MAVIS edition 103
Published 26 July 2017
1. News
1.1 Invitation to attend the Open Meetings of the Veterinary Medicines Directorate (VMD) and the Veterinary Products Committee (VPC)
The VMD and VPC will hold their Open Meetings on Friday 29 September 2017 at the Animal and Plant Health Agency, Weybridge, Woodham Lane, New Haw, Addlestone, Surrey, KT15 3NB. The VMD Open Meeting will begin at 10.30am followed by the VPC Open Meeting and close by 1pm. Admission is free but will be by ticket only.
VMD staff will give presentations based on the advance questions received followed by presentations by VPC members and then an open question and answer session.
Tea and coffee will be available before and after the meeting.
Questions and requests for tickets should be sent by Friday 25 August 2017 to Chris Abbott openmeeting@vmd.defra.gsi.gov.uk. Please include the names of all attendees.
1.2 First DNA vaccine authorised in the EU
A new vaccine to protect Atlantic salmon against Salmon Pancreas Disease, for which the VMD played the lead assessment role, has been authorised across the EU through the centralised procedure. Clynav is the first DNA vaccine to be authorised in the EU.
The VMD steered the authorisation application through challenging regulatory hurdles involving complex scientific debates and negotiation, championing the adoption of this innovative DNA vaccine technology for veterinary use.
The authorisation of this vaccine has the potential to herald a new era of novel vaccines to protect animals against disease.
Clynav is marketed by Elanco Europe Ltd.
For further information contact Matthew Isted m.isted@vmd.defra.gsi.gov.uk
1.3 EU Exit stakeholder workshops – Autumn 2017
We are organising workshops for each of our stakeholder groups to discuss the implications of the UK’s exit from the EU.
Topics could include:
- Prescribing cascade
- Pharmacovigilance
- Joint labelling
- Residues surveillance plan (samples)
- Ongoing applications at time of EU exit
- Inspection of non-UK based manufacturers
Our aim is to listen to our stakeholders to understand what you consider to be the key risks and opportunities from leaving the EU and to plan for the future.
In order for us to gauge attendance levels, please express your interest by emailing events@vmd.defra.gsi.gov.uk and include any topics, in order of preference, that you would like to be discussed on the day. You will receive further information by email.
1.4 Accredited Internet Retailer Scheme 5th Anniversary
The VMD’s Accredited Internet Retailer Scheme (AIRS) has now been running for five years since its launch on 25 May 2012.
AIRS was developed in response to the general public’s concerns about buying veterinary medicines over the internet. The aim of the scheme is to provide assurance to the public and professional keepers of animals, that by purchasing their veterinary medicines from an accredited internet retailer they are:
- buying medicines from a reputable UK-based retailer
- at less risk of buying unauthorised, inappropriate or ineffective medicines for their animals
- confident that the retailer meets the requirements of the Scheme and the law
There are now 41 accredited websites operated by 32 businesses and further applications are in the pipeline. Companies whose websites have been accredited are diverse and include some well-known organisations. An up to date list can be found on GOV.UK
We have worked closely with retailers to explain the assessment criteria and what changes they need to make to their websites for compliance with both AIRS and the Veterinary Medicines Regulations. Whilst doing this, the VMD has built some very good working relationships with retailers and has gained a better understanding about how internet businesses operate. We have used this information to develop our guidance and to help applicants understand how to meet the accreditation standards.
Changes to the Scheme
With the aims of the Scheme in mind, we reviewed its requirements in 2016 and made a number of changes, in particular:
- Accreditation is now focussed on the retail supply of authorised veterinary medicines (classes POM-V, POM-VPS and NFA-VPS) only
- Non-medicinal products are no longer included in the assessment. Instead, applicants have to declare that they do not make medicinal claims for non-medicinal products on their websites
- Applicants have to acknowledge that continued accreditation is contingent on a commitment to promptly correct all non-compliances brought to their attention
We believe that these changes have simplified the accreditation process whilst maintaining the appropriate controls on the sale of medicines by internet retailers.
AIRS is open to all UK-based registered veterinary practice premises, registered pharmacies and approved SQP retailers that supply POM-V, POM-VPS and NFA-VPS veterinary medicines online.
Membership is voluntary and it’s free. Retailers who meet the accreditation criteria display the special ‘VMD Accredited Retailer’ logo with their unique accreditation number:

Accredited Retailer logo
If you wish to apply to become an AIRS member, application forms and other guidance is available on GOV.UK.
