JCVI final statement on phase 2 of the COVID-19 vaccination programme: 13 April 2021
Updated 13 April 2021
Introduction
The Joint Committee on Vaccination and Immunisation (JCVI) is an independent expert advisory committee which advises the UK health departments on vaccination. In December 2020, JCVI advised the vaccination of 9 key priority groups against COVID-19[footnote 1], covering all adults aged 50 years and over, and younger adults with underlying health conditions that put them at specific risk from COVID-19. This part of the programme, termed phase 1, began rollout in the UK from 8 December 2020. Phase 1 aims to reduce mortality from COVID-19, along with the protection of UK health and social care systems.
The programme has been a success in terms of delivery, with over 30 million people having received their first vaccine dose so far[footnote 2]. The COVID-19 vaccines in use in the UK are highly effective and have been shown to substantially reduce the risk of infection and severe disease from COVID-19[footnote 3]. It has been estimated by Public Health England (PHE) that over 10,000 deaths have been averted by the COVID-19 immunisation in the first 3 months of the programme[footnote 4].
The successful delivery of the phase 1 programme can be attributed to the exceptional efforts of the NHS, volunteers and community organisations and the operational simplicity of the programme. Programmes that are less complicated to organise are more readily delivered at speed and are more likely to achieve high vaccine coverage.
Data on hospitalisations due to COVID-19 indicate that a substantial number of admissions occur in people under the age of 50 years who would not have been vaccinated in the first phase of the vaccination programme.[footnote 5] [footnote 6] [footnote 7] [footnote 8] [footnote 9]
JCVI has been asked by the Department for Health and Social Care (DHSC) to formulate advice on the optimal strategy to further reduce mortality, morbidity and hospitalisations from COVID-19 disease in the next phase of the programme.
Options considered for the next phase (phase 2) of the programme include:
- direct protection of those at higher risk of serious disease and hospitalisation, including groups associated with an increased risk
- targeted vaccination to reduce transmission of COVID-19 in the population
- vaccination of occupational groups at higher risk of exposure
These options are not mutually exclusive.
Mathematical modelling of vaccination strategies for phase 2 indicates that rapid vaccine deployment is the most important means to maximise public health benefits against severe outcomes from COVID-19.[footnote 10] [footnote 11]
A strategy aimed primarily at reducing transmission of infection would take longer to achieve reductions in hospitalisations and would require very high vaccine uptake in the target populations. There is evidence that some occupations have an increased risk of morbidity due to COVID-19 and that males aged 40 to 49 years are more likely to be employed in these occupations.[footnote 12] [footnote 13]
However, a mass vaccination strategy centred specifically on occupational groups would be more complex to deliver and may require new vaccine deployment structures which would slow down vaccine delivery to the population as a whole, leaving some individuals unvaccinated for longer. Operationally, simple and easy-to-deliver programmes are critical for rapid deployment and high vaccine uptake.
Advice
This advice refers to COVID-19 vaccines authorised for use in the UK. Progress into phase 2 of the programme should be accompanied by continued efforts to extend coverage among those prioritised in phase 1 but who remain unvaccinated, and to complete delivery of second doses to all those given first doses in phase 1.
There is good evidence that the risks of hospitalisation and critical care admission from COVID-19 increase with age. In occupations where the risk of exposure to SARS-CoV-2 is potentially higher, persons of older age are also those at highest risk of severe outcomes from COVID-19. JCVI therefore advises that the offer of vaccination during phase 2 is age-based starting with the oldest adults first and proceeding in the following order:
-
all those aged 40 to 49 years
-
all those aged 30 to 39 years
-
all those aged 18 to 29 years
In individuals aged 18 to 49 years there is an increased risk of hospitalisation in males, those from certain ethnic minority backgrounds, those with a body-mass index (BMI) of 30 or more (obese or morbidly obese), and those experiencing socio-economic deprivation.[footnote 5] [footnote 6] [footnote 7] [footnote 8] [footnote 9] [footnote 14]
JCVI strongly advises that individuals in these groups promptly take up the offer of vaccination when they are offered, and that deployment teams should utilise their understanding of local health systems and demographics, combined with clear communications and outreach activity, to promote vaccination in these groups.
