MAVIS Newsletter edition 104
Published 25 October 2017
1. News
1.1 The VMD Customer Satisfaction Survey 2018: Improving our service to you
On 30 October 2017 the VMD will launch its customer satisfaction web survey 2018. If you work for, or on behalf of, Marketing Authorisation holders and holders of Manufacturing, Wholesaler Dealer, Autogenous Vaccine, Blood Bank, Equine Stem Cell and Overseas Manufacturing Authorisations you are invited to complete this survey.
The deadline for completion of the survey is 20 November 2017.
We would welcome returns from any individual in your company who regularly deals with the VMD. Seeking feedback from those people who use the Regulatory services of the VMD is something extremely important to us. We are very keen to understand better how well we meet your needs; what we do well and where we could improve. In this way we, both VMD and you as stakeholders, can be confident that we are striving to continuously improve our service in line with your needs and expectations.
Mo Gannon & Associates Ltd, an independent market consultancy which specialises in agriculture and animal health, is carrying out this survey on our behalf. Mo Gannon has been involved in the previous surveys so can compare and contrast the findings with those from previous years to help us collect evidence for the areas where we have improved in response to previous feedback and also where further improvement or remedial action may still be needed. Anonymity is guaranteed so that no individual or company will be identified against any statement. However, the VMD will be informed of how many individuals from each particular company have completed the survey. Once Mo Gannon & Associates Ltd has closed the web survey and analysed the responses, they may make a request to those of you who agreed to further contact, to add clarity to your responses. The VMD will not be involved, and the feedback given to the VMD will be anonymous.
Ultimately, we will use the findings from the survey and any follow up discussions to improve the services we provide. We will also make the results of the survey and any planned improvements available to you.
If you have not received an email with a link to the survey, or have any questions about any aspect of the survey, please contact David Lewsey in our business support team on 01932 338332 or by email d.lewsey@vmd.defra.gsi.gov.uk.
1.2 Membership of the Veterinary Products Committee
We started the process to appoint ten new members to the Veterinary Products Committee (VPC) on 11 September 2017 and are looking to appoint:
- Chair
- Veterinary surgeon (public health)
- Veterinary surgeon (pigs)
- Veterinary nurse
- Pharmacologist
- Specialist in novel therapies (in particular stem cells)
- Lay member
- Food safety risk assessor (consumer safety)
- Pharmacist
- Statistician/Epidemiologist
Please contact Chris Abbott for more information and an application pack c.abbott@vmd.defra.gsi.gov.uk.
The closing date for receipt of applications is 27 October 2017.
2. Licensing
2.1 Marketing Authorisations
We no longer publish information about varied Marketing Authorisations (MAs). Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
Information on newly authorised and expired MAs is available in the Product Information Database.
You can see lists on GOV.UK of:
- recently authorised MAs in the previous 6 months
- expired products
2.2 Quarterly reporting against VMD Published Standards for 2016/17 licensing work
This report is published on a monthly basis under Our Statistics.
For further information contact Natalie Shilling n.shilling@vmd.defra.gsi.gov.uk and put ‘MAVIS’ in the subject line.
2.3 Validation during the Christmas period 2017
New Marketing Authorisation applications
The last validation meeting to discuss applications for new Marketing Authorisations will take place on 21 December 2017. Applications to be considered for validation must be received on or before 18 December 2017. Weekly validation meetings will resume week commencing 1 January 2018.
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
Manufacturing and Wholesale Dealers Authorisation applications (new and variations)
The last day for validation of applications for Authorisations for Manufacturers, Blood Banks, Equine Stem Cell Centres and Wholesale Dealers (new and variations) will be 15 December 2017. To be considered for validation by this date, please ensure that your application reaches us by 13 December 2017. The validation discussions will resume week commencing 1 January 2018.
For further information contact Justin Murphy inspections@vmd.defra.gsi.gov.uk
Export Certificates and Batch Release requests
The last day for receiving export certificates and batch release requests will be Tuesday 19 December 2017. Any requests received after this date will be processed during the week commencing 1 January 2018.
For further information contact batchr@vmd.defra.gsi.gov.uk or exportcert@vmd.defra.gsi.gov.uk
2.4 Top ten imported veterinary medicines quarterly report from 1 July to 30 September 2017
We provide a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Ingredient | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,804 |
| Vet-Goid | Allergens | 261 |
| Stimovar Injection | Serum Gonadotrophin | 152 |
| Greer Allergenic Extract Patient Prescription | Allergens | 126 |
| Oncept (Canine Melanoma Vaccine) | Canine Melanoma DNA | 94 |
| Staphage Lysate (SPL) | Staphylococcus Aureus | 86 |
| Ekyflogyl 125mls | Prednisolone Acetate Lidocaine Hydrochloride Dimethyl Sulphoxide | 84 |
| Genta-Ject 10% | Gentamicin Sulphate | 81 |
| ACTT Allergy Drop | Allergens | 81 |
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice Inspection Team (GMPIT)
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
Guidance is available on obtaining a UK manufacturing authorisation (ManA) for an authorised veterinary medicine or an authorisation for an extemporaneous product (special) (ManSA).
