Vaccine update: issue 347, July 2024, RSV special
Published 2 August 2024
Applies to England
Introduction of new NHS vaccination programmes against respiratory syncytial virus (RSV)
Following guidance from the Joint Committee on Vaccination and Immunisation (JCVI), from 1 September 2024 there will be 2 new RSV vaccination programmes, during pregnancy for infant protection and for older adults.
RSV has been described as “maybe the most common virus you have never heard of” by GP and journalist Dr Mark Porter. RSV, usually undiagnosed, will have caused great concern – and sometimes tragedy – for many parents whose babies have struggling to breathe and feed due to bronchiolitis or pneumonia. 20,000 infants are admitted to hospitals in England each winter due to RSV. The vast majority have no underlying medical conditions. Many need intensive care, and 20 to 30 die. RSV has wider impacts too, cancelling major paediatric surgery because critical care beds are needed to keep children with RSV breathing.
The maternal vaccine is a major step forward for child health. It offers 70% protection against severe RSV lower respiratory tract infection over the most vulnerable first 6 months of life. The Royal College of Paediatrics and Child Health has called it “game changing”.
For older adults the clinical impact is less obvious, RSV can present as exacerbations of long-term conditions as well as acute respiratory infection syndromes, but it is no less serious. The latest estimates suggest around 80,000 GP attendances, around 9,000 admissions and over 2,000 deaths a year in the over 75s. Vaccine protection for older adults looks even more promising at around 80%. Monitoring is now being done internationally to see how for many years a single jab keeps older adults protected.
So, it is fantastic to be introducing life-saving RSV vaccines to the routine immunisation schedule:
- in every pregnancy in week 28 or soon after, to protect newborn babies
- at the age of 75 years, with a catch-up programme for current 75 to 79 year olds (age on 1 September 2024)
The RSV Pre-F vaccine (Abrysvo®) has already demonstrated a good safety profile in the USA where it has been given to around 300,000 pregnant women and 3 million older adults, and we expect it will have an important impact on RSV in England this season and beyond.
The success of this programme will be due to the vital work of teams across maternity services, GP surgeries and pharmacies, including everyone advising on or offering vaccination to patients and everyone organising vaccination, from inviting patients to ordering vaccine and supporting the clinical front line.
Thank you all in advance. This is an ambitious new immunisation programme against a heavyweight pathogen, and your work will make a vital difference.
Programme for pregnant women to protect infants
All women who are at least 28 weeks pregnant on 1 September 2024 should be offered a single dose of the RSV vaccine, through commissioned services. After that, pregnant women will become eligible as they reach 28 weeks gestation, and remain eligible up to birth. The ideal opportunity to offer vaccination would be at the 28-week antenatal contact (ANC), following prior discussion at the 20-week ANC. Providers should aim to vaccinate those already eligible on 1 September as soon as possible.
Programme for older adults aged 75 to 79 years old
All adults turning 75 years old on or after 1 September 2024 will be eligible for the routine programme and should be offered a single dose of the RSV vaccine on or after their 75th birthday. A one-off catch-up campaign for those already aged 75 to 79 years old on 1 September 2024 should be undertaken at the earliest opportunity with the aim of completing the majority by 31 August 2025.
To offer the best protection, we are asking systems and providers to vaccinate as many people as possible during September and October 2024 prior to the expected RSV season. In line with JCVI guidance, individuals will remain eligible until the day before their 80th birthday, with the exception of people who turn 80 in the first year who have until 31 August 2025 to get vaccinated.
Information and guidance for healthcare practitioners in preparation for RSV vaccination
As there are two different RSV vaccination programmes starting in September (one for pregnant women for infant protection and a separate one for older adults), there are different supporting resources available for each. It is important that anyone involved in RSV immunisation is aware of which resources to use. More details on RSV and RSV vaccination are in the green book, chapter 27a: respiratory syncytial virus. Many clinical questions and scenarios are also addressed in the two information for healthcare practitioners guidance pages.