Unvaccinated individuals who are at increased risk of severe outcomes from COVID-19 on account of their occupation, male sex, obesity or ethnic background are likely to be vaccinated most rapidly by an operationally simple vaccine strategy.
Reactogenicity
Typical side effects from vaccination include injection site pain, headache or mild fever. Such side events are relatively common and are usually mild to moderate in intensity and short-lived.[footnote 15] The frequency of these events is associated with age, with the effects seen most commonly in younger adults. Events typically resolve within a few days of vaccination. Given the frequency of mild to moderate reactions in younger adults, JCVI advises that those attending for vaccination should be informed of the frequency of reactions and that paracetamol can be used in ameliorating symptoms.
Special considerations
There have been reports of an extremely rare adverse event of concurrent thrombosis (blood clots) and thrombocytopenia (low platelet count) following vaccination with the first dose of AstraZeneca ChAdOx1 nCoV-19 vaccine (AZD1222). There has been no signal for thrombosis with thrombocytopenia following receipt of other COVID-19 vaccines approved for use in the UK (Pfizer/BioNTech and Moderna). Given the very low numbers of events reported overall, there is currently a high level of uncertainty in estimates of the incidence of this extremely rare adverse event by age group. The available data suggests there may be a trend for increasing incidence of this adverse event at younger ages. In contrast, the risk of severe disease associated with COVID-19 increases steeply with age, with the youngest adults at lowest risk. There are currently no known risk factors for this extremely rare condition, which appears to be an idiosyncratic reaction on first exposure to the AstraZeneca COVID-19 vaccine.
Alternatives to the AstraZeneca COVID-19 vaccine currently approved for use in the UK include the Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 vaccines. JCVI has weighed the relative balance of benefits and risks, and advise that the benefits of prompt vaccination with the AstraZeneca COVID-19 vaccine far outweigh the risk of adverse events for individuals aged 30 years and over or those at any age who have underlying health conditions which put them at higher risk of severe COVID-19 disease.
JCVI currently advises that in phase 2, it is preferable for adults aged 18 to 29 years without underlying health conditions that put them at higher risk of severe COVID-19 disease, to be offered an alternative to the AstraZeneca COVID-19 vaccine, if available. People may make an informed choice to receive the AstraZeneca COVID-19 vaccine to receive earlier protection.
Further details on contra-indications, precautions and scheduling will be outlined in the Green Book: immunisation against infectious disease.
Operational flexibility
Having reviewed evidence on vaccine uptake by region, age, ethnicity, gender, and index of multiple deprivation, there are concerns around lower uptake in some groups, particularly certain ethnic minority groups. Issues affecting uptake can include access to vaccination and confidence in the programme. JCVI supports flexibility in delivery of the programme to ensure every opportunity is utilised to offer vaccine in groups with lower uptake. For example, this could include a wider offer of vaccine in settings such as religious and ethnic minority community centres, or when vaccine is delivered to those in multigenerational households. Local and national public health agencies should be consulted around how best to utilise this flexibility to maximise uptake in groups with low vaccine uptake.
