Guidelines for completing Sloane main form
Updated 19 December 2019
Overview
This document explains the Sloane Project: main form. It includes written guidance and images to help with the completion of each section.
Eligible cases
Cases are eligible for submission to the Sloane Project if the patient has a diagnosis of:
- flat epithelial atypia (FEA)
- atypical intraductal epithelial proliferation (AIDEP)
- atypical ductal hyperplasia (ADH)
- atypical lobular hyperplasia (ALH)
- lobular carcinoma in situ (LCIS)
- pleomorphic lobular carcinoma in situ (PLCIS)
Patients must also:
- be screen-detected within the NHS Breast Screening Programme
- be diagnosed from 1 April 2012 onwards
- have no previous atypia, ductal carcinoma in situ (DCIS), or invasive disease in either breast
- have no concurrent DCIS or invasive disease in either breast
Completing the Sloane Project: main form
Complete one data form for each episode of FEA, AIDEP, ADH, ALH, LCIS, or PLCIS.
Complete the form once the patient episode has been completed. This includes:
- standard core biopsy
- widespread vacuum-assisted mammotomy
- surgical excision
Questions 1 to 9: patient details
In this section, indicate the:
- patient date of birth
- patient NHS number
- screening unit
- screening (SX) number
- date of screening mammogram
- screening round (number of times the patient has been screened as part of the breast screening programme)
- hospital name (hospital of pathology if no surgical treatment given)