Training
As with any new programme, immunisers should have completed all the relevant training in line with the national minimum standards for immunisation before vaccinating. Like many other UK routine immunisation programmes, UKHSA has produced specific slide sets to assist with training delivery. For both RSV programmes, these training slide sets can be found alongside many other RSV resources on the gov.uk immunisation collection: RSV immunisation. These slide sets contain information about RSV, who it affects most severely and when, the JCVI recommendations about the vaccination programmes, the Abrysvo® RSV vaccine, how it works, preparation, administration and wider considerations. These slide sets are designed for both individual use and for use or adaptation during the delivery of RSV vaccination training. Trainers should select the slides required depending on the background and experience of the immunisers they are training and according to the role they will have in delivering the programme. The older adults RSV vaccine programme training slide set can be downloaded from khub. The RSV vaccination of pregnant women for infant protection training slide set can be downloaded from khub.
Ordering vaccines
Vaccines for the national RSV vaccination programmes should be ordered in England via the ImmForm website. Healthcare practitioners should refer to this website for current information on vaccine availability. As both programmes involve a year-round and not a seasonal offer of vaccination, vaccines should be ordered regularly throughout the year. To minimise wastage due to fridge failures or expiry, healthcare practitioners are reminded to order no more than 2 weeks’ worth of stock rather than over-ordering or stockpiling vaccines. Vaccines should be ordered, stored and monitored as described in the green book chapter 3: storage, distribution and disposal of vaccines. Please note, although the same Abrysvo® vaccine will be used for both the older adult and the vaccination of pregnant women programmes, they will be listed as separate items on ImmForm and the vaccine allocated for each programme should be managed independently where possible. More information can be found on ImmForm and in the RSV bipartite letter.
Patient Group Directions
Most RSV vaccines administered as part of the UK routine immunisation programmes will be administered under a Patient Group Direction (PGD). An RSV PGD template has been produced by UKHSA. It has been signed by a doctor, nurse and pharmacist, from UKHSA’s national immunisation team, who have been involved in the PGD’s development. You can view this on the PGD collection page. All PGD templates produced by UKHSA require further local authorisation in section 2 of the document before they can be used. These PGDs are not legal or valid without signed authorisation by an appropriate person, for details see the PGD collection page.
Vaccine preparation
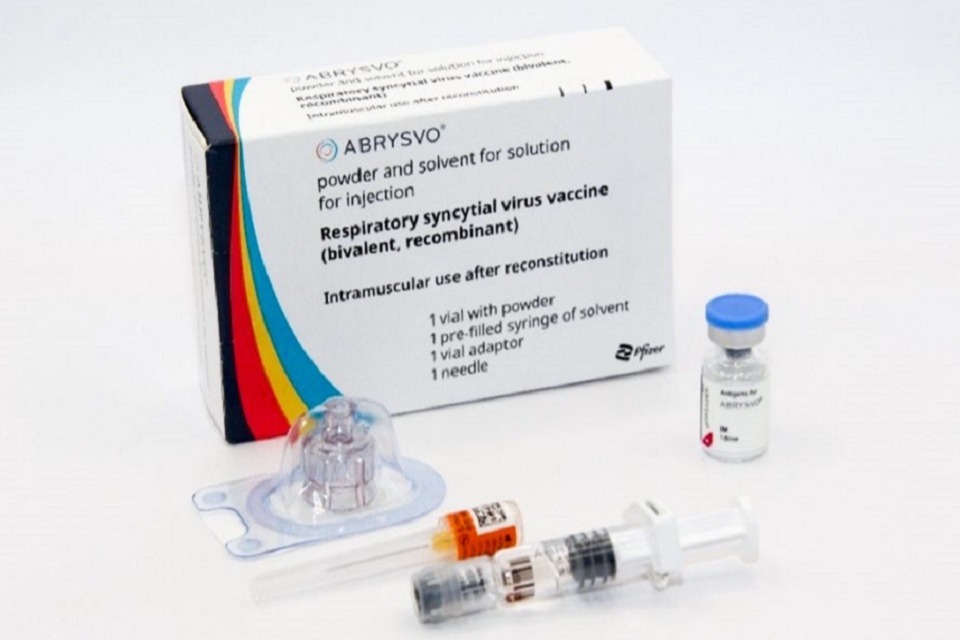
The vaccine procured and recommended for use in both programmes is the Abrysvo® vaccine which is manufactured by Pfizer. Unlike any other vaccine currently in use in the UK routine immunisation schedule, Abrysvo® requires reconstitution using a vial adaptor (which is included in the pack contents, pictured above). Immunisers are strongly advised to watch the manufacturer’s instructional video before preparing and administering the vaccine.
