HPR volume 11 issue 38: news (27 October)
Updated 15 December 2017
Tuberculosis in England 2017
PHE has published its annual report on tuberculosis that presents data – to the end of 2016 – describing the epidemiology of TB in England [1]. In addition, the report presents data on the UK pre-entry screening programme, the national roll-out of systematic latent TB infection (LTBI) testing and treatment, and BCG vaccination coverage estimates.
The information contained in the report, and accompanying monitoring data displayed on Fingertips (due to be released on 7 November 2017) [2], can be used to support action to achieve the aims for TB control set out in the Collaborative Tuberculosis Strategy for England 2015-2020 [3].
Epidemiology
In 2016, there were 5,664 TB cases notified, down from 5,727 in 2015. Following annual declines of at least 10% in the number of cases between 2012 and 2015, the decline in 2016 has slowed to 1%. The incidence rate in 2016 was 10.2 per 100,000 (CI 10.0-10.5), compared with 10.5 per 100,000 (CI 10.2-10.7) in 2015, and is the lowest incidence in England since the start of enhanced TB surveillance in 2000 (see figure).
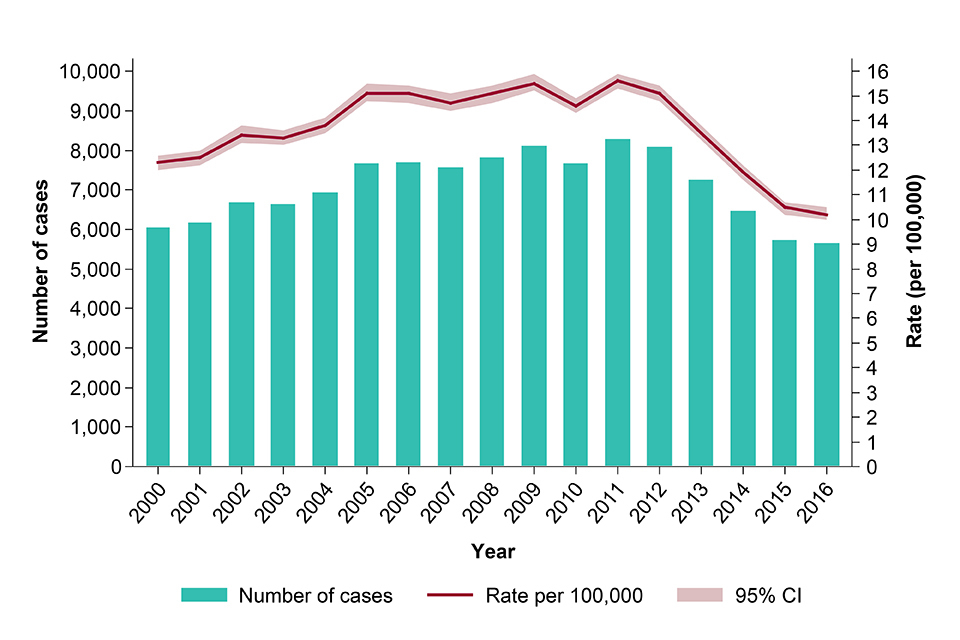
TB case notifications and rates, England, 2000-2016
There was a small decline in the number of cases in the UK born population, while the incidence rate of TB remained low (3.2 per 100,000). In the non-UK born population, there was no decrease in the number of TB cases (4,096 in both years), and the rate (49.4 per 100,000, CI 47.9-50.9) was similar to 2015 (51.3 per 100,000, CI 49.7-52.9). Similar to previous years, the rate of TB in the non-UK born population was 15 times higher than the UK born population, and almost three-quarters (74%) of all cases were born outside the UK.
Outcomes in drug sensitive TB cases
The proportion of drug sensitive TB cases notified in 2015 who completed treatment within 12 months decreased to 83.4%, down from 85.6% for cases notified in 2013. There was a small increase in the proportion of drug sensitive TB cases who died at the last recorded outcome, from 4.7% for cases notified in 2013, to 6.1% for cases notified in 2015; most of these deaths occurred in those aged 65 and older.
Antimicrobial resistance
The number and proportion of TB cases in the drug resistant cohort (confirmed or treated as multi-drug resistant/rifampicin resistant TB (MDR/RR-TB)) has remained relatively stable over the last three years, with 68 notified cases in 2016. The number (59) and proportion (1.7%) of TB cases with initial confirmed MDR/RR-TB increased slightly compared with 2015 (53, 1.5%). Ten cases of XDR-TB were notified in 2016, the same number as in 2015, but higher than in previous years.
