MAVIS Newsletter edition 105
Published 1 February 2018
1. News
1.1 Membership of the Veterinary Products Committee
The Secretary of State has appointed the following new members to serve on the Veterinary Products Committee from January 2018 until 31 December 2021:
- Enrique Vega is a veterinary surgeon with experience of public health and food safety
- Mark White is a veterinary surgeon and pig consultant
- Yu-Mei Ruby Chang is a chartered statistician
- Andrea Tarr is a pharmacist
- Helen Ballantyne is a dual qualified registered nurse in veterinary and human health
- Rachel Bennett is a qualified veterinary surgeon with experience of clinical pharmacology
The current member, Professor Malcolm Bennett, has been appointed as Chair.
Six existing members have been reappointed to the committee to serve a further term of four years.
Requests for further information on membership of the VPC should be made to Sandra Russell s.russell@vmd.defra.gsi.gov.uk.
2. Licensing
2.1 Marketing Authorisations
Information on newly authorised and expired MAs is available in the Product Information Database.
Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
You can see lists on GOV.UK of:
- recently authorised MAs (previous 6 months)
- recently updated MAs (last 30 days)
- expired products
2.2 Quarterly reporting against VMD Published Standards for 2017/18 licensing work
This report is published on a monthly basis under Our Statistics.
For further information contact Natalie Shilling n.shilling@vmd.defra.gsi.gov.uk and put ‘MAVIS’ in the subject line.
2.3 Approved labelling text (QRD) to be published online
Guidance for Marketing Authorisation Holders
The approved labelling known as Quality Review of Documents (QRD) will be made available online from 1 April 2018. This will help increase the availability of product information to end users. Marketing Authorisations that do not have approved mock-ups will have a QRD text document watermarked as ‘No Approved Packaging’.
Maintaining your QRD
A QRD is now required for all veterinary medicines which is maintained throughout the product’s life.
You should submit a draft QRD:
- for all new MA applications
- for all variation-extension, renewal and variation applications, where the QRD is affected.
When QRD and mock-ups are not requested, we will annotate the agreed changes onto the latest authorised QRD and mock-ups and issue these to you.
If you wish to submit revised QRD text and mock-ups for assessment that is not covered by a specific variation category, i.e. to update the QRD using the latest template, you need to submit an application under cover of a Type IB variation (C.II.6(b)) which will be dealt with under normal variation procedures.
For further information email postmaster@vmd.defra.gsi.gov.uk and put ‘National QRD’ in the subject line.
2.4 Top ten imported veterinary medicines quarterly report from 1 October to 31 December 2017
A quarterly list of the ten products for which the most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Ingredient | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,655 |
| Vet-Goid | Allergens | 216 |
| Spectrum Hyposensitisation Vaccine – Injectable Solution | Allergens | 213 |
| Greer Allergenic Extract Patient Prescription | Allergens | 136 |
| Equine Rotavirus | Equine Rotavirus | 124 |
| ACTT Allergy Drops | Allergens | 94 |
| Hipravit AD3E Forte | Vitamin A Alpha Tocopheryl Acetate Cholecalciferol | 93 |
| Adequan i.m. | Polysulphated glycosaminoglycan | 88 |
| Staphage Lysate (SPL) | Staphylococcus aureus | 82 |
| Artuvetrin® Test, injection fluid for intracutaneous use in dogs | Allergens | 72 |
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
Guidance is available on obtaining a UK manufacturing authorisation (ManA) for an authorised veterinary medicine or an authorisation for an extemporaneous product (special) (ManSA).
Guidance is also available on obtaining a manufacturing authorisation for an equine stem cell centre (ESCCA), a non-food animal blood bank (NFABBA) or an autogenous vaccine (AVA).
