Spinal muscular atrophy type 1: NCARDRS data briefing
Published 4 September 2019
Background
During 2018 and 2019 the National Congenital Anomaly and Rare Disease Registration Service (NCARDRS) began to look at the value of using available, routinely collected data to support rare disease data collection. This report contains preliminary prevalence data on spinal muscular atrophy type 1 (SMA1) based on Hospital Episode Statistics (HES), Office of National Statistics (ONS) mortality data and patient data held by the NCARDRS. Further information on the background to this work and technical notes on the methods can be found in Appendix 1.
Spinal muscular atrophy (SMA)
Spinal muscular atrophy (SMA) is a progressive neuromuscular disorder caused by mutations in the SMN1 gene with autosomal recessive inheritance. The SMA phenotype ranges from mild to severe. The different types of SMA are classified by clinical severity, with the most severe type being Type 0, which presents in the prenatal or perinatal period to Type IV, which presents in adulthood.
We chose SMA1 as an exemplar for the evaluation of HES and ONS mortality data because it has a relatively specific ICD-10 code, presumably a high hospitalisation rate, and a high mortality rate. Also, treatment options for SMA1 are limited and expensive. Nusinersen (Spinraza) has recently been approved by the National Institute for Health and Care Excellence (NICE) for treatment of pre-symptomatic SMA, or SMA types 1, 2 or 3 in England. [footnote 1]
SMA occurs in an estimated one in 10,000 births. SMA1 accounts for around 60% of all cases of SMA. [footnote 2]
Prevalence estimates
Using HES, ONS mortality data and registration data, we estimate that one in 16,230 babies born in England have SMA1 (around 42 babies a year). The estimated prevalence of 6.2 per 100,000 births (95% CI: 5.6-6.8) aligns with other published figures.
We identified 106 people in England who were living on 1 July 2016 with SMA1 born since 2000. This gives a population prevalence of 1.9 per million (95% CI: 1.6-2.3). Over the 10 years 2008 to 2016, the estimated birth prevalence (incidence) was 6.2 per 100,000 births (95% CI: 5.6-6.8) or approximately one per 16,350 births (Table 1).