- hospital number
- histology report number
Some patients may have undergone multiple biopsies for diagnosis and therapeutic purposes. It is not necessary to provide all this data. Indicate the last histology report number for reference as the main number.
Question 10: which breast is disease in?
Indicate left or right breast. Complete a separate form for each side if the patient has bilateral disease.
For multi-focal disease in a single side, complete only one form.
Question 11: background pattern
Using the BI-RADS classification, indicate which of the following 4 images best describes the background pattern:
Fatty
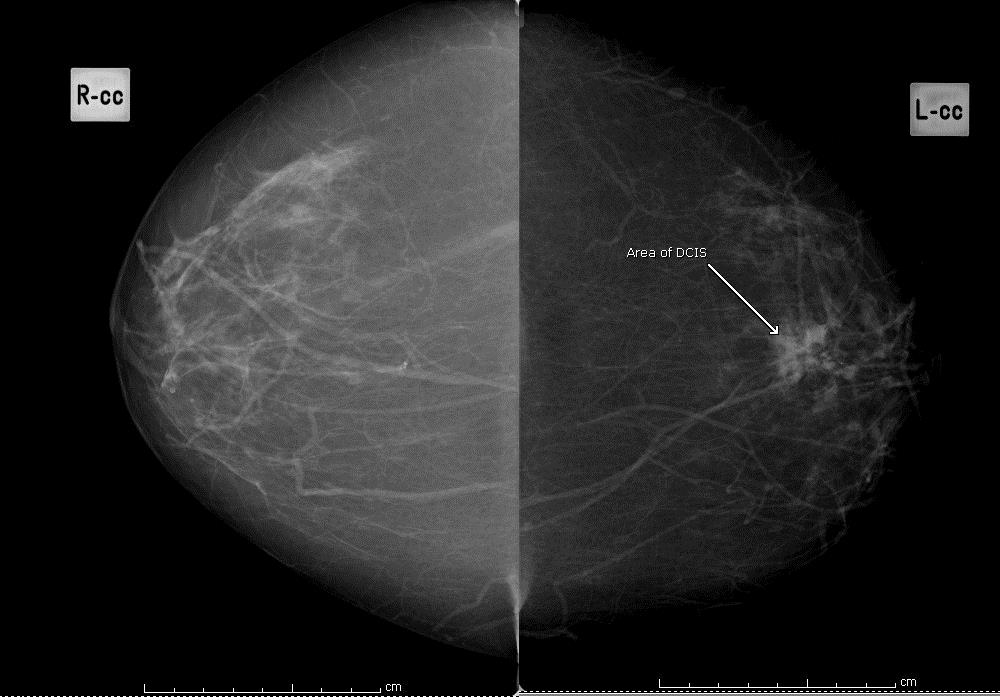 Figure 1: fatty background
Figure 1: fatty background
Scattered fibroglandular densities
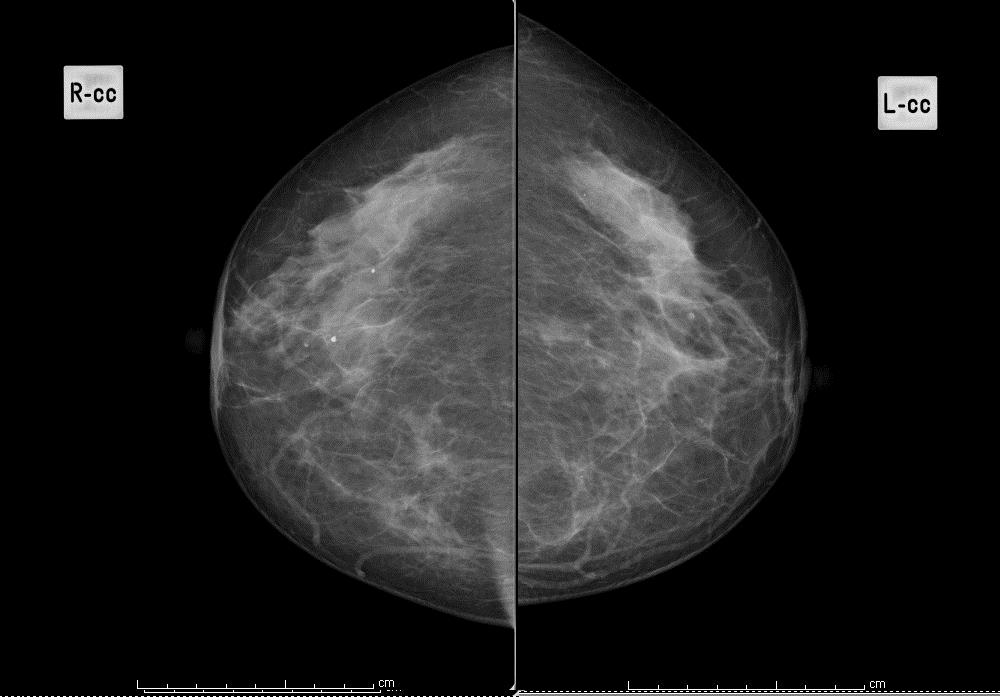 Figure 2: scattered fibroglandular tissues
Figure 2: scattered fibroglandular tissues
Heterogeneously dense
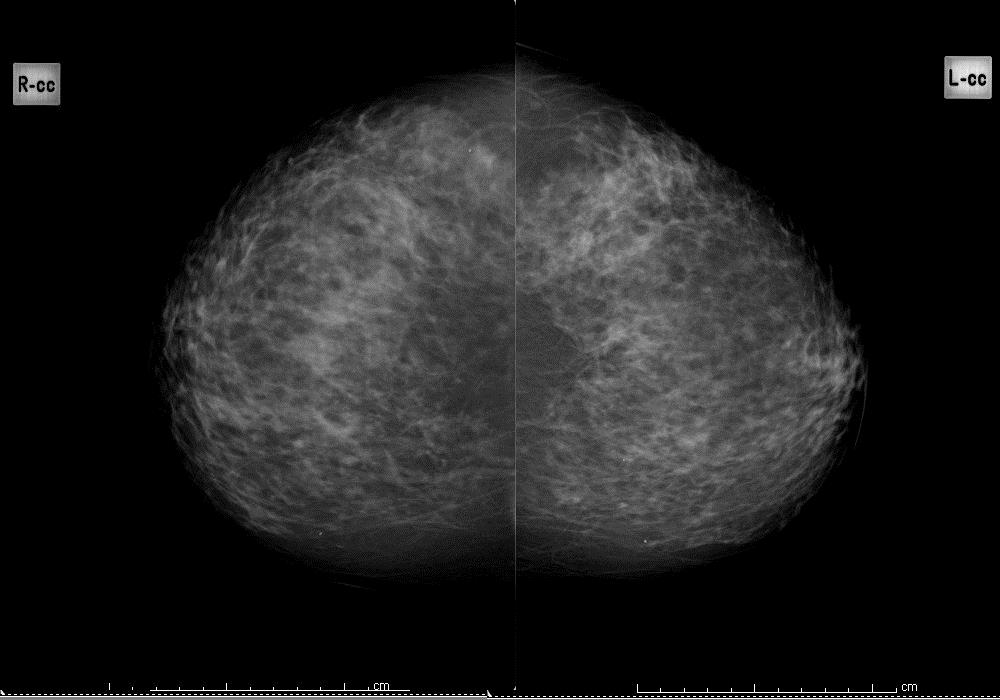 Figure 3a: heterogeneously dense
Figure 3a: heterogeneously dense
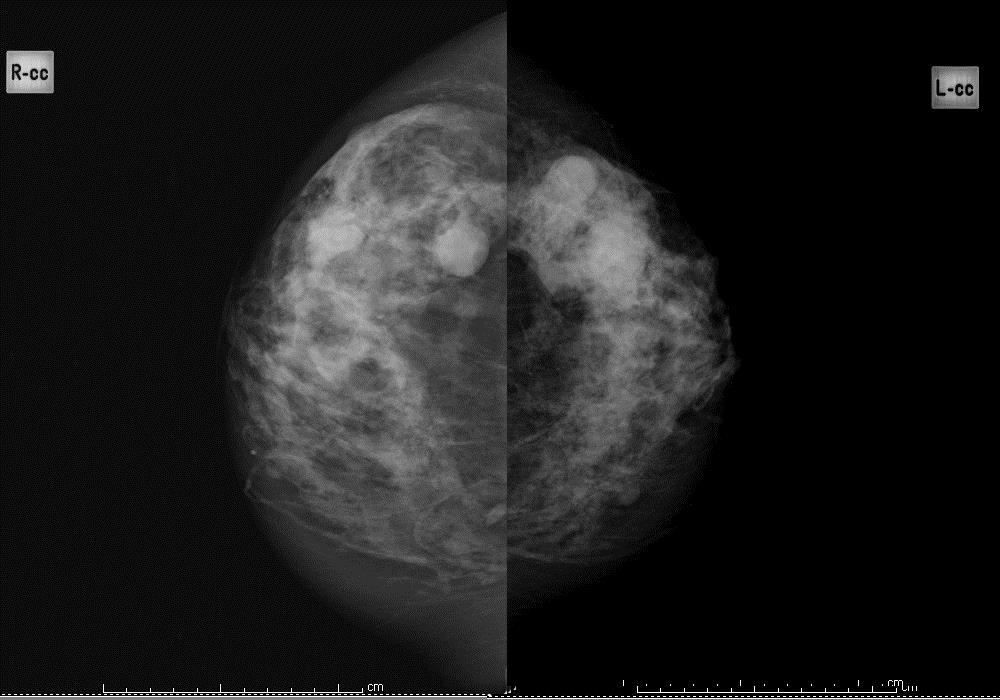 Figure 3b: heterogeneously dense
Figure 3b: heterogeneously dense
Extremely dense
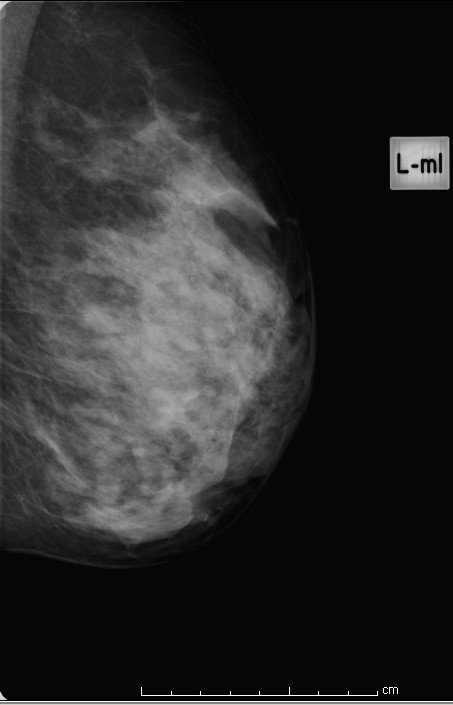
Figure 4: extremely dense
Question 12: predominant radiological feature
Choose the predominant radiological feature on the mammogram and classify as appropriate.
Select only one box of the following features: calcification, parenchymal distortion, mass: well-defined, mass: ill-defined, spiculate mass or none of these.