3.2 GMP inspection deficiency findings April to December 2017
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
‘Other’ Deficiencies Apr 17 - Dec 18
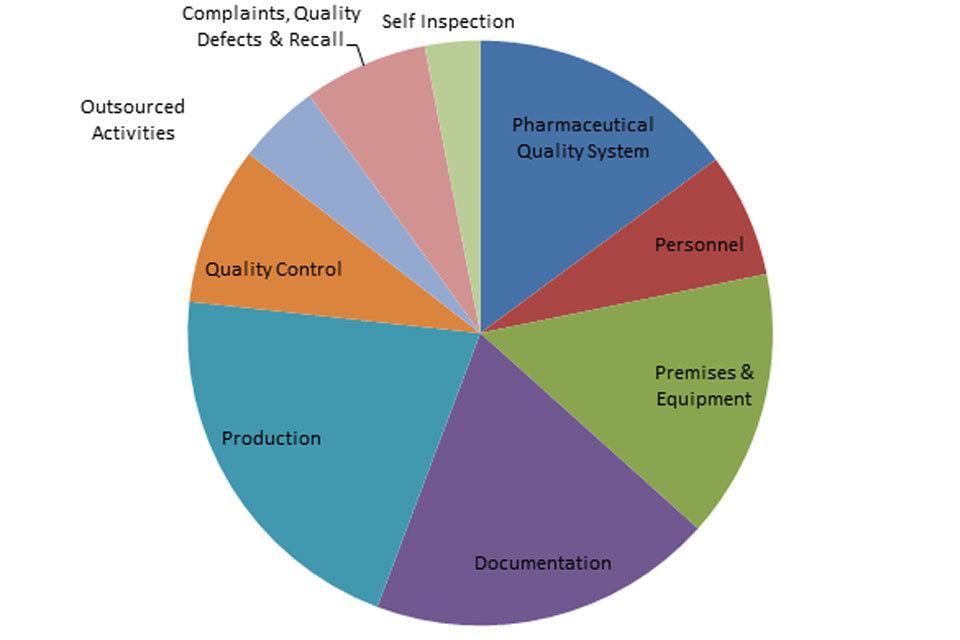
GMP Inspections Other Deficiencies April 2017 to December 2017
‘Major’ Deficiencies Apr 17 - Dec 18
3.3 Wholesale, retail and feedingstuffs
Guidance is available for:
- manufacturers and distributors of animal feedingstuffs containing veterinary medicines and/or coccidiostats (referred to in the Veterinary Medicine Regulations as specified feed additives; SFAs)
- veterinary-only wholesale dealers to assess compliance against Good Distribution Practice businesses whose premises are approved for the retail supply of POM-VPS and NFA-VPS medicines by suitably qualified persons (SQP retailers)
- veterinary practice premises (VPPs), other than those accredited by the Royal College of Veterinary Surgeons (RCVS) under its voluntary Practice Standards Scheme (PSS)
Changes to the approval process for SQP Retailers
For the past three months we have been trialling a new process for approving SQP Retailer premises.
Under the revised process applicants are required to submit details of their retail premises, including photographs and floor plans, showing where restricted products will be stored. Provided this information is satisfactory, the premises is given ‘Conditional Approval’ which allows the business to start trading immediately. We will then carry out an on-site inspection within 3 months and, providing all is in order, grant full Approval. The aim of this revised process is to minimise the delay before an applicant can begin supplying VPS medicines whilst assuring ourselves that the premises and storage areas are suitable. It will also mean that, unlike the previous process where we often inspected an empty premises and then returned for a further inspection 6-12 months’ later, we will now be able to inspect the business ‘in operation’ and set a standard re-inspection date.
This change has received very positive feedback from SQP Retailers.
Changes to the risk rating descriptions for retailers
Following an inspection we currently rate veterinary practice premises (VPPs) and SQP Retailers as ‘Good’, ‘Acceptable’, ‘Poor’ or ‘Unacceptable’ depending on the number and type of non-compliances (deficiencies) observed. The rating then determines the premises’ re-inspection interval.
From 1 April 2018, we will be moving to a numerical rating system which will allow better analysis of inspection trends. The ratings will be from 1 – 4, with a score of 1 being equivalent to ‘Poor’ and 4 being equivalent to ‘Good’.
This change will have no impact on current inspection intervals.
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 September to 30 November 2017, the VMD received 2,208 animal suspected adverse event reports. Of the 2,834[footnote 1] products involved in these reports, 2,725 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,452 | Prescription Only Medicine Veterinarian (POM V) |
| 179 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 55 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 39 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 109 products were:
| Amount | Type |
|---|---|
| 42 | Authorised human medicines |
| 5 | Medicine used in a trial under an Animal Test Certificate |
| 12 | Veterinary products without any medicinal claim |
| 3 | Authorised medicines imported from other countries |
| 4 | Medicines sold under the exemption for small pet animals |
| 1 | Specified feed additive |
| 16 | Specially formulated veterinary medicines |
| 26 | Incompletely identified products |
During this period 37 reports of human suspected adverse reactions were received. Of the 44[footnote 1] products involved in these reports, 44 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 29 | Prescription Only Medicine Veterinarian (POM V) |
| 8 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 2 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 1 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining four products were:
| Amount | Type |
|---|---|
| 4 | Authorised human medicines |
No environmental incident reports were received during this period.