1.5 The VMD Annual Report and Accounts 2016/17
The VMD’s Annual Report and Accounts were presented to the House of Commons in accordance with Section 7(3)(c) of the Government Resources and Accounts Act 2000 and ordered by the House of Commons to be printed 12 July 2017.
The overview within the document consists of the Chief Executive’s Statement, About the Veterinary Medicines Directorate and the Performance Summary and Analysis.
The remainder of the document includes sections on Accountability, Remuneration and Staff Reports and the Parliamentary Accountability and Audited Report, and Financial Statements for the VMD.
Annual Report & Accounts 2016/17
1.6 Resignation of the Director of Authorisations
Due to resigning her position as Director of the Authorisations Division, Marie-Odile Hendrickx will be leaving the VMD at the end of July. The vacancy has been advertised. We will make any interim arrangements that may be necessary for temporary cover of the Authorisations Director post.
2. Licensing
2.1 Marketing Authorisations
We no longer publish information about varied Marketing Authorisations (MAs). Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
Information on newly authorised and expired MAs is available in the Product Information Database (PID). You can:
- go to the homepage and select the ‘Recently Authorised MAs’ tab for a list of MAs authorised in the previous 6 months
- go to the Expired tab to see a list of expired MAs
2.2 Reminder about the scheme allowing Marketing Authorisation Holders (MAHs) to obtain e-data
On 1 April 2012 MAVIS 82, we introduced a new scheme allowing MAHs to obtain electronic copies of all supporting data for their UK authorised products. This article is simply to remind you about the scheme.
Please send requests for data to s.response@vmd.defra.gsi.gov.uk including the product name and Vm number in the covering email. You will be sent an invoice for the appropriate fee by email, so please also include the email address of where you would like the invoice sent.
| Type | Price |
|---|---|
| All supporting data for one product where the data ARE NOT held on microfiche | £250 |
| All supporting data for one product where some or all of the data are held on microfiche | £750 |
For further information contact s.response@vmd.defra.gsi.gov.uk and put ‘Data Request’ in the subject line.
2.3 Summary of Company visit questionnaires from 1 April 2016 to 31 March 2017
Background
This is the eighth full year of the VMD seeking feedback from companies who request a meeting with us, on the effectiveness, accuracy and relevance of the advice provided. The outcomes for 2016/17 are similar to previous years, with consistently high levels of satisfaction being achieved.
These qualitative results complement the many quantitative measures we have in place and help to provide a more rounded summary of the performance and service that industry can expect to receive.
For the 2016/17 financial year, the VMD set a target that the overall median score from meeting questionnaires for individual VMD company meetings should be not less than 4 for at least 90% of the meetings.
In addition, any feedback received is used to enable the VMD to continue to provide a service that meets the industry need and helps to identify areas where improvements can be made.
Meetings
Between 1 April 2016 and 31 March 2017 a total of 66 meetings were held at the request of companies in order to discuss potential projects. A total of 33 completed questionnaires were received from companies reporting on the experiences that they had in arranging and attending meetings, which was a lower return rate than in previous years. In addition feedback was provided on the effectiveness, accuracy and relevance of the advice given.
The VMD would like to thank those who took the time to respond to this questionnaire. Your feedback is valued and we will be looking at the individual comments made to see where we can improve further. We are disappointed not to have received more completed questionnaires. We appreciate everyone is very busy and this is an additional task but we would like to encourage all companies on all occasions to provide us with feedback.
Results
The questionnaire relies on a simple scoring system from 1 to 5 with 1 being at the lower end of the scale and 5 at the top end.
-
31 out of the 33 respondents rated the overall usefulness of these meetings as 4 or above, with two others giving a rating of 3. The average score was 4.62.
-
Ease of arranging meetings – all companies rated this as 4 or above with the average being 4.76.
-
Respondents thought that the VMD staff were well prepared for these meetings with the average score being 4.59.
-
The VMD returned the draft set of minutes with our comments to the company within an average of 11.42 calendar days from receipt.