Question 13: microcalcification
Classification is based only on the shape of the individual calcifications. For instance, the presence or absence of a prominent ductal distribution should not influence the classification.
1. Casting/linear: this should include any case where there is even a single linear or branching calcification even if the rest are granular or punctate in shape.
2. Granular/crushed stone: this should include cases which have irregularly shaped calcifications but no linear forms. Some or even most of the calcifications may be punctate.
3. Powdery/punctate: all calcifications are either round or oval in shape.
Examples of the 3 groups are shown in figures 5, 6 and 7, below.
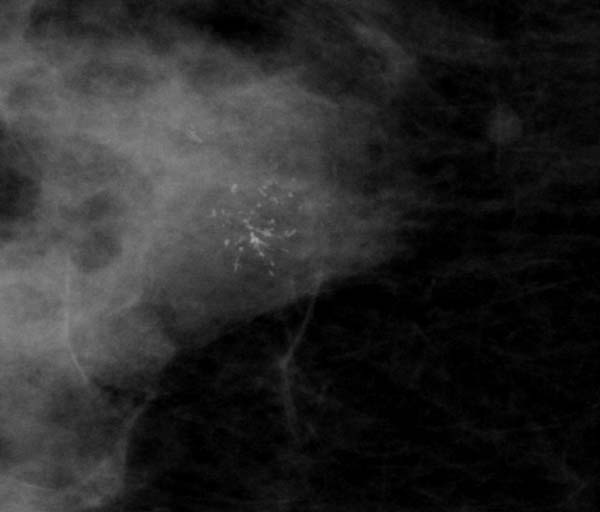
Figure 5: localised casting type calcification
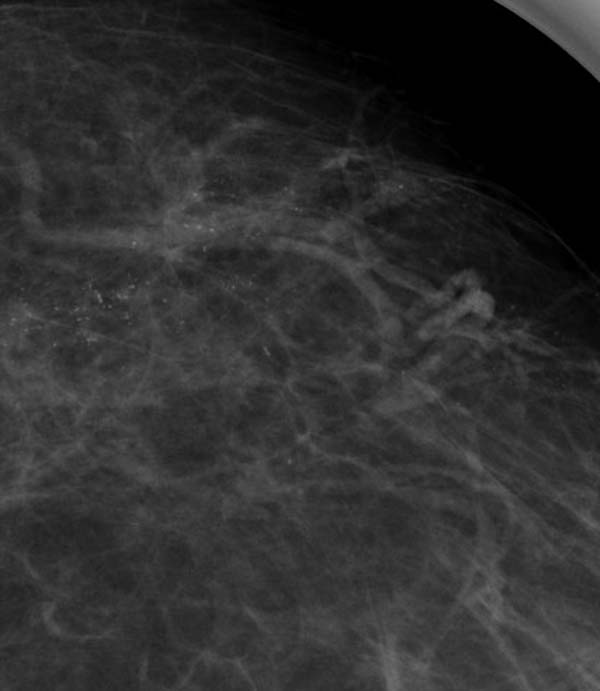
Figure 6: extensive granular (Nottingham classification) or crushed stone (Tabar classification)
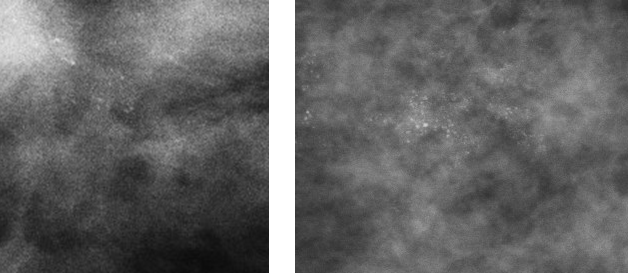
Figure 7: punctate (Nottingham classification) or powderish (Tabar classification) calcification
Question 14: estimated size of lesion on mammograms
On both the oblique and the craniocaudal (CC) views of the breast.
Measure:
- the maximum length of lesion (mm) (see figure 8 for example)
- the maximum diameter of lesion at 90 degrees to long axis of lesion (mm) (see figure 9 for example)
Indicate on the form the maximum overall size (mm) of these 4 measurements. Measurements of distortions are not required.
In cases where there are two distinct areas separated by apparently normal breast tissue, the measurement should be from one end of one site to the furthermost end of the other site, along with all the apparently normal breast tissue in between.
This is because it reflects how such cases are clinically managed. For instance, if DCIS is found in two sites with apparently normal breast tissue in between, the patient will generally be subjected to mastectomy (see figure 10).