Information for healthcare practitioner guidance
Healthcare practitioner information and guidance to support both the RSV programme for the older adults programme and the vaccination of pregnant women to protect infants programme has been produced and can be accessed on the RSV page of the UKHSA immunisation collection.
The information for healthcare practitioners’ guidance pages are useful, practical resources and are recommended reading for healthcare practitioners involved in administering or providing advice about RSV vaccination. They include information about the vaccination programme and examples of questions and scenarios which may arise for staff involved in delivering the RSV vaccination programmes. There is information available on healthcare practitioners’ guidance for the older adults programme. There is also information available for healthcare practitioners guidance for the vaccination of pregnant women for infant protection programme.
Publications to support and promote the RSV vaccination programmes
Posters
- It’s time for your RSV vaccine poster for older adults. Available to order and download for free using product code RSVOAEN.
- Protect your baby from RSV poster for pregnant women. Available to order and download for free using product code RSVPGEN
RSV for older adults vaccination programme leaflet
Available to order and download for free using product code C24RSV01EN.
This leaflet is available in the following languages: Albanian, Arabic, Bengali, Bulgarian, Chinese (simplified), Chinese (traditional), Dari, Estonian , Farsi , French, Greek, Gujarati, Hindi , Italian, Latvian, Lithuanian, Nepali, Panjabi, Pashto, Polish, Portuguese, Romanian, Romany, Russian , Somali , Spanish, Tagalog, Tigrinya , Turkish, Twi, Ukrainian, Urdu , Yiddish and Yoruba.
This leaflet is also available in a range of accessible formats: Audio, Braille, British Sign Language and Large Print.
RSV maternal vaccination programme leaflet
Available to order and download for free using product code C24RSV03EN.
Albanian, Arabic, Bengali, Bulgarian, Chinese (simplified), Chinese (traditional), Dari, Estonian , Farsi , French, Greek, Gujarati, Hindi , Italian, Latvian, Lithuanian, Nepali, Panjabi, Pashto, Polish, Portuguese, Romanian, Romany, Russian , Somali , Spanish, Tagalog, Tigrinya , Turkish, Twi, Ukrainian, Urdu , Yiddish and Yoruba.
This leaflet is also available in a range of accessible formats:
Audio, Braille, British Sign Language and Large Print.
Vaccine Supply
Routine vaccination programme
Introduction of Abrysvo® RSV vaccine for older adults and during pregnancy for infant protection
From 1 September 2024, RSV programmes will be introduced for older adults and during pregnancy for infant protection. The same RSV vaccine, Abrysvo®, will be used for both programmes. Abrysvo® is now available to order via ImmForm.
Scottish customers should refer to local ordering guidance. Abrysvo® ordered via ImmForm will be supplied as a single-dose pack, containing one vial of vaccine, diluent for reconstitution and patient information leaflet.
Each pack will also contain one 25G x 25mm (1 inch) needle. Guidance on the choice of needle size can be found in the green book: chapter 4. If required, other sizes of needles should be obtained locally.
The pack of Abrysvo® vaccine is physically larger than most other vaccines supplied via ImmForm. Please ensure you have enough fridge capacity before placing any orders. To help with planning storage requirements, each single-dose pack measures 73mm x 35mm x 116mm (H x W x D).
When ordering Abrysvo®, orders should be placed for the ImmForm product that is specific to each RSV protection programme.
Product ordered for the infant RSV protection programme (offered to pregnant women) should not be used for the older adult RSV protection programme and vice versa. Account holders will see two different product lines and should order accordingly.
High-level ordering controls will be in place to reduce the risk of ordering errors only. These are not intended to restrict activity. Any further updates to ordering information will be published online as an ImmForm news article.
To minimise wastage due to fridge failures, please order no more than 2 weeks’ worth of stock. Details about the RSV vaccination programmes is published inthe green book: chapter 27a .
Further details about Abrysvo® vaccine can be found via the EMC Contact helpdesk@immform.org.uk for ordering queries.
Registering for a new or updating your existing ImmForm vaccine ordering account
When you register for or update an existing ImmForm account, UKHSA as a wholesaler of vaccines needs to verify the requesting customer. Please ensure you have your professional regulatory body registration number or wholesaler dealer licence and an organisation code which can be verified when requesting updates or requesting a new vaccine ordering account. For more information please see the ImmForm Helpsheet: how to register.
