Joint Committee on Vaccination and Immunisation (JCVI) statement on COVID-19 vaccinations in 2022: 21 February 2022
Published 21 February 2022
Applies to England
Advice
The Joint Committee on Vaccination and Immunisation (JCVI) recognises that there remains considerable uncertainty with regards to the likelihood, timing and severity of any potential future wave of coronavirus (COVID-19) in the UK. There may be a transition period of a few years before a stable pattern, such as a regular seasonal wave of infection, is established. Advances in vaccine technologies and therapeutic agents in the meantime are ongoing.
Autumn vaccination programme
Despite the known uncertainties, in the year ahead, winter will remain the season when the threat from COVID-19 is greatest both for individuals and for health communities. It is JCVI’s interim view that:
-
an autumn 2022 programme of vaccinations will be indicated for persons who are at higher risk of severe COVID-19; such as those of older age and in clinical risk groups
-
precise details of an autumn programme cannot be laid down at this time
-
this advice should be considered as interim and for the purposes of operational planning
Spring vaccination programme
Many of the oldest adults, and therefore most vulnerable, will have received their most recent vaccine dose in September or October 2021. These individuals are at higher risk of severe COVID-19, and with the lapse of time, their immunity derived from vaccination may wane substantially before autumn. Therefore, as a precautionary strategy for 2022, JCVI advises a spring dose, around 6 months [footnote 1] after the last vaccine dose, should be offered to:
-
adults aged 75 years and over
-
residents in a care home for older adults
-
individuals aged 12 years and over who are immunosuppressed, as defined in the Green Book
Eligible persons aged 18 years and over may be offered booster vaccination with 30mcg Pfizer-BioNTech (Comirnaty) vaccine or 50mcgModerna (Spikevax) vaccine. Eligible persons aged between 12 and 18 years may be offered booster vaccination with 30 mcg Pfizer-BioNTech (Comirnaty) vaccine. New vaccine products, including variant vaccines which are more closely matched to future circulating virus(es), may become licensed and available in 2022. Advice on the vaccine product will be reviewed and updated accordingly.
Background
A timeline of previous advice on adult vaccinations can be found in the statement from JCVI dated 7 January 2022.
The need for, and timing of, vaccinations to protect against severe disease (hospitalisation and mortality) is influenced by various factors, including the degree of match between vaccine and virus, the duration of vaccine induced immunity and the timing of any future wave of infection. As an increasing proportion of the population experiences and recovers from milder, non-severe COVID-19 infection, the role of natural immunity (level, breadth and duration of protection and its interaction with vaccine-induced immunity) will become increasingly relevant as well. Studies on all these fronts are continuing and evidence will be reviewed by JCVI as appropriate.
Autumn booster programme effectiveness
Thus far, the 2021 autumn booster programme has provided high levels of protection against severe disease from COVID-19 due to both Delta and Omicron variants across the population. Data from the UK Health Security Agency (UKHSA) indicate that in relation to the Omicron variant, vaccine effectiveness against severe disease (hospitalisation) following the 2021 autumn booster in older adults aged 65 years and over is around 90% shortly after vaccination, with this reducing slightly by 10 to 14 weeks. [footnote 2] For the Delta variant, corresponding values are higher, likely reflecting differences in the match between currently available vaccines and the different variants. How well matched any future newly dominant variant of concern might be to available vaccines (current and new) cannot be determined at this time. Projections from immunological data suggest relatively good levels of protection against severe disease due to the Omicron variant may be expected to be maintained in older persons for 6 to 9 months (consensus study data – unpublished). However, these estimates are imprecise due to a lack of standardised correlates of protection between measured immune responses and clinical vaccine effectiveness.