JCVI appreciates that a flexible approach, where decisions are taken in consultation with national or local public health experts, may also be required for operational considerations such as:
-
vaccine product storage, transport and administration constraints
-
exceptional individualised circumstances
-
availability of suitable approved vaccines, for example for specific age cohorts
-
minimisation of vaccine wastage
Background and considerations
Hospitalisations
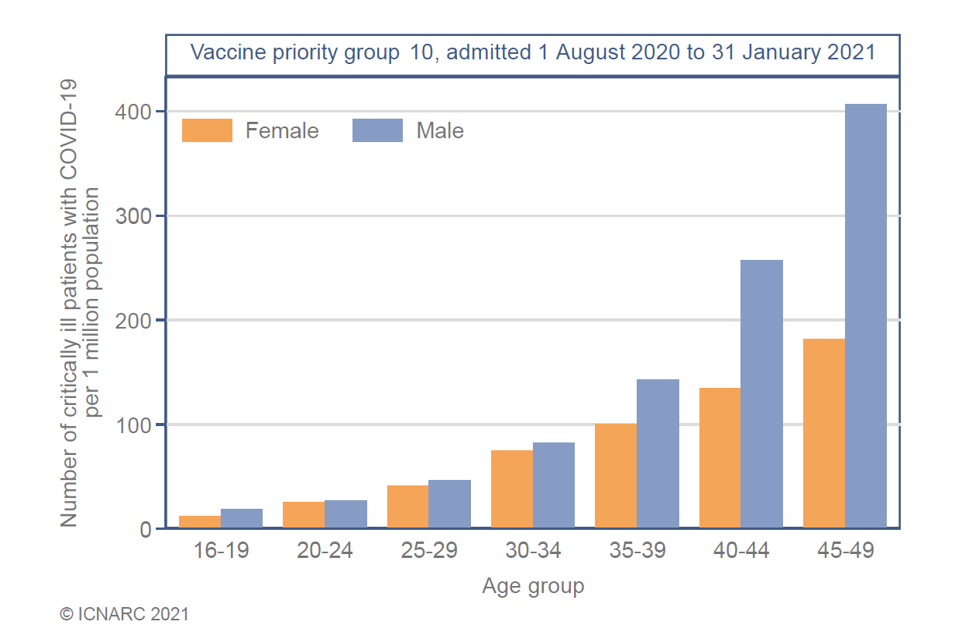
Evidence from ICNARC, ISARIC/CO-CIN, SARi-watch, and OpenSAFELY([footnote 5] [footnote 6] [footnote 7] [footnote 8] [footnote 9]) all indicate that the risk of hospitalisation and critical care admission from COVID-19 increases with age. Those at highest risk of hospitalisation outside of cohorts 1 to 9 (phase 1) are those aged 40 to 49 years, with the risk reducing with descending age. An example of this evidence is provided in Figure 1.
The evidence also indicates that males, persons from certain ethnic minority backgrounds, people in lower socioeconomic groups, and those with a body-mass index (BMI) of 30 or over, are at higher risk of hospitalisation (see below).
Figure 1: profile of critical care admissions with COVID-19, 1 August 2020 to 31 January 2021 [footnote 16]
Male sex
Evidence on hospitalisations indicates an increased risk of hospitalisation in males, particularly those aged 40 to 49 years (Figure 1). This risk may be associated with male sex itself, exposure to infection, occupation, and/or other factors.
JCVI strongly advises that males promptly take up the offer of vaccination.
Persons from ethnic minority backgrounds
People from certain ethnic minority backgrounds are at higher risk of hospitalisation from COVID-19.[footnote 5] [footnote 6] [footnote 7] [footnote 8] [footnote 9] [footnote 14] This follows a similar pattern to data on mortality reviewed in considerations relating to phase 1 of the programme. There is no strong evidence that ethnicity by itself (or genetic characteristics) is the sole explanation for observed differences in rates of severe illness and deaths. Between waves 1 and 2 of the pandemic, changes in the risk of infection and mortality in people from ethnic minority backgrounds, and within ethnic minorities, have been observed[footnote 17]. Such large rapid changes are unlikely to be due to biological factors and are more likely to be related to environmental and behavioural changes.
Discussions with ethnic minority communities and opinion leaders highlight the importance of building trust and the avoidance of stigmatisation or discrimination when planning the delivery of vaccination programmes.[footnote 18] [footnote 19] Thus far, in phase 1 of the vaccination programme, slower coverage has been noted in persons from certain ethnic minority backgrounds.[footnote 20]
Well-recognised factors that impact on vaccine uptake are:
- access to vaccination
- vaccine confidence (safety and efficacy)
- perception of risk from disease
The reasons for slower coverage in people from certain ethnic minority backgrounds are unclear at present, but there is evidence that addressing structural issues related to access and involving community influencers can have a positive impact.
