MAVIS Newsletter edition 106
Published 25 April 2018
1. News
1.1 New Director of Authorisations
The VMD is pleased to announce that the new Director of the Authorisations Division is Abigail Seager who will take up her position on 1 May 2018. Since 2016 Abigail has been Head of the EU Exit and International Office at the VMD. Before this, from 2006, she was Head of the General Assessment and Imports Team. Her previous roles include: Deputy Head of Fisheries Legislation (Defra), where she led on the establishment of the Marine Fisheries Agency (2004-6), and Senior Policy Officer in the Criminal Justice Fine Enforcement team, Ministry of Justice, where she took the new policy on fine enforcement through to primary legislation (Courts Act Bill) and implementation of the new legislation (2002-4).
1.2 Changes to Restrictions on Schedule 6 (Exemptions for small pet animals) active substances
The VMD published a position statement in MAVIS 102 precluding active substances approved for use in fish under the Small Animal Exemption Scheme being marketed for the purpose of anaesthesia. In response to feedback received and taking into consideration the availability of medicines for these species the VMD has considered the matter further and decided not to restrict the use of these active substances for anaesthesia based on the available evidence at this time.
2. Licensing
2.1 Marketing Authorisations
Information on newly authorised and expired MAs is available in the Product Information Database.
Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
You can see lists on GOV.UK of:
- recently authorised MAs (previous 6 months)
- recently updated MAs (last 30 days)
- expired products
2.2 Quarterly reporting against VMD Published Standards for 2017/18 licensing work
This report is published on a monthly basis under Our Statistics.
For further information contact Natalie Shilling n.shilling@vmd.defra.gsi.gov.uk and put ‘MAVIS’ in the subject line.
2.3 Publication of Public Assessment Reports for homeopathic products
Public Assessment Reports for homeopathic MAs are now available on the Product Information Database.
2.4 Changes to Marketing Authorisation holder, named distributor and local representative details
There are some differences in the way member states deal with changes to MA holder, distributor and local representative details. These differences are summarised below.
Guidance about partial MA holder transfers is also provided.
MA holder
In the UK, changing the legal entity of the MA holder is a Type IB variation (category A.z) dealt with on a national basis regardless of whether the MA is mutually recognised or nationally authorised. A change in legal entity will result in a change to the ‘company number’, which forms part of a product’s Vm number.
The procedure for making this change may differ between member states. Guidance on how each member state deals with this type of change is available on the HMA website.
A change to the name and/or address of an MA holder (same legal entity) is a Type IA variation, which will be dealt with on an EU basis for mutually recognised products (submitted to the RMS and affected CMSs only), or on a national basis for nationally authorised products. This is consistent across all member states.
Partial MA holder transfer
Following initial authorisation of a mutually recognised product, the MA holder may change in some member states resulting in more than one MA holder being responsible for the same authorisation. Further information is available on HMA website.
2.5 Distributor and local representative
Local representatives and named distributors perform different roles, although a single company may perform both roles for a specific MA.
In the UK, changing distributor details is a Type IB variation (category A.z) dealt with on a national basis regardless of whether the MA is mutually recognised or nationally authorised.
Whilst some member states adopt a similar approach to the UK, others deal with this change via a Type IA (C.II.6(a)) variation, which is dealt with on an EU basis for mutually recognised products (submitted to the RMS and affected CMSs only).
A change to local representative details is a Type IA (C.II.6(a)) variation dealt with on an EU basis for mutually recognised products (submitted to the RMS and affected CMSs only), or on a national basis for nationally authorised products. This is consistent across all member states.
Guidance about making the above changes is available on GOV.UK.
Further information about changes to MA holder details is also available on the HMA website.
