GP surgeries or clinics could:
- Include LGBT+ or trans-specific posters in waiting and examination rooms
- Have a note addressed to trans and/or non-binary patients on the website.
- Check which name the patient would prefer you to use
- Consider the reactions of other patients. While you can’t control how other people may react, you can check in with your trans and/or non-binary patients and be ready to step in if you witness transphobia or discrimination
Misgendering and using incorrect language
Misgendering is referring to someone by the wrong gender.
To address this, sample takers could say:
- I use these pronouns [share your pronouns – for example, ‘she/her’]. What pronouns should I use for you?
- I may need to write notes about you regarding this visit. What pronouns would you like me to use?
- What gender should I note down for you / What is your gender?
If the individual is known to the service and has transitioned or started transitioning, the sample taker could ask:
- Remind me of your name?
- Remind me what pronouns I should use for you?
- Listen to what they tell you and mirror their language.
Dysphoria - gender dysphoria describes a sense of unease or distress that a person may feel because of a mismatch between their sex assigned at birth and their gender identity.
Not everyone experiences dysphoria, but it is important to be mindful of it. It’s also okay to ask the individual whether they do, as it may be difficult for them to talk about.
Offering practical and emotional support for dysphoria:
- Agree on the language you will use
- Ask ‘How would you like me to refer to this body part?’
- Ask ‘Are there any words or language you’d like me to avoid?’
- Avoid personalising body parts. For example, instead of ‘your cervix’ say ‘the cervix’.
- Tell them they can bring someone they trust
- Provide the option of seeing and touching the equipment
- Provide the option of inserting the speculum themselves
Barriers to screening due to testosterone replacement therapy (TRT)
Vaginal atrophy can make inserting the speculum during cervical screening much more uncomfortable or painful. There are ways to lessen this, including:
- Offering a smaller speculum size
- Offering more lubrication
- Using topical oestrogen prior to screening - it is important to recognise that, as a female hormone, it may not be acceptable for a trans man and/or non-binary person
TRT can cause the clitoris to grow. As you would with all patients, avoid commenting on or reacting to their genitals unless there is a health issue you need to address.
Ensuring correct sample processing
To help avoid samples from being rejected, contact the laboratory ahead of time and let them know a sample marked as male will be sent for testing. Seek consent from the person before their screening test to ensure they are aware of this process. A record of the conversation should be recorded in the individual’s medical record.
Sample takers must include in the clinical details section of the cervical screening request form, that the sample is from a trans man and/or non-binary person with a cervix and include details of any previous screening history and treatment.
If a sample is rejected, contact the laboratory as soon as you are notified. They may still be able to retrieve and test it.
If the person is registered as female they will automatically receive the test result via the call and recall service to the address they are registered at. The implications of this will need to be discussed with the person at the time of their cervical screening appointment.
If the person is registered as male, the laboratory will return the test result to the GP surgery or clinic as they will not be uploaded onto the call and recall system. The sample taker should confirm the patient address where GP should send the result letter to and the name the patient would like the result letter addressed to. By doing this, it is avoiding unintentionally outing someone or potentially putting them in danger.
For people registered as male, the sample taker and GP surgery or clinic is responsible for communicating cervical screening results to a trans man and/or non-binary patient. In England, this includes:
-
inviting back in 3 or 5 years (1 year if HIV positive), depending on age, if the results do not show high-risk HPV
-
inviting back in 1 year if the results show high-risk HPV without cell changes
If required, the laboratory will make a direct referral to colposcopy. It is the responsibility of the GP practice to follow up with the colposcopy service and the person to ensure that the referral has been arranged and if there are any special needs to address.
Ceasing from the screening programme if/when gender reassignment surgery is complete
If an individual has undergone a total hysterectomy (including removal of the cervix) during gender reassignment surgery, they are able to withdraw from the cervical screening programme. The individual does not have to mention they are transgender if they do not wish to (it is not necessary for anyone to provide a reason for withdrawing). Anyone wishing to withdraw from the programme should follow the process for voluntary withdrawal described in the NHS Cervical Screening Ceasing Guidance.
Further information about screening for transgender people is available including reducing cervical screening inequalities for transgender people.
14.3 People with disabilities and domiciliary cervical screening
14.3.1 People with learning disabilities
For someone who lacks mental capacity to make a decision about whether or not to have screening, a best interests decision can be made on their behalf by the appropriate people (such as the person’s family, carer, and or GP).
Sample takers should understand that people with a learning disability:
- have the same right of access to cervical screening as other eligible people
- cannot be assumed to be sexually inactive, and even if they are sexually inactive, they are still entitled to cervical screening
- are entitled to accessible information to help make their own decision about cervical screening
- may require reasonable adjustments to support them having screening
If a person is not able to make a decision themselves despite additional support offered, the relevant people can make and record a best interest decision on their behalf. Sample takers should seek specialist advice if necessary. The community learning disability team should be able to support this.
An ‘easy read’ leaflet is available which was designed by and for people with a learning disability, for use with their family members or carers. The leaflet aims to help them to make their own decisions about cervical screening, and to prepare them for the screening process.
Sample takers can also direct people who cannot read or do not like written words to the Beyond Words cervical screening picture story, which includes a suggested storyline for family members, carers or health professionals to refer to.
An editable easy read version of the cervical screening invitation letter is available to download. This can be used as a template to invite people who have a learning disability. If requested, the easy read invitation letter should be sent by the GP practice along with the easy guide in addition to the standard national invitation letter and leaflet.
14.3.2 Preparation for screening someone with a learning disability
Sample takers should be aware of the importance of:
- establishing that the person has an understanding of cervical screening
- preparing the person to have the test
Inviting the person to a preliminary visit to the practice or clinic before they come for screening may be helpful. Scheduling a longer appointment time may also be beneficial, so that the person has more time to relax and become familiar with their surroundings and with the sample taker.
When the person attends for a test, the sample taker should check for behavioral signs of compliance with the procedure. This is especially important when screening people for whom it may be difficult to ascertain their level of understanding or consent. There are 6 behavioral signs to look for:
- the person is relaxed and cooperative
- the person is willing to get undressed
- the person is willing to be positioned
- the person is able to keep still
- the person is willing to accept having the speculum passed
- the person maintains awareness throughout
Lack of co-operation by the person, or distress in any way, must be recognised as their choice not to have the test. The person should be offered another appointment if they need more preparation and reassurance.
Guidance for supporting people with learning disabilities to access cervical screening is available.
14.3.3 People with a physical disability
Sample takers who work with individuals with a physical disability should be confident and experienced in taking cervical samples. A person’s physical disability may prevent them from achieving a position where the cervix can be visualised and a cervical sample taken. This may include people with severe arthritis or morbid obesity. The sample taker should consider:
- · access to the venue (can an alternative be offered?)
- · the height of the couch
- · the person’s physical limitations
- · the possibility of a domiciliary visit
- the need for assistance – a companion (e.g. relative/ carer/ friend) or a professional from the practice
- · seeking specialist advice if necessary
Where a service cannot be provided in primary care, the sample taker may need to make special arrangements, for example with the local colposcopy service, to take a sample at a clinic where a hoist is available.
14.4 14.4 Domiciliary cervical screening
GP practices have a duty of care to provide reasonable adjustments to allow all women and people with a cervix to participate in cervical screening. A risk assessment must be undertaken on a case by case basis to determine what support the practice can provide locally. Consider arrangements for onward referral where needed.