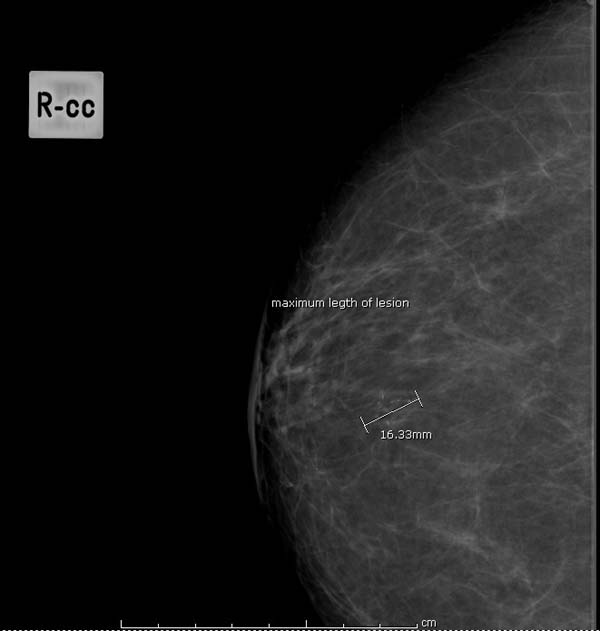
Figure 8: measuring maximum length of lesion (mm)
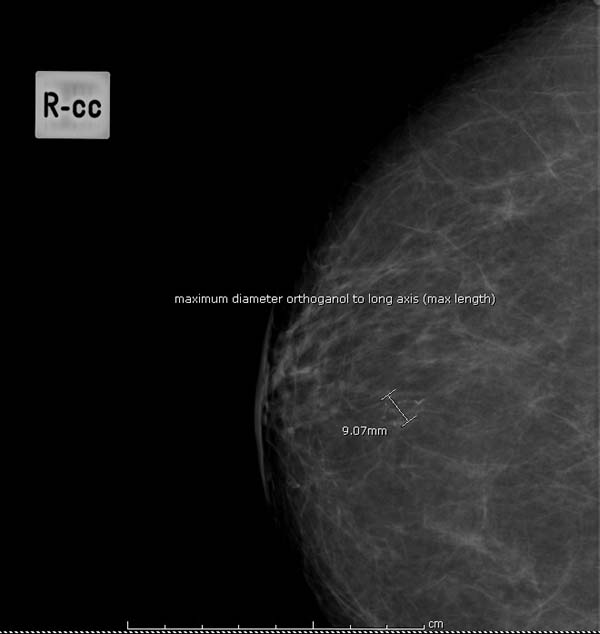
Figure 9: measuring maximum diameter of lesion (mm)
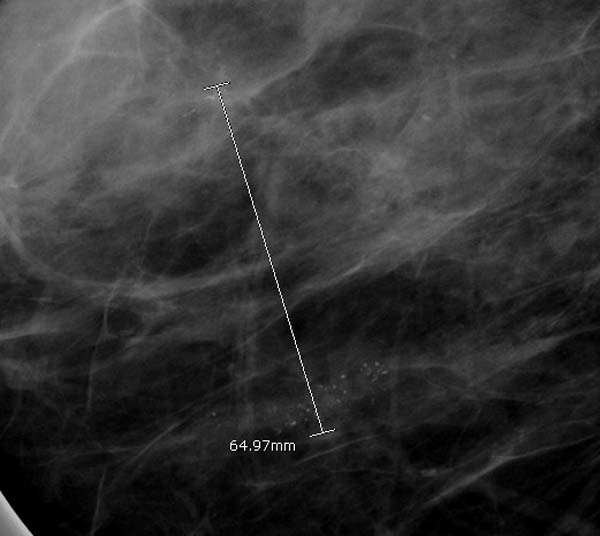
Figure 10: measuring multi-focal disease
Question 15: standard core biopsy
Indicate if a core biopsy is carried out. If so, indicate the number of cores taken.
Indicate whether any of the following are present in the core: AIDEP, FEA, Lobular Neoplasia, PLCIS. Example images of each of these specimens appear further down this page.
Do not include any cases where there is associated invasive disease or DCIS present in any of the specimens from that patient episode.
Question 16: diagnostic vacuum assisted biopsy
Indicate if a vacuum assisted biopsy is carried out. If so, indicate the number of cores taken and the gauge of the core.
Indicate whether any of the following are present in the core: AIDEP, FEA, Lobular Neoplasia, PLCIS. Example images of each of these specimens appear further down this page.
Do not include any cases where there is associated invasive disease or DCIS present in any of the specimens from that patient episode.
Question 17: therapeutic vacuum assisted excision
Indicate if a vacuum assisted excision is carried out. If so, indicate the number of cores taken and the gauge of the core.
Indicate whether any of the following are present in the core: FEA, ADH, ALH, LCIS, PLCIS. Example images of each of these specimens appear further down this page.
Do not include any cases where there is associated invasive disease or DCIS present in any of the specimens from that patient episode.
Question 18: calcification present on core
Indicate whether calcification was present on any of the core biopsies undertaken. Identify the site of the lesion: atypical lesion, benign lesion, combination of atypia and benign, or site unknown.
Question 19: other benign lesion present on core
Indicate whether any other benign lesion was present on any of the core biopsies undertaken. If yes, identify the type of benign lesion: fibroadenoma, papilloma, radial scar, other. If other, specify type.
Question 20: surgical specimens
Indicate whether surgery was carried out. If yes, choose all surgical specimens that apply and the date the surgical specimen was taken.
Diagnostic open biopsy
Includes lumpectomy, microdochectomy (microductectomy) and other procedures taken for diagnostic rather than therapeutic purposes.
Therapeutic excision
Includes lumpectomy/wide local excision, wedge excision, segmentectomy, partial mastectomy and therapeutic mammoplasty specimens with the aim of complete excision of the lesion.
Bed biopsy/cavity shave
A specimen shaved from one or more edges of the biopsy cavity of a therapeutic excision sent separately by the surgeon.
Immediate re-excision specimen
A re-excision specimen, of a specified margin, taken at the time of therapeutic procedure.
Delayed re-excision specimen
A re-excision specimen, usually of biopsy cavity and a specified margin, taken at a subsequent surgical procedure.
Completion mastectomy
Mastectomy performed for the purposes of obtaining complete excision as a subsequent surgical procedure.
Mastectomy
Mastectomy performed for the purposes of obtaining complete excision including simple, skin-sparing and subcutaneous mastectomy specimens.
Question 21: disease present in surgical specimens
Indicate whether ADH, ALH, LCIS, PLCIS, and/or FEA are present in the specimen(s). Tick all that are identified. If no atypical proliferative disease is evident in the surgical specimen, as it appears to have been removed by the core biopsy, indicate in this section.