Available data from the UK and internationally over the course of the pandemic suggest that older persons are at highest risk from waning of vaccine-induced protection; they are more likely to experience waning of immunity due to immunosenescence, and much more likely to experience severe disease if infected. Practically, because they were prioritised for vaccination at the start of the COVID-19 vaccine programme, older persons are also now furthest in time from their last vaccine dose. Data on hospital admissions in the UK are consistent with the clinical risk being highest in those aged 75 years and older. [footnote 3]
Figure 1: weekly hospital admission rate by age group for new COVID-19 positive cases reported through SARI Watch
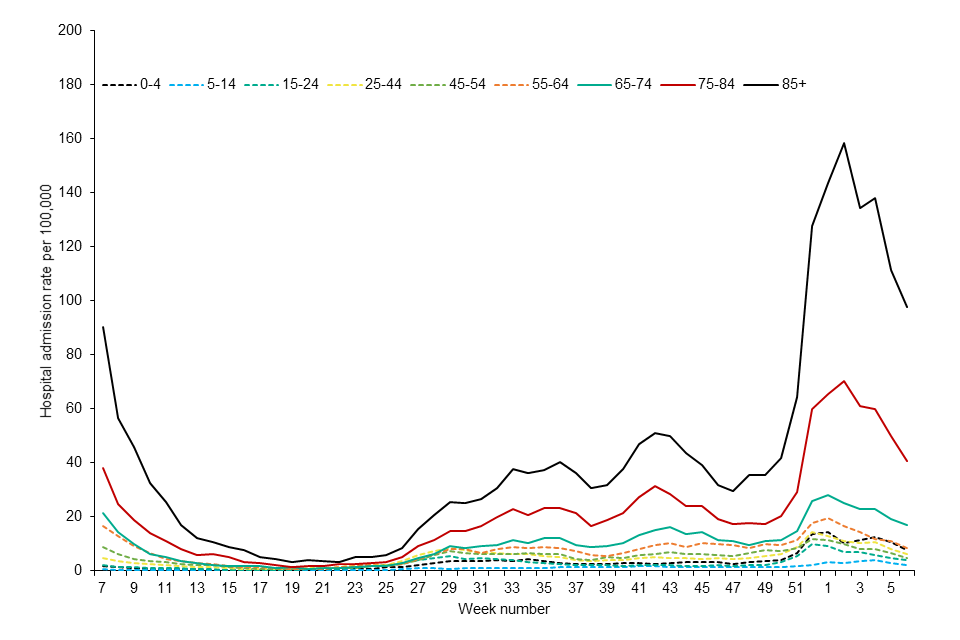
Precautionary programme for protection across 2022 for the most vulnerable
As COVID-19 isolation measures are relaxed in the UK, and population immunity against milder, non-severe infection wanes, elimination of SARS-CoV2 virus from the population is not anticipated. There is no precise threshold of vaccine effectiveness against severe disease below which extra vaccinations should be triggered. A balance between frequency of vaccination, likely vaccine coverage and additional vaccine derived benefits is important. Increasingly, scientific evidence indicates that a longer interval between vaccine doses, compared to a shorter interval, is beneficial in terms of improving the level of immunity provided by vaccination, widening the breadth of immunity against different variants, increasing the duration of immunity and reducing the amount of vaccine related reactions (consensus study data – unpublished). [footnote 4][footnote 5][footnote 6]
As a precautionary measure, JCVI’s view is that a spring dose for the most vulnerable persons in the population, is a proportionate response in the current circumstances.
JCVI will continue its rolling review of the vaccination programme and the epidemiological situation, particularly in relation to the timing and value of doses for less vulnerable older adults and those in clinical risk groups ahead of autumn 2022. Rapid response measures may be required if there are substantial changes in our understanding of vaccine protection against the Omicron variant, or major changes in the unfolding epidemiology of COVID-19. JCVI will provide more definitive advice regarding an autumn booster programme in due course.
-
Operational flexibility around the timing of the spring dose in relation to the last vaccine dose is considered appropriate. More information on operational flexibility will be provided in the Green Book: Immunisation against infectious disease. ↩
-
UKHSA data 10th February and unpublished analyses by age. ↩
-
UKHSA – National influenza and COVID-19 surveillance report ↩