JCVI strongly advises that priority is given to the deployment of vaccination in the most appropriate manner to promote vaccine uptake in persons from ethnic minority backgrounds who have not yet been vaccinated.
This may include planning to enable easy access to vaccination sites, supported engagement with local ethnic minority community and opinion leaders, and tailored communication with local and national coverage. As appropriate, these efforts should consider a longer-term view beyond the current COVID-19 mass vaccination programme and seek to address inequalities which already exist across the wider immunisation programme.
JCVI strongly advises persons from ethnic minority backgrounds who have not yet been vaccinated to promptly take up the offer of vaccination.
Underlying health conditions
Evidence indicates that individuals aged 18 to 49 years who have a BMI over 30 (obese or morbidly obese) ([footnote 5] [footnote 6] [footnote 7] [footnote 8] [footnote 9]) are at higher risk of hospitalisation compared with age matched peers. Only very modest increased risks for hospitalisation were identified in persons with certain other underlying health conditions not already covered in phase 1 of the programme. As the risk is also age associated, JCVI continues to advise that an age-based programme is the best way of delivering vaccine to these populations.
Occupational exposure
While many have been able to work at home during the pandemic, some occupations are not compatible with home working and cannot be undertaken without interaction with other people. In these circumstances individuals may be exposed to SARS-CoV-2. These include workers who have public-facing roles, or who work in close contact with co-workers. Commuting to a workplace using public transport constitutes a potential additional risk of infection.
JCVI has reviewed data to understand the association between occupation and the risk of exposure to SARS-CoV2, the risk of COVID-19 disease and the risk of COVID-19 related severe outcomes, including mortality.
The evidence indicates that certain occupations have a higher risk of exposure, and these are more likely to be occupations involving frequent contact with multiple other people in enclosed settings. These encompass the elementary occupations, manufacturing, processing and those working in the caring, leisure and a broad range of service occupations. Where increased risk of serious disease is evident, this is considered likely to be associated with a combination of various risk factors for a) exposure and b) poorer outcomes including:
- older age
- an overrepresentation of certain underlying health conditions in those undertaking certain jobs
- socioeconomic deprivation
- household size
- inability to work from home
Occupational risk associated with poorer outcomes from COVID-19 has predominantly affected men aged 40 to 49 years (please see Annex A for more details).
Delivery of a programme targeting occupational groups is recognised to be operationally complex given a number of key factors:
-
robust data on the infection exposure risk for every occupational group, or in every occupational setting, are not available
-
occupation is not routinely recorded within primary care records and these records may not be up to date
-
advice to target certain occupations could be considered discriminatory towards those in occupations where no data are available or that are not accurately listed within primary care records
-
workplaces that may be associated with higher exposures to infection may include individuals from multiple occupational groups
Overall, JCVI considers that an operationally simple, and thereby rapidly deliverable, age-based programme starting with those aged 40 to 49 years is the optimal way to protect individuals, working in jobs with a potentially higher risk of exposure to SARS-CoV2, from severe disease related to COVID-19.
Transmission
Data is accumulating to indicate that vaccination results in some reduction in asymptomatic infection. This in turn may be inferred as evidence to suggest some impact on reducing transmission of infection as well.[footnote 21] [footnote 22] [footnote 23] Accordingly, vaccination may not only protect the person being vaccinated, but also reduce transmission to others, including to older and more vulnerable adults.