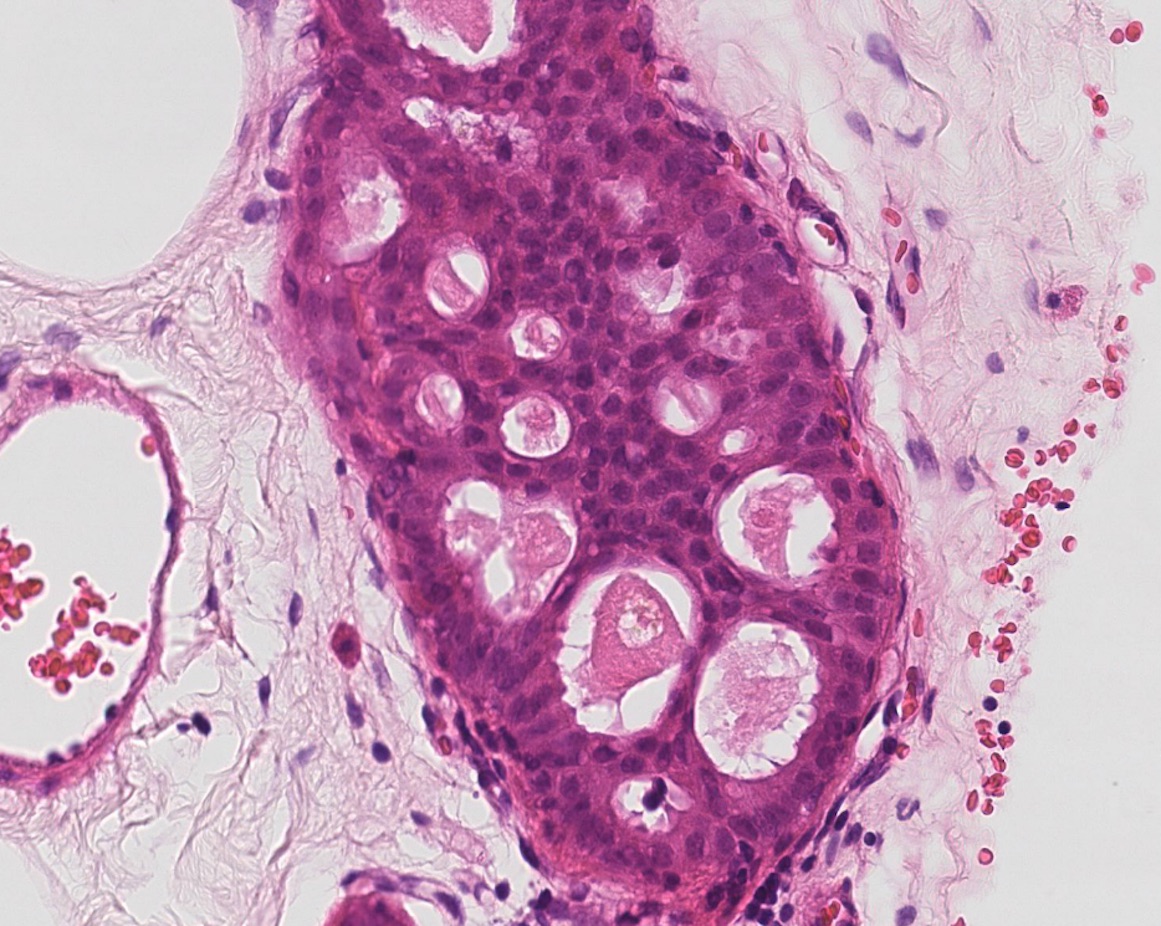
Figure 11: atypical intraductal epithelial proliferation (AIDEP)
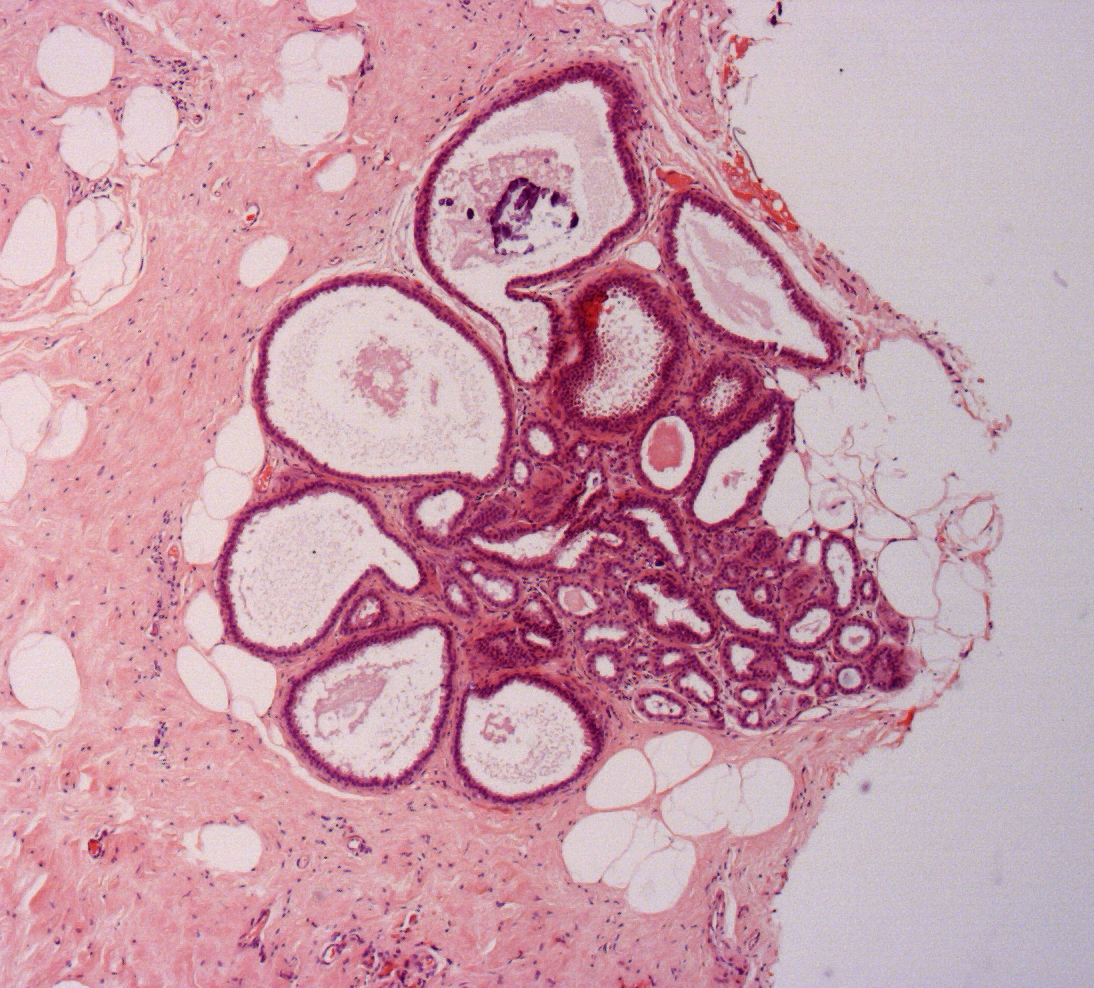
Figure 12: flat epithelial atypia (FEA)
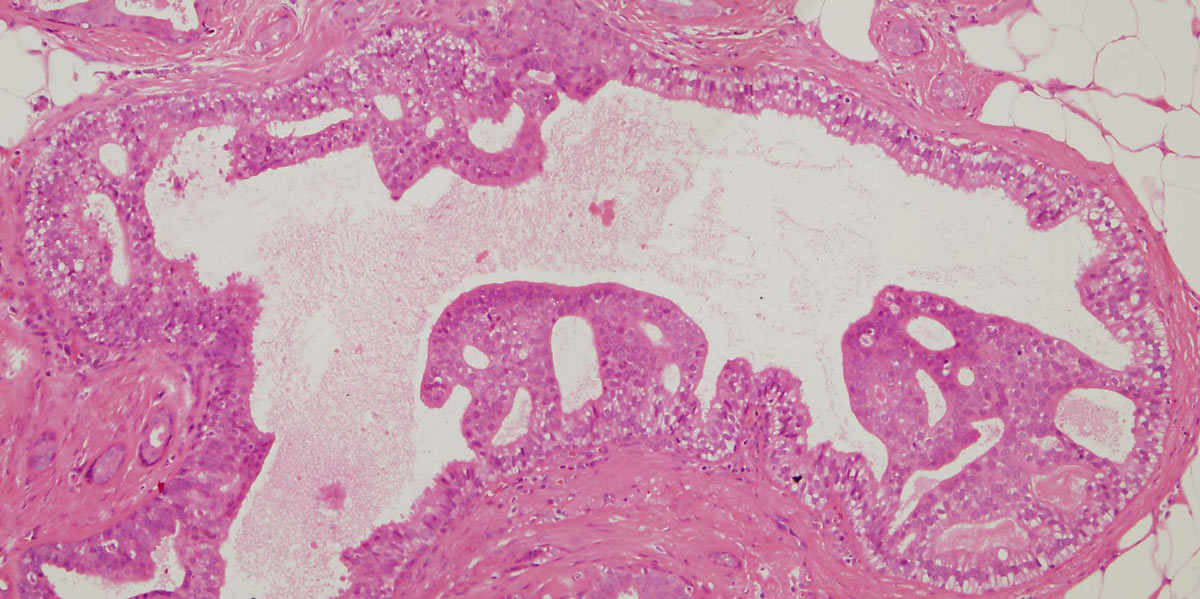 Figure 13: a small focus of ADH. Note partial involvement of the duct with focally a monotonous population of epithelial cells showing rigid structures and low grade cytology
Figure 13: a small focus of ADH. Note partial involvement of the duct with focally a monotonous population of epithelial cells showing rigid structures and low grade cytology
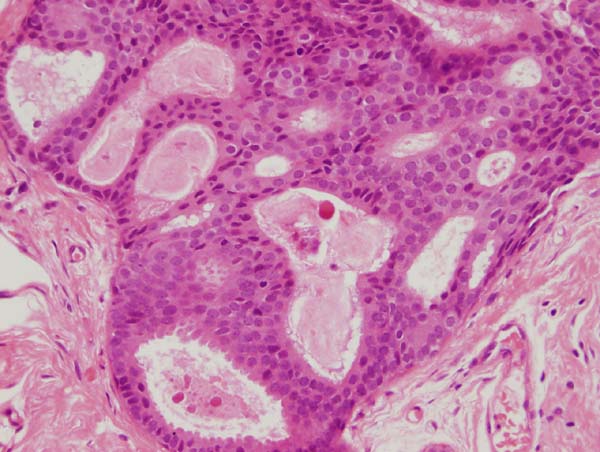
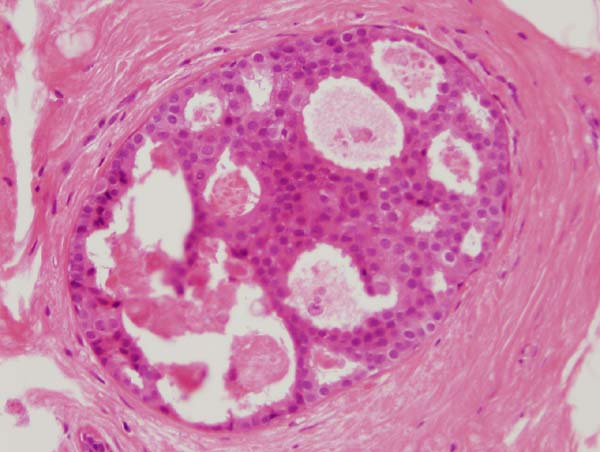
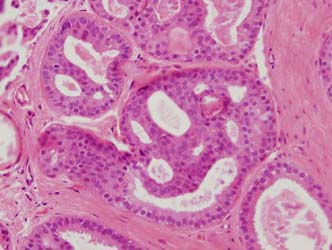
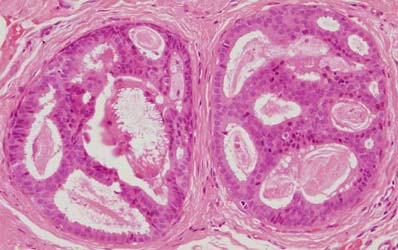
Figure 14: four images showing different views of a small focus of ADH. Note partial involvement of the duct, particularly in the top image, a monotonous population of epithelial cells showing rigid structures across ducts and low grade cytology. Luminal microcalcifications are present