Guidance is also available on obtaining a manufacturing authorisation for an equine stem cell centre (ESCCA), a non-food animal blood bank (NFABBA) or an autogenous vaccine (AVA).
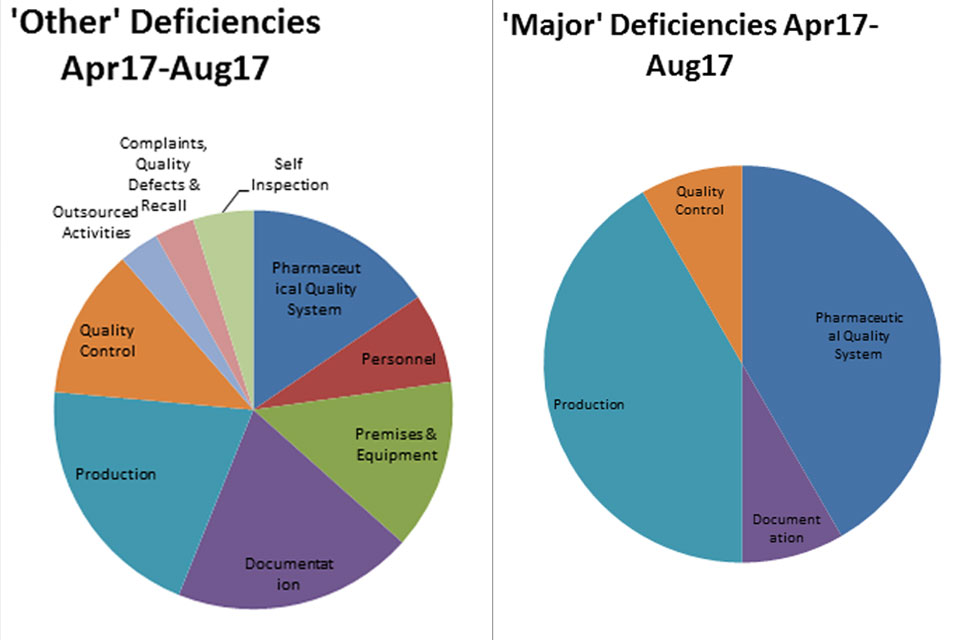
GMPIT inspection deficiency findings April to August 2017
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
3.2 GMP Update: Annex 16 – Certification by a Qualified Person and Batch Release
This revised annex became operational on the 15 April 2016. This now contains updated guidance on:
- Scope
- General Principles
- The Process of Certification
- Relying on GMP assessments by Third Parties, for example Audits
- Handling of unexpected Deviations
- The Release of a Batch
Qualified Persons should ensure that they are familiar with the guidance as compliance with the above expectations is evaluated during GMP inspections.
4. The Inspections and Investigations Team (IIT)
Guidance is available for:
- manufacturers and distributors of animal feedingstuffs containing veterinary medicines and/or coccidiostats (referred to in the Veterinary Medicine Regulations as specified feed additives; SFAs)
- veterinary-only wholesale dealers to assess compliance against Good Distribution Practice businesses whose premises are approved for the retail supply of POM-VPS and NFA-VPS medicines by suitably qualified persons (SQP retailers).
- veterinary practice premises (VPPs), other than those accredited by the Royal College of Veterinary Surgeons (RCVS) under its voluntary Practice Standards Scheme (PSS)
The VPP and SQP inspection criteria have recently been published on GOV.UK. These documents explain the legal requirements and best practice for VPPs and SQP retailers.
The SQP Code of Practice has also been updated on GOV.UK. The Code now includes a requirement for a Nominated SQP to be responsible for professional standards within approved premises. The nominated SQP should take overall responsibility for how veterinary medicines are obtained, stored, supplied and disposed of. They should also ensure that colleagues recognise the professional responsibilities of SQPs.
A business with multiple approved premises may nominate a single SQP to be responsible for all premises, or have individually nominated SQPs for each premises.
4.1 The Inspections Administration Team (IAT)
The IAT provides administrative support for the GMPIT and the IIT including:
- processing applications (including variations) for authorisations to manufacture or wholesale veterinary medicines
- processing applications for approval of premises as feed business operators or SQP retailer premises
- arranging inspections.
The IAT also co-ordinates the assessment of product defect reports submitted by Marketing Authorisation Holders and rapid alert notifications circulated by other National Competent Authorities. The VMD provides an analysis of quality product defects received 2016-17.