Delay between symptom onset and start of treatment
A high proportion of cases continue to experience delays between symptom onset and the start of treatment. In 2016, 31% of pulmonary TB cases experienced a delay of more than four months, increasing from 28% in 2015. Treatment delays continue to be more common among UK born cases than non-UK born cases, with 34% of UK born cases experiencing a delay of four months or more, compared with 29% of non-UK born cases.
Social risk factors
There was a small decrease in the proportion of TB cases with at least one social risk factor (homelessness, drug or alcohol misuse or imprisonment) from 11.7% in 2015 to 11.1% in 2016. TB cases notified in 2015 with at least one social risk factor were more likely to have pulmonary disease, drug resistance and worse outcomes, being almost twice as likely to have died or be lost to follow-up after 12 months compared to cases without a risk factor.
Conclusions and recommendations
The recent steep decline in notifications of TB in England slowed to 1% in 2016. The reasons for this are as yet unclear and likely to be multifactorial. We continue to monitor the situation to ascertain whether this rate of decline continues
To continue to achieve year-on-year reductions in TB incidence, and return to the steep declines seen in previous years, effort needs to be maintained to deliver all 10 key areas for action in the national strategy and strengthen TB control. Recommendations to achieve this are outlined at the end of the annual report. Specifically, it will be important to focus on:
- reducing active TB in recent new migrants through the UK TB pre-entry screening programme
- preventing reactivation of TB among migrants through LTBI testing and treatment
- continuing efforts to reduce diagnostic delay through awareness raising in communities affected by TB and among health professionals
- maintaining the quality of TB diagnostic, treatment and care services to ensure high rates of culture confirmation and treatment completion
- maintaining a focus on the social factors associated with TB and ensuring an integrated approach to the specific needs of under-served populations.
References
- PHE (October 2017). Tuberculosis in England: 2017 report (presenting data to end of 2016).
- PHE website. TB Strategy Monitoring Indicators.
- PHE (January 2015). Collaborative tuberculosis strategy for England 2015-2020.
English Surveillance Programme for Antimicrobial Utilisation and Resistance fourth report
The fourth annual report of the English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR), published by PHE on 23 October [1], provides data on antibiotic use and resistance in the calendar years 2012 to 2016. It also reviews antimicrobial stewardship quality improvement activities pursued in primary and secondary care and considers personal and professional engagement activities, as anticipated by the five-year AMR strategy for England published in 2013 [2].
Key findings of the fourth annual report are that:
On resistance
- four in 10 patients with an E.coli bloodstream infection in England cannot be treated with the commonest antibiotic (co-amoxiclav) used in hospitals. In addition, almost one in five of these bacteria were resistant to at least one of five other key antibiotics
- of the 1 million antibiotic-resistant UTI bacteria identified in NHS laboratories in 2016, trimethoprim resistance was very common (averaging 35% across acute healthcare and community settings) but the current recommended first line treatment, nitrofurantoin remains effective (3% resistance across both sectors).
On usage
- between 2012 and 2016, antibiotic prescribing reduced by 5%, when measured as defined daily doses per 1000 inhabitants per day
- the number of antibiotic prescriptions dispensed in General Practice decreased by 13% between 2012 and 2016 (-2% from 2015 to 2016)
- dental practices dispensed 1 in 5 fewer prescriptions in 2016 compared to 2012 and more than 99% of prescribed antibiotics were in accordance with dental treatment guidelines
- hospital prescribing has increased year on year, but has reduced use of the last resort antibiotics (piperacillin/tazobactam and carbapenems) by 4% between 2015 and 2016
- the national importance of reducing unnecessary and inappropriate antibiotic use was demonstrated through NHS antimicrobial stewardship initiatives, namely the Quality Premium (QP), from 2014/15 in primary care, and Commissioning for Quality and Innovation (CQUIN), from 2016/17 in secondary care
- over the first two years of the QP, 88% of CCGs met their objective to reduce antibiotic consumption and 83% reduced broad-spectrum antibiotic use to the target level
- in the first year (2016/17) of the CQUIN, 37%, 33% and 52% of NHS acute Trusts met their objectives to reduce total antibiotic, piperacillin/tazobactam and carbapenem consumption respectively, to 2013/14 levels; though significantly more reduced their piperacillin/tazobactam and carbapenem compared to 2015/16 levels (66% and 67% respectively).