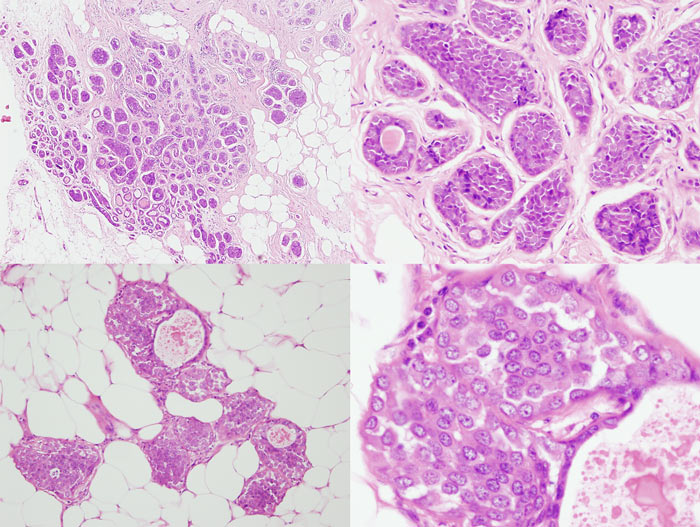 Figure 15: ALH examples
Figure 15: ALH examples
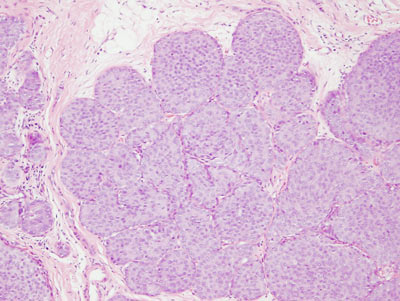
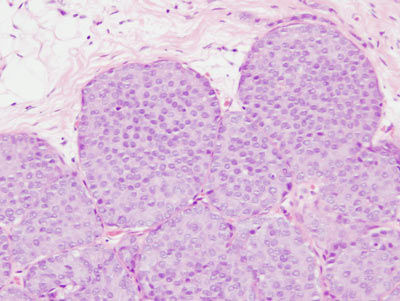
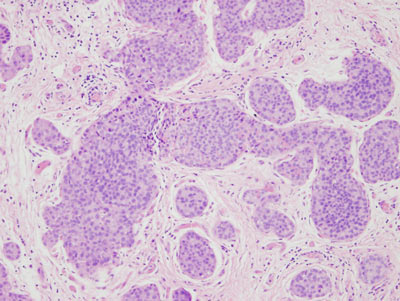
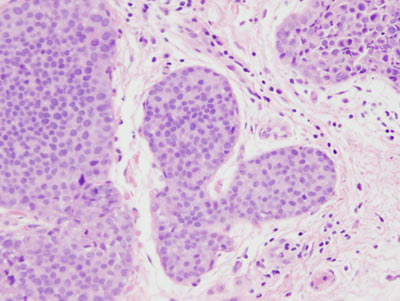
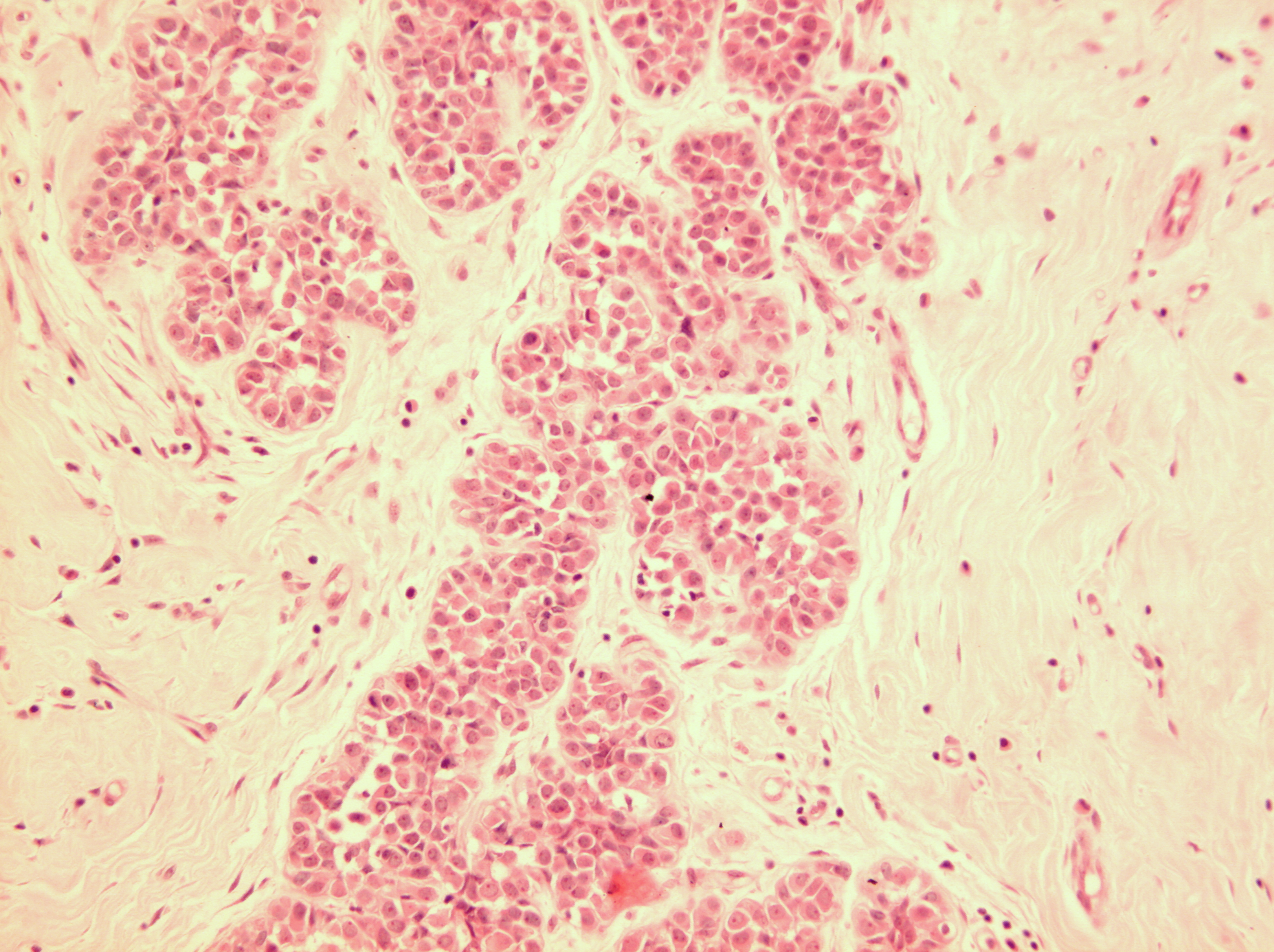
Figure 16: LCIS examples
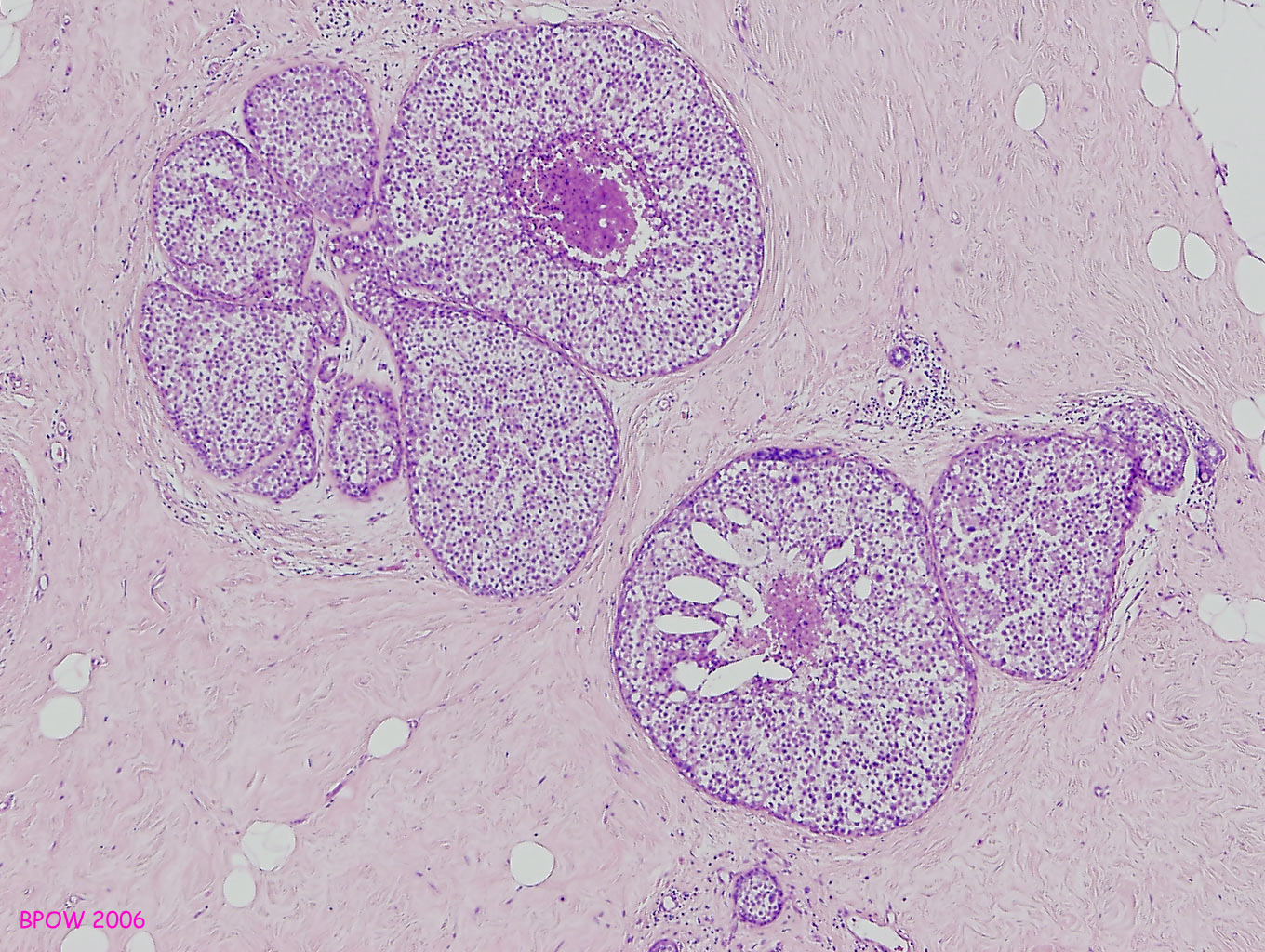
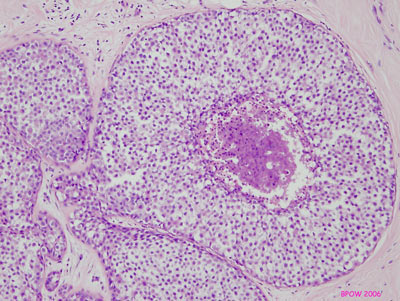
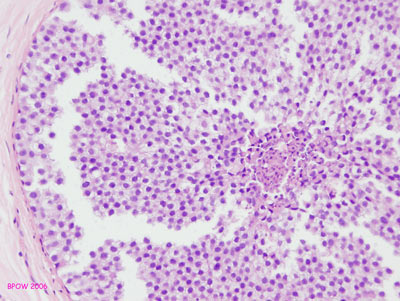
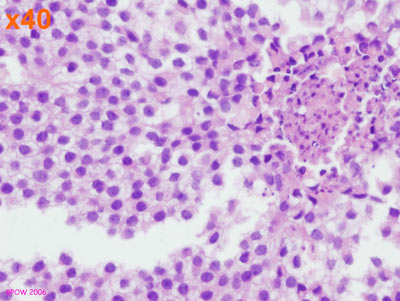
Figure 17: PLCIS/florid LCIS examples
Question 22: maximum (total) size of lesion on surgical specimen (mm)
Indicate the total size of the disease present in the lesion, if known. This may be a summation from the total in several specimens, for example diagnostic, therapeutic and re-excision samples, and may be an approximation.