The EU Falsified Medicines Directive and Delegated Regulation as applicable to UKHSA-supplied vaccines for the national immunisation programme
The EU Falsified Medicines Directive (FMD) and Delegated Regulation impose legal obligations on the EU medicines supply chain to prevent entry of falsified medicinal products into the supply chain. The Delegated Regulation was implemented in all EU member states on 9 February 2019. Following the UK’s departure from the EU, the Delegated Regulation ceased to apply in Great Britain from 31 December 2020, but continues to apply in Northern Ireland.
Information for customers in Northern Ireland
FMD-barcoded packs of routine immunisation programme vaccines that are centrally supplied by UKHSA continue to be supplied with active FMD serialisation, and should be decommissioned by end users in Northern Ireland. Customers in Northern Ireland who access centrally supplied vaccines are encouraged to review local guidance on implementation of the EU FMD.
Non routine vaccine supply
Hepatitis A vaccine
Adult:
- GSK: supply of Havrix Adult PFS singles and packs of 10 are currently available
- Sanofi: Avaxim PFS singles are currently available. Avaxim packs of 10 are currently unavailable
- MSD: VAQTA Adult is available
Paediatric:
- GSK: supply of Havrix Paediatric singles and packs of 10 are currently available
- MSD: VAQTA Paediatric is available
- Sanofi: Avaxim Junior singles are currently available
Hepatitis B vaccine
Adult:
- GSK: Engerix B PFS singles and packs of 10 are currently available
- GSK: supply of Fendrix is currently available
- MSD: HBVAXPRO 10 micrograms is available
- MSD: HBVAXPRO 40 micrograms is available
- Valneva: PreHevbri is available
Paediatric:
- GSK: supply of Engerix B Paediatric singles is currently available
- MSD: HBVAXPRO 5 micrograms is available
Combined hepatitis A and B vaccine
- GSK: Twinrix Adult singles and packs of 10 are available
- GSK: Twinrix Paediatric is currently available
- GSK: Ambirix is available
Combined hepatitis A and Typhoid vaccine
Sanofi: Viatim is now a discontinued product and no longer available for sale.
Typhoid vaccine
- Sanofi: Typhim singles and packs of 10 are available
- Patientric: Vivotif is available
Rabies vaccine
- Valneva; Rabipur is available
- Sanofi: Verorab currently out of stock
Pneumococcal polysaccharide vaccine (PPV)
MSD: supply of Pneumovax 23 (PPV23) PFS is available.
Pneumococcal polysaccharide conjugate vaccine (PCV)
- Pfizer: Prevenar 13 is currently available
- MSD: Vaxneuvance is currently available
Varicella zoster vaccine
- GSK: VARILRIX is currently available
- MSD: VARIVAX is available
- MSD: ZOSTAVAX is a discontinued product
Diphtheria, tetanus, poliomyelitis (inactivated) vaccine
Sanofi: Revaxis is available.
Diphtheria, tetanus, pertussis (acellular) and (inactivated) vaccine
- GSK: supply of Boostrix-IPV is currently available
- Sanofi: Repevax is currently available
MMR
- MSD: MMR Vaxpro is currently available
- GSK: Priorix is currently available
Meningitis ACWY vaccine
- GSK: Menveo is currently available
- Pfizer: Nimenrix is currently available
- Sanofi: MenQuadfi is available
Yellow fever
Sanofi : Stamaril is available.
Human papillomavirus (HPV) vaccine
- MSD: GARDASIL has been discontinued
- MSD: Gardasil 9 is currently available
- GSK: Cervarix has been discontinued
Cholera vaccine
- Valneva: Dukoral is available
- Patientric: Vaxchora is available
Japanese encephalitis vaccine
Valneva: Ixiaro is available
Meningococcal group B vaccine
GSK: Bexsero is currently available
Diphtheria, tetanus, pertussis, hib vaccine and poliomyelitis
GSK: Infanrix IPV+Hib is currently available
Hib + meningococcal group c combined vaccine
GSK: Menitorix is currently available
Live attenuated rotavirus vaccine
GSK: Rotarix is currently out of stock – expected recovery Q4
Herpes zoster vaccine
GSK: Shingrix is currently available
Diphtheria, tetanus and pertussis
Sanofi: ADACEL is available to order without restrictions
Dengue tetravalent vaccine
Takeda: Qdenga is currently available