National point prevalence survey of healthcare associated infections and antimicrobial use in acute hospitals
The ESPAUR report includes a chapter presenting the results of the fifth national point prevalence survey of HCAIs in England (for 2016). Despite an older population with increased co-morbidities and surgery, there was no significant change in the prevalence of HCAI, or in AMU, between the last survey in 2011 and 2016. In 2016, 1 in 15 patients in acute hospitals had an HCAI and 1 in 3 were on antibiotics on the day of survey. A higher level of antibiotic resistance was seen in those with an HCAI compared to the rates observed in the all-patient GNBSI and UTI surveillance data, suggesting that HCAIs are more likely to be antibiotic resistant than community infections. Infections were most prevalent among patients on intensive care units (ICUs).
Antifungal use and resistance
A chapter is dedicated to antifungal resistance, focussing on key antifungals in moulds and yeasts and emerging resistance within those species, including an update in PHE’s response to the emerging threat posed by Candida auris. The chapter highlights the variation in testing performed in both reference and clinical laboratories and notes the low frequency of antifungal susceptibility testing.
Antimicrobial stewardship
This report features process evaluations of antimicrobial stewardship toolkits for dental and GP sectors, demonstrating their wide global uptake and utility in helping clinicians prescribe antibiotics appropriately. Activities aimed at increasing engagement, and encouraging both professional and public behaviour change – such as the Antibiotic Guardian campaign – are described. ESPAUR has also worked closely with PHE marketing team on an eight-week national public campaign – Keep Antibiotics Working – that was launched on 23 October 2017.
References
- PHE website. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report.
- PHE (December 2015). UK five year antimicrobial resistance strategy 2013 to 2018.
Integrated guidance for health clearance and management of HCWs infected with HIV, hepatitis B or hepatitis C
Public Health England, in conjunction with Health Protection Scotland, has released a new publication entitled Integrated Guidance on the Management of Healthcare Workers Infected with Blood-borne Viruses (HIV, hepatitis B and/or hepatitis C) (2017) [1].
The guidance provides up-to-date, evidence-based recommendations intended to reduce the risk of healthcare worker-to-patient transmission of blood borne viruses and reduce the future burden of patient notification exercises. The guidance also provides advice on key operational and service delivery issues that need to be addressed to ensure that healthcare workers infected with blood-borne viruses who perform exposure prone procedures (EPPs) are managed in a manner that safeguards their confidentiality and employment rights.
The document brings together detailed guidance on:
- health clearance for hepatitis B, hepatitis C and HIV for new healthcare workers
- the management of healthcare workers infected with hepatitis B, hepatitis C and/or HIV, including monitoring arrangements for those performing EPPs
- guidance and arrangements for undertaking patient notification exercises following the diagnosis of a healthcare workers with hepatitis B, hepatitis C and/or HIV including definitions of EPPs
- roles and responsibilities of the UK Advisory Panel for Healthcare Workers with Blood-borne Viruses (UKAP) and other parties involved in the management of healthcare workers infected with hepatitis B, hepatitis C and/or HIV.
While this guidance does not represent a policy change, there are some changes in the arrangements for testing and reporting of results for hepatitis B.
Previous guidance for hepatitis B (HBV) infected healthcare workers specified a cut-off of 103 geq/mL, above which healthcare workers were not allowed to perform exposure-prone procedures. HBV DNA testing was restricted to two designated laboratories (the West of Scotland Specialist Virology Centre and the Public Health Laboratory, Birmingham) who were able to benchmark healthcare worker-derived samples against a WHO International Standard known to contain 103 geq/mL.
In the years since issuance of that guidance, commercially available HBV viral load assays have been developed that use a WHO International Standard for Hepatitis B Virus Nucleic Acid Amplification Techniques. The International Standard and CE marked quantitative HBV DNA PCR assays calibrated to this standard are now widely available and it is now standard practice for HBV viral load assay results to be reported in international units per millilitre (IU/mL).
A set of quick reference guides for management of common situations are published alongside the main guidance document on the website [1].
Reference
- PHE website. “BBVs in healthcare workers: health clearance and management”.
Infection reports in this issue of HPR
The following infection report is published in this issue of HPR.