Question 23: distance to margins in therapeutic excision
Does ADH, ALH, LCIS, PLCIS or FEA extend to the margin of the surgical specimen? If known/recorded, tick all disease that is present at the margin.
Question 24: calcification present in surgical specimen
Indicate whether calcification was present in any of the surgical specimens. Identify the site of the lesion: in the atypical lesion, benign lesion, combination of atypia and benign, or site unknown.
Question 25: comedo necrosis present
Indicate whether there is central necrotic debris present. Individual cell death is not considered to represent necrosis and should not be recorded.
Question 26: other benign lesion present on surgery
Indicate whether any other benign lesion was present on any of the surgical specimens undertaken. If yes, identify the type of benign lesion: fibroadenoma, papilloma, radial scar, other. If other, specify type.
Questions 27 to 29: nodes
Most of the patients in the atypia audit will not have had lymph nodes removed. However, some may have done and this is worthy of analysis.
Record the number of nodes examined, the number positive from the axilla and those that are sentinel nodes or others, such as internal mammary nodes. If no nodes were examined, indicate zero for each type of node.
You must also record at which operation the nodes were taken.
Questions 30 to 31: treatment strategy (non-surgical)
Indicate whether the treatment strategy following core biopsy or surgery included any of the following: radiotherapy, endocrine therapy, no further adjuvant therapy, other.
If endocrine therapy was given, indicate type. If other therapy was given, indicate type.
Questions 32 and 33: date and person completing form
Record the date the form was completed and print your name.