On average respondents scored the advice given by each discipline as follows:
| Disipline | Score |
|---|---|
| Biologicals | 4.8 |
| Quality | 4.5 |
| Safety | 4.54 |
| Efficacy | 4.42 |
| Admin | 4.59 |
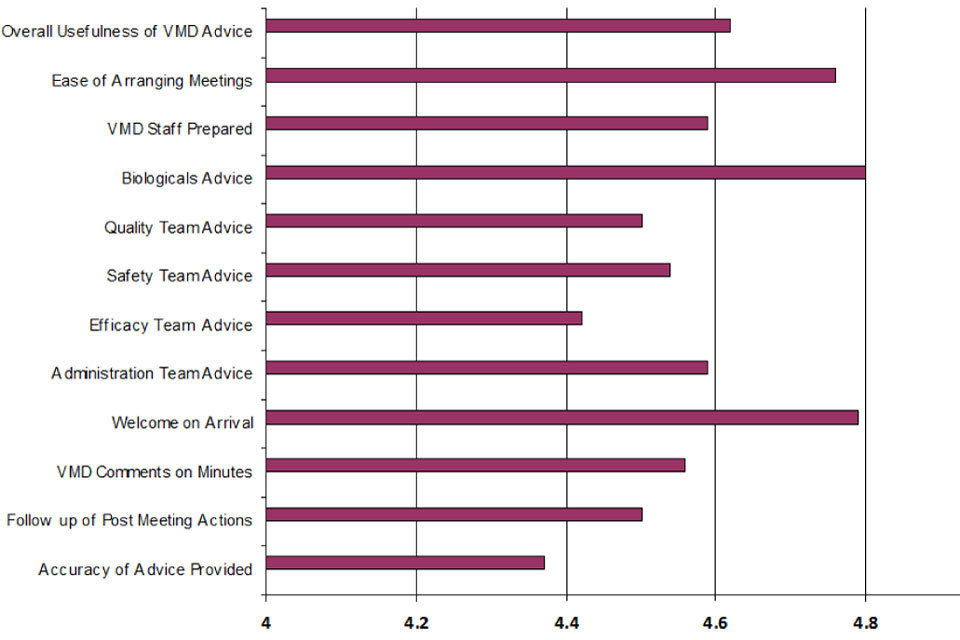
Summary of company visits questionnaire by disipline
As a balance to the company views, the VMD also completes a questionnaire after each company meeting. This questionnaire seeks views on the quality of the agenda provided; whether all the agenda points were covered or any additional ones added at the meeting; on the engagement of the company during the meeting; and also on the quality of the minutes provided.
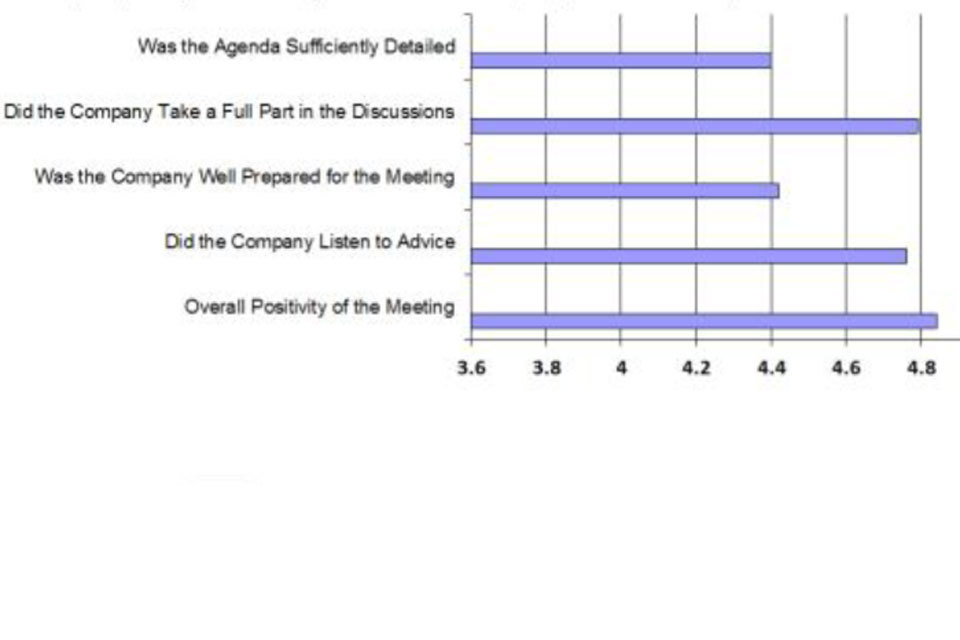
Summary of VMD views for company visits
Conclusions
As with previous years the clear message is that industry welcomes the VMD’s open approach to meetings. These are easy to arrange and usually within the timescale requested by the company. Appropriate qualified people attend these meetings which enables constructive debate around the issues that are of importance to the company. The feedback is that the advice offered by VMD staff across all disciplines is valued, relevant and of good quality; and that companies come well prepared and willing to discuss and exchange views.