For further information contact Roy Savory r.savory@vmd.defra.gsi.gov.uk
5. Enforcement
We publish a list of prosecutions and notices involving illegal activity with veterinary medicines within the last year.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 7 December 2017 to discuss the recent trends in antibiotic resistance in bacteria of importance to human and animal health. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined the work towards the upcoming 5 year UK AMR strategy, with contributions from the devolved administrations. Minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
6.2 Sales Data and Antibiotic Resistance Surveillance Report
The UK-Veterinary Antibiotic Resistance and Sales Surveillance 2016 report was published on 27 October 2017 and launched at the RUMA-VMD conference. The report presented combined data on veterinary antibiotic sales and antibiotic resistance in bacteria from animals in the UK.
The report highlighted some important milestones: In response to the O’Neill review, the government committed to reduce antibiotic use in livestock and fish farmed food to a multi-species average of 50 mg/kg by 2018, from 62 mg/kg in 2014. This has been achieved two years early with a reported 27% decrease of antibiotic usage to 45 mg/kg. For the first time, the report also included antibiotic usage data from the pig, meat, poultry, egg, gamebird and dairy industries.
The 2016 report, highlights report and supplementary materials are also available.
6.3 RUMA-VMD Conference Antibiotic Resistance – ‘Facing up to the AMR challenge’
On 27 October 2017, RUMA and the VMD hosted an interactive conference which addressed key subjects relating to antimicrobial resistance in animal health. The conference featured notable speakers such as Lord Gardiner, Gwyn Jones, Nigel Gibbens, Paul Cosford and Pete Borriello, directing discussions on the use of antimicrobials in food-producing animals and outlining future actions on ensuring responsible and sustainable practices.
The sector specific targets for reducing antibiotic use across the key livestock sectors were announced at the conference. These targets were developed by a ‘Targets Task Force’ facilitated by RUMA and published in Targets Task Force Report 2017.
6.4 UK 5 year AMR strategy high level steering group meeting
Cross government representatives met in November to discuss the early framework for the next UK 5 year antimicrobial resistance strategy. The group met to consider the recent publication of the UK-VARSS report and sector specific targets, as well as discuss any implications of EU exit on the future strategy.
For further information contact Alexandra Pickering a.pickering@vmd.defra.gsi.gov.uk
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in September 2017. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
For further information contact Sandra Russell s.russell@vmd.defra.gsi.gov.uk
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2017 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
9. Organogram as at 31 January 2018
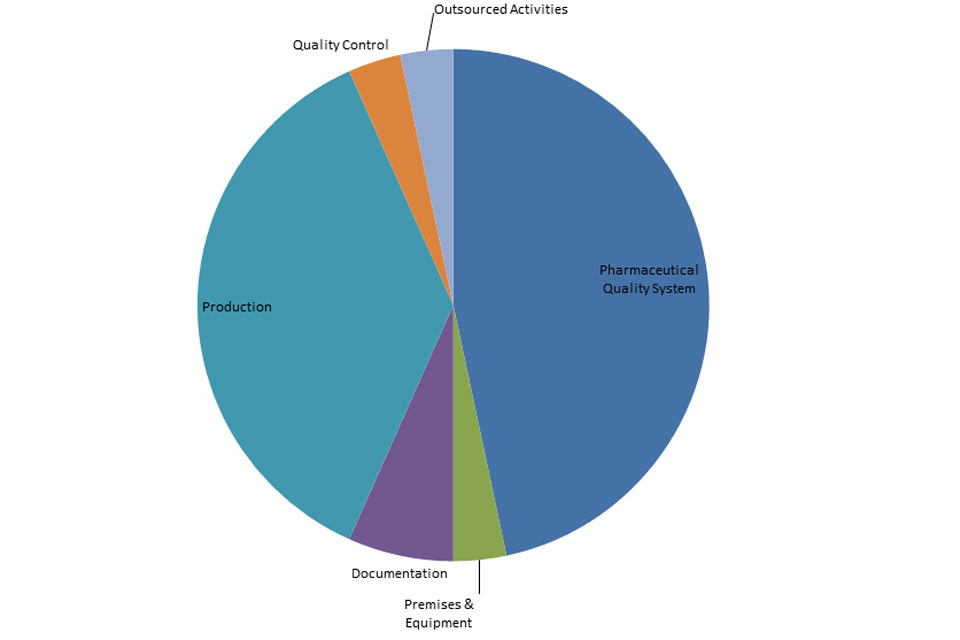
GMP Inspections Major Deficiencies April 2017 to December 2017