5. Pharmacovigilance reports
5.1 Quarterly report
During the period 1 June to 31 August 2017, the VMD received 1,736 animal suspected adverse event reports. Of the 2,634[footnote 1] products involved in these reports, 2,510 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,311 | Prescription Only Medicine Veterinarian (POM V) |
| 111 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 57 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 31 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 124 products were:
| Amount | Type |
|---|---|
| 59 | Authorised human medicines |
| 6 | Medicine used in a trial under an Animal Test Certificate |
| 25 | Veterinary products without any medicinal claim |
| 5 | Authorised medicines imported from other countries |
| 6 | Medicines sold under the exemption for small pet animals |
| 9 | Specially formulated veterinary medicines |
| 14 | Unidentified products |
During this period 50 reports of human suspected adverse reactions were received. Of the 59[footnote 1] products involved in these reports, 55 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 45 | Prescription Only Medicine Veterinarian (POM V) |
| 7 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 1 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 2 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining four products were:
| Amount | Type |
|---|---|
| 4 | Authorised human medicines |
One environmental incident report was received during this period which involved an incompletely identified product.
For further information contact Roy Savory r.savory@vmd.defra.gsi.gov.uk
6. Enforcement
A list of prosecutions and notices involving illegal activity with veterinary medicines in the last year can be found on GOV.UK.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
7. Antimicrobial Resistance
7.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 14 September 2017 to discuss recent trends in antibiotic resistance in bacteria of importance to human and animal health. The group received an update on sector-specific antibiotic consumption data projects currently under development, and discussed international collaborations in which they took part. The afternoon included topical presentations focused on the existing evidence for dissemination of antibiotic resistance in the environment, including slurry management systems and river catchments. Minutes of this meeting and previous meetings will be published on GOV.UK in due course.
7.2 Sales Data and Antibiotic Resistance Surveillance Report
Data for the 2016 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report are currently being collated for publication towards year end. The report collates data on antibiotic sales from UK Marketing Authorisation Holders and antibiotic resistance data from the VMD’s surveillance programmes.
UK-VARSS 2015 and previous reports are available on GOV.UK.
For further information contact Stacey Brown s.brown@vmd.defra.gsi.gov.uk
7.3 RUMA-VMD Conference Antibiotic Resistance – ‘Facing up to the AMR challenge’
On 27 October 2017, RUMA and the VMD will host an interactive conference which will address key subjects relating to antimicrobial resistance in animal health. Notable speakers and chairs such as Lord Gardiner, Gwyn Jones, Nigel Gibbens, Paul Cosford and Pete Borriello will prompt discussions on the use of medicines in food producing animals and future actions to take to ensure safe food and sustainable food producing practices.
For further information contact rumaconference@btinternet.com
8. Veterinary Products Committee
8.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in September 2017. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
The VPC held its Open meeting on 29 September 2017. Presentations given by two members, Professor Jacqueline Matthews and Declan O’Rourke are available online.
For further information contact Sandra Russell s.russell@vmd.defra.gsi.gov.uk
9. Residues controls and monitoring
9.1 Results of Statutory Surveillance
Sampling commenced in January 2017 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
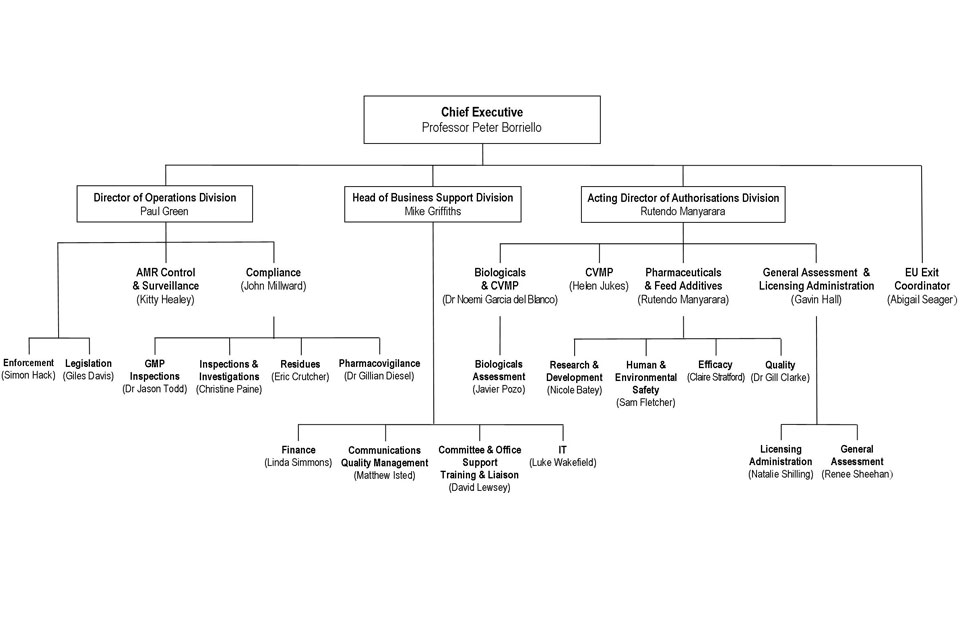
10. Organogram as at 1 October 2017
VMD Organogram as at 1 October 2017