The VMD welcomes the early provision of agendas with key points or questions for discussion clearly indicated. The VMD is grateful that companies, in the majority of cases, provide agendas at least one week prior to the meeting which provides sufficient time for preparation, enabling more focused discussions to take place. Furthermore, the VMD encourages companies to provide draft minutes so that these can be reviewed, ratified and consequently retained as a record of the discussions and of any agreements which may have been reached. It is important that when completing the minutes that sufficient detail and key points/agreements are recorded. Often there can be a gap between the meeting itself and the project being progressed to the point of submission or in compiling the dossier. The minutes provide a valuable reference point for both parties, especially when personnel may have changed during the intervening period.
For further information contact Gavin Hall g.hall@vmd.defra.gsi.gov.uk
To arrange a meeting contact Chris Abbott c.abbott@vmd.defra.gsi.gov.uk
2.4 Quarterly reporting against VMD Published Standards for 2016/17 licensing work
This report is published on a monthly basis under Our Statistics on GOV.UK.
For further information contact Natalie Shilling n.shilling@vmd.defra.gsi.gov.uk and put ‘MAVIS’ in the subject line.
2.5 Reminder about the Batch Release Request scheme for an immunological veterinary medicine
Please be reminded that a batch of Immunological Veterinary Medicinal Products (IVMP) may not be placed onto the market without prior consent from the VMD. Therefore, please ensure you submit a batch release request to the VMD and obtain the necessary approval BEFORE you place your product on the market. Failure to do so may result in a batch recall. Information about the Batch Release scheme is available on GOV.UK.
For further information contact the Batch Release team on batchr@vmd.defra.gsi.gov.uk
2.6 Top ten imported veterinary medicines quarterly report from 1 April to 30 June 2017
The VMD provides a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Ingredient | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,759 |
| Nobivac Myxo-RHD, lyophilisat et solvant pour suspension injectable pour lapins | Myxoma vectored RHD virus (live) | 1275 |
| Filavac VHD K C+V | Rabbit Haemorrhagic Disease (Inactivated) | 377 |
| Vet-Goid | Allergens | 342 |
| Spectrum Hyposensitisation Vaccine - Injectable Solution | Allergen | 252 |
| Botulism Vaccine | Clostridium botulinum type C & type D toxoid | 182 |
| Smartshot B12 Prime Lamb | Hydroxocobalamin | 134 |
| Greer Allergenic Extract Patient Prescription | Allergens | 131 |
| European Viper Venom Antiserum (Antytoksyna Jadu Zmij) | European Viper Venom Antiserum | 113 |
| Calmivet Solution Injectable | Acepromazine Maleate | 84 |
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice Inspection Team (GMPIT)
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
Guidance is available on obtaining a UK manufacturing authorisation (ManA) for an authorised veterinary medicine or an authorisation for an extemporaneous product (special) (ManSA) on GOV.UK.
Guidance is also available on GOV.UK on obtaining a manufacturing authorisation for an equine stem cell centre (ESCCA), a non-food animal blood bank (NFABBA) or an autogenous vaccine (AVA).
3.2 The Inspections and Investigations Team (IIT)
Guidance is available for:
- manufacturers and distributors of animal feedingstuffs containing veterinary medicines and/or coccidiostats (referred to in the VMR as specified feed additives – SFAs)
- veterinary-only wholesale dealers to assess compliance against GDP
- premises for the retail supply of POM-VPS and NFA-VPS medicines by suitably qualified persons (SQPs)
- veterinary practice premises (VPPs), other than those accredited by the Royal College of Veterinary Surgeons (RCVS) under its voluntary Practice Standards Scheme (PSS).