2.6 Top ten imported veterinary medicines quarterly report from 01 January to 31 March 2018
A quarterly list of the ten products for which the most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Substance | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,582 |
| Botulism Vaccine | Clostridium botulinum type C toxoid Clostridium botulinum type D toxoid | 298 |
| Vet-Goid | Allergens | 269 |
| Spectrum Hyposensitisation Vaccine – Injectable Solution | Allergens | 261 |
| Emedog 1mg/ml Solution Injectable Pour Chiens | Apomorphine | 227 |
| Neovit B Complex oldatos injekció A.U.V | Dexpanthenol (Vitamin B5) Pyrodixine (Vitamin B6) Riboflavin (Vitamin B2) Thiamine (Vitamin B1) Cyanocobalamin (Vitamin B12) Nicotinamide (Vitamin B3) | 147 |
| Greer Allergenic Extract Patient Prescription | Allergens | 123 |
| ACTT Allergy Drops | Allergens | 88 |
| Oncept (Canine Melanoma Vaccine) | Canine Melanoma DNA | 87 |
| Antepsin 1g Tablets Sucralfato | Sucralfate | 81 |
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
Guidance is available on obtaining a UK manufacturing authorisation (ManA) for an authorised veterinary medicine or an authorisation for an extemporaneous product (special) (ManSA).
Guidance is also available on obtaining a manufacturing authorisation for an equine stem cell centre (ESCCA), a non-food animal blood bank (NFABBA) or an autogenous vaccine (AVA).
3.2 GMP inspection deficiency findings 01 April 2017 to 31 March 2018
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
‘Other’ Deficiencies Apr 17 - Mar 18
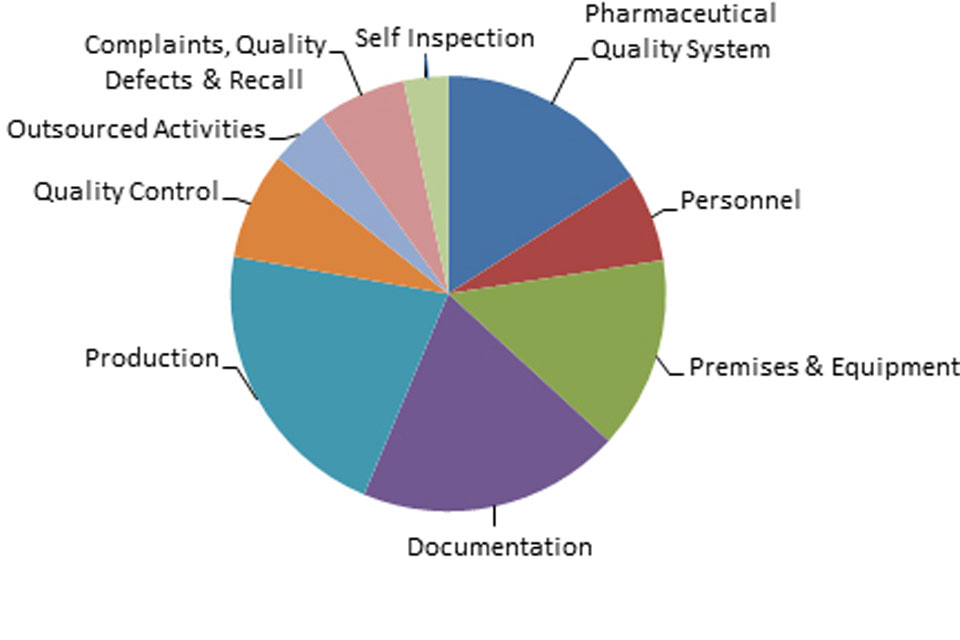
GMP Inspections Other Deficiencies 1 April 2017 to 31 March 2018
‘Major’ Deficiencies Apr 17 - Mar 18
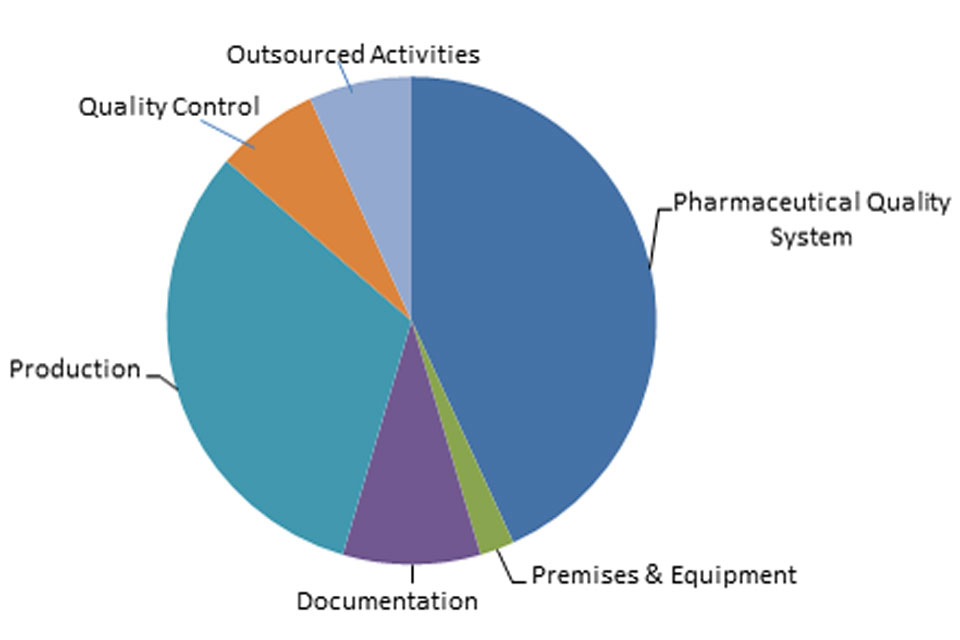
GMP Inspections Major Deficiencies March18
3.3 Wholesale, retail and feedingstuffs
Guidance is available for:
- manufacturers and distributors of animal feedingstuffs containing veterinary medicines and/or coccidiostats (referred to in the Veterinary Medicine Regulations as specified feed additives; SFAs)
- veterinary-only wholesale dealers to assess compliance against Good Distribution Practice businesses whose premises are approved for the retail supply of POM-VPS and NFA-VPS medicines by suitably qualified persons (SQP retailers)