The probable effect of vaccination on transmission suggests that targeting groups more likely to interact with multiple other individuals could have some added impact on limiting the spread of COVID-19. Data on mixing patterns generally indicate that people mainly mix with others of the same age group, with a higher frequency of contact among those who are younger.[footnote 24] In addition, members of certain occupations are required to interact with the general public or have frequent close contact with co-workers. However, which groups contribute most to viral transmission is currently not well defined. Furthermore, mathematical modelling of strategies for phase 2 vaccination indicates that an approach designed to protect the population primarily through interruption of transmission would afford modest benefit at best. Speed of vaccine deployment is the single most important factor for an optimal programme that maximises public health benefit. As evidenced in phase 1, a simple age-based programme is considered the keystone of rapid vaccine deployment. Maintaining this structure through phase 2 of the programme should enable the high pace of vaccine deployment to continue, with any consequent reduction in transmission considered a potentially important complementary benefit.
Children
JCVI has started to consider evidence on the risk of serious disease in children, the role children may play in transmission, and the safety and efficacy of COVID-19 vaccines in children. Following infection, almost all children will have asymptomatic infection or mild disease. No vaccines are currently authorised for use in children. As evidence becomes available it will be reviewed and advice offered as appropriate.
Post-acute COVID-19 syndrome
Prolonged symptoms after acute infection with SARS-CoV2 have been described in people of all ages, including healthy young adults. At present there are limited data on the aetiology, extent, duration and severity of these symptoms. Studies are ongoing to better define the nature of post-acute COVID-19 syndrome, and to identify any factors which could predispose individuals to it. Existing evidence is inadequate to structure a vaccination policy specifically targeting populations at risk of post-acute COVID-19 syndrome.[footnote 25]
As COVID-19 vaccination reduces the risk of acquiring SARS-CoV2 infection, it will reduce the risk of vaccinated individuals developing post-acute COVID-19 syndrome.
References
-
Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 30 December 2020. ↩
-
PHE monitoring of the effectiveness of COVID-19 vaccination. ↩
-
COVID-19 vaccines have prevented 10,400 deaths in older adults. ↩
-
ICNARC data on hospitalisations in those aged under 50 years (unpublished). ↩ ↩2 ↩3 ↩4 ↩5
-
BMJ. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. ↩ ↩2 ↩3 ↩4 ↩5
-
ISARIC data on hospitalisation in those aged under 50 years (unpublished). ↩ ↩2 ↩3 ↩4 ↩5
-
National flu and COVID-19 surveillance reports. ↩ ↩2 ↩3 ↩4 ↩5
-
OpenSAFELY analysis on the risk of hospitalisation in those aged under 50 (unpublished). ↩ ↩2 ↩3 ↩4 ↩5
-
Moore S et al. Vaccination and non-pharmaceutical interventions: When Can the UK Relax About COVID-19? SSRN; 2021. DOI: 10.2139/ssrn.3753372. ↩
-
University of Warwick modelling on options for phase 2 of the COVID-19 programme (unpublished). ↩
-
SAGE-EMG-Transmission Working Group paper – COVID-19 risk by occupation and workplace. ↩
-
Coronavirus vaccine – weekly summary of Yellow Card reporting. ↩
-
Harrison D, Rowan K – ICNARC data (unpublished) ↩
-
Bhaskaran K, OpenSAFELY. Ethnicity and COVID-19 death in the early part of the COVID-19 second wave in England: an analysis of OpenSAFELY data from 1 September to 9 November 2020 (unpublished). ↩
-
COVID-19 vaccine and health inequalities: considerations for prioritisation and implementation. ↩
-
SPI-B consideration of priorities for phase 2 of the programme. ↩
-
Voysey et al. Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine. ↩
-
Hall V et al. Effectiveness of BNT162b2 mRNA vaccine against infection and COVID-19 vaccine coverage in healthcare workers in England, multicentre prospective cohort study (the SIREN study). ↩
-
Shah et al. Risk of hospitalisation with coronavirus disease 2019 in healthcare workers and their households: a nationwide linkage cohort study. MedRxiv. ↩
-
Mossong et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. ↩
-
Maxwell E. Living with COVID-19: a dynamic review of the evidence around ongoing COVID-19 symptoms. NIHR CED 30 September 2020. ↩