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 March to 31 May 2017, the VMD received 1,620 animal suspected adverse event reports. Of the 2,378* products involved in these reports, 2,289 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,122 | Prescription Only Medicine Veterinarian (POM V) |
| 104 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 38 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 25 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 89 products were:
| Amount | Type |
|---|---|
| 35 | Authorised human medicines |
| 2 | Medicine used in a trial under an Animal Test Certificate |
| 12 | Veterinary products without any medicinal claim |
| 9 | Authorised medicines imported from other countries |
| 10 | Medicines sold under the exemption for small pet animals |
| 10 | Specially formulated veterinary medicines |
| 11 | Unidentified products |
During this period 52[footnote 1] reports of human suspected adverse reactions were received. Of the 57 products involved in these reports, 52 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 41 | Prescription Only Medicine Veterinarian (POM V) |
| 8 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 1 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 2 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 5 products were:
| Amount | Type |
|---|---|
| 2 | Authorised medicines imported from other countries |
| 3 | Unidentified products |
No environmental incident reports were received during this period.
For further information contact Roy Savory r.savory@vmd.defra.gsi.gov.uk
5. Enforcement
A list of prosecutions and notices involving illegal activity with veterinary medicines in the last year can be found on GOV.UK.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 UK Antimicrobial Resistance (AMR) Strategy
The most recent meeting of the High Level Steering Group (HLSG) for the AMR Strategy took place on 5 July 2017. Activities to deliver the aims of the Strategy are being implemented in line with the guidance of the HLSG. Discussions on future plans to look into ‘AMR in the Environment’ and on the content of the next AMR Strategy’s plan took place.
6.2 Defra Antimicrobial Resistance Co-ordination (DARC)
The DARC group met on 29 June 2017 to discuss recent trends in antibiotic resistance in bacteria of importance to human and animal health. The group received an update on sector-specific antibiotic consumption data projects currently under development, and discussed international collaborations they took part in. The afternoon included topical presentations focused on the existing evidence of antimicrobial usage and resistance in aquaculture. Minutes of DARC group meetings can be found on GOV.UK.
6.3 Sales Data and Antibiotic Resistance Surveillance Report
Data for the 2016 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report are currently being collated for publication towards year end. The report collates data on antibiotic sales from UK Marketing Authorisation Holders and antibiotic resistance data from VMD’s surveillance programmes.
UK-VARSS 2015 and previous reports are available on GOV.UK.
For further information contact Stacey Brown s.brown@vmd.defra.gsi.gov.uk
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in May 2017. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2016 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
For further information contact Myles Munroe m.munroe@vmd.defra.gsi.gov.uk
9. Organogram as at 1 July 2017
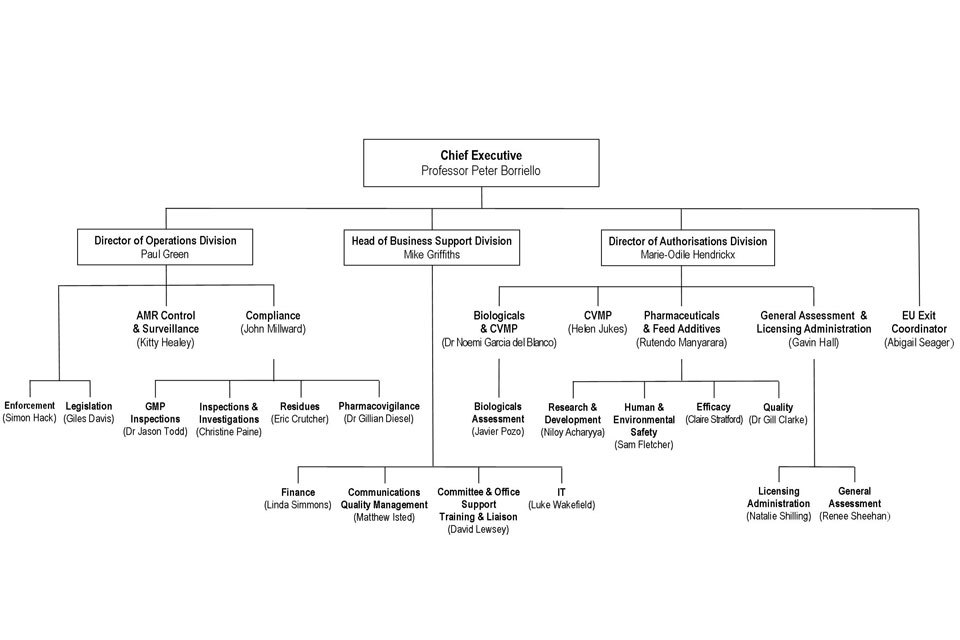
VMD Organogram as at 1 July 2017
-
Individual reports may involve more than one product ↩