- veterinary practice premises (VPPs), other than those accredited by the Royal College of Veterinary Surgeons (RCVS) under its voluntary Practice Standards Scheme (PSS)
3.4 Reporting Product Defects
The notification form for Marketing Authorisation (MA) holders to report product defects will soon be updated to include a short self-assessment of the risk of the defect. MA holders will be asked to declare if they consider the ‘risk’ to the product to be High, Moderate or Low. If the risk is assessed as High, further information should be submitted with the report so that a recall of the affected product can proceed quickly. A full explanation of the self-assessment method is included in the form.
An analysis of product defect reports received in 2016-17 has recently been updated.
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 December 2017 to 28 February 2018, the VMD received 1,559 animal suspected adverse event reports. Of the 2,381[footnote 1] products involved in these reports, 2,252 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,113 | Prescription Only Medicine Veterinarian (POM V) |
| 84 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 40 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 15 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 129 products were:
| Amount | Type |
|---|---|
| 54 | Authorised human medicines |
| 4 | Medicine used in a trial under an Animal Test Certificate |
| 14 | Veterinary products without any medicinal claim |
| 13 | Authorised medicines imported from other countries |
| 7 | Medicines sold under the exemption for small pet animals |
| 1 | Disinfectant product |
| 2 | Pesticide products |
| 5 | Specially formulated veterinary medicines |
| 29 | Incompletely identified products |
During this period 27 reports of human suspected adverse reactions were received. Of the 44[footnote 1] products involved in these reports, 30 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 28 | Prescription Only Medicine Veterinarian (POM V) |
| 2 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
No environmental incident reports were received during this period.