Over the period 2009 to 2016, we estimate the infant mortality rate in patients with SMA1 to be 4.2 per 100,000 live births (95% CI: 3.7-4.8) (Table 2). We calculated infant mortality rate (IMR) for cases where SMA, SMA0 or SMA appeared anywhere on the death certificate for the years 2008 to 2017. In 2017, there appears to have been a drop the infant mortality rate in babies where SMA0, SMA1 or SMA appear on the death certificate. Nusinersen became available in the UK through the Expanded Access Programme in 2017, though access was limited.[footnote 3]
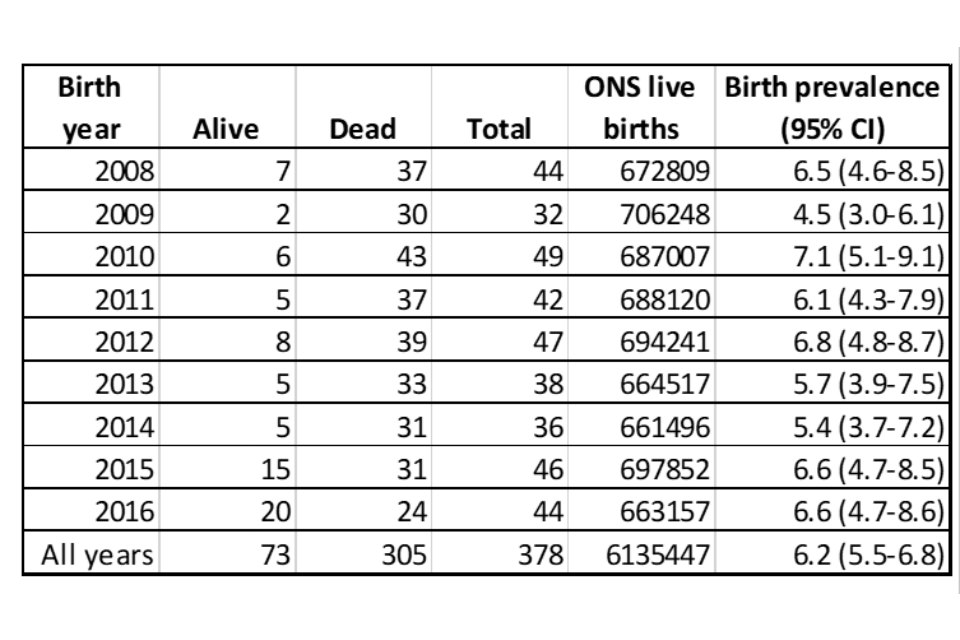
Table 1. Birth prevalence (per 100,000 live births) with 95% confidence intervals for SMA0, SMA1 and SMA based on death certificate and hospital admissions data. Vital status per March 2019
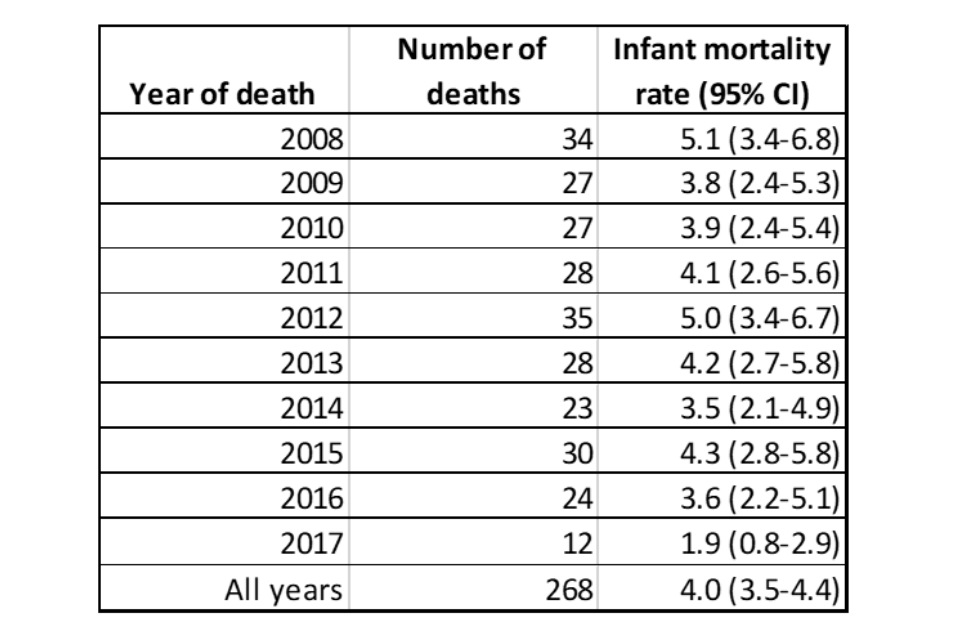
Table 2. Infant mortality rate (per 100,000 live births) with 95% confidence intervals for SMA0, SMA1 and SMA based on death certificate data.
There is the possibility of misclassification of cases using both ONS mortality data and HES data. Ascertaining SMA cases from mortality data requires using both coded and free text cause of death fields. Ideally, these cases should be validated against another data source, particularly if the type of SMA is not specified. HES data did not capture all the cases that were identified using mortality data. Also, HES cases that contained a code indicating SMA1 were not all confirmed when hospital patient information was checked.
These findings demonstrate the value of ONS mortality and HES data to support rare disease registration. They also reinforce the need to apply a multi-source approach to case ascertainment and completion. By using available routinely collected data, and expanding existing and creating new NCARDRS data feeds, we will increase the accuracy and usefulness of rare disease data for healthcare planning and research.
References
-
National Institute of Clinical Excellence. (2019) NICE recommends first ever treatment for children with rare muscle-wasting condition. Last accessed 28 May 2019. ↩
-
Verhaart IEC, Robertson A , Wilson IJ, and others. Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy – a literature review. Orphanet J Rare Dis 2017; 12:124 ↩
-
Spinal Muscular Atrophy UK. (2019) UK Access Summary: From Autumn 2016 to May 2019. Last accessed 11 June 2019. ↩