For further information contact Roy Savory r.savory@vmd.defra.gsi.gov.uk
5. Enforcement
We publish a list of prosecutions and notices involving illegal activity with veterinary medicines in the last year.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 21 February 2018 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to human and animal health. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined latest research and development in AMR with contributions from the Animal and Plant Health Agency and University of Nottingham. Minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
6.2 Sales Data and Antibiotic Resistance Surveillance Report
Data for the 2017 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report are currently being collated for publication later in the year. The report collates UK data on antibiotic sales from Marketing Authorisation Holders and antibiotic resistance data from the VMD’s surveillance programmes.
UK-VARSS 2016 and previous reports are also available.
6.3 UK AMR strategy High Level Steering Group meeting
The most recent meeting of the High Level Steering Group (HLSG) for the AMR Strategy took place on 23 March 2018. HLSG members discussed ambitions and objectives to include in the next UK AMR Strategy. The meeting is chaired by the Chief Medical Officer and is represented by cross-government department leaders, including devolved administrations.
6.4 Communications
During February, the VMD supported the Responsible Use of Medicines in Agriculture Alliance (RUMA) in their #ColostrumIsGold campaign. Using social media platforms, facts were broadcast to raise awareness on how quality colostrum delivered at the right time and of the right quantity can reduce the need for antibiotics during animals’ lifetimes, especially during the early stages. The campaign stimulated much discussion within the farming industry. The campaign was concluded with a poll inviting farmers to consider their colostrum practices and share what had been learnt from #ColostrumIsGold. Almost half of participants acknowledged they had scope to improve their practices.
In addition to this campaign, the VMD endorsed and promoted the Bella Moss Foundation survey of the public’s perception on antibiotic use in companion animals. The VMD also contributed with Defra and the British Veterinary Association (BVA) to develop and launch the “trust your vet” campaign in April this year.
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in February 2018. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2018 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
9. Organogram as at 01 May 2018
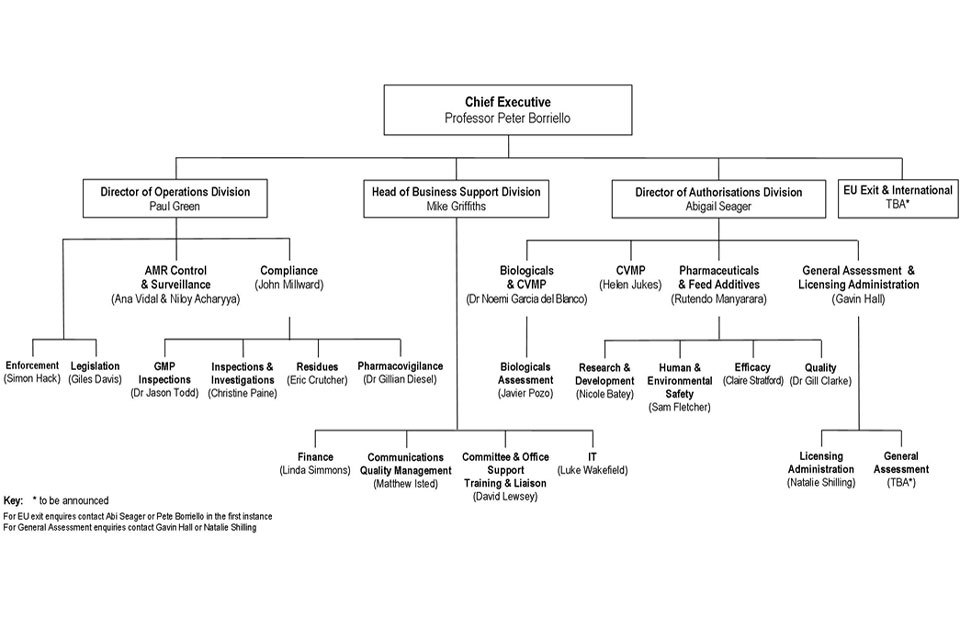
VMD Organogram as at 1 May 2018
