MAVIS Hub Edition 116
Published 9 November 2020
1. Product news
Johnson’s 4Fleas 80mg Spot-on Solution for Cats - Product defect recall alert
Prednidale 5 mg Tablets - Product defect recall alert 2
Availability of Lactating Cow Intramammary Antibiotics
Carprieve 50 mg/ml Solution for Injection for Cattle - Product defect recall alert
Gallifen 200 mg/ml Suspension, Pigfen 200 mg/ml Suspension - Product defect recall alert
2. Enforcement
Animal medicine improvement notice: Wynnstay Group
Prosecution of Mr Michael Dawson, trading as Element Bullys Limited, Plymouth
Animal medicines seizure notices: Element Bullys Ltd
Animal medicines seizure notice: Susan Bello-Pearson, Dezinerbullz Ltd (Jan)
Animal medicines seizure notice: Susan Bello-Pearson, Dezinerbullz Ltd (April)
3. AMR (Antimicrobial Resistance)
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The September DARC meeting did not take place due to other work commitments and current circumstances. The next meeting will be taking place on Tuesday 24 November.
Minutes from previous meetings are available.
3.2 Communications
Preparations for communications and the launch of VARSS 2019 are taking place, which will coincide with European Antibiotic Awareness Day and World Antimicrobial Awareness Week (WAAW) 2020. This will be the VMD’s main output for the week as well as sharing other stakeholder’s communications.
VMD are working with Cefas, PHE and APHA to create a leaflet promoting the UK’s Reference Centres and a One Health approach to tackling AMR.
4. Inspections
Due to COVID-19 the Inspections and Investigations Team suspended all on-site inspections on the 18 March. After a considerable review and implementation of appropriate safety measures, on-site inspections resumed on the 24 August with some aspects taking place remotely.
There were a very limited number of remote inspections carried out between 18 March and 24 August. As there is little data for the period 1 June to 31 August no report is available. These data will be included in the next reporting period and in the next edition of MAVIS.
For the latest information on VMD inspections please refer to the VMD website
5. Guidance Updates
VMD temporary enforcement policy under specific COVID‐19 circumstances
The VMD web services will be unavailable from Sat 22 to Sun 23 August
VMD on-site inspections to re-start 24 August
Coronavirus (COVID-19) VMD News and Guidance
Controlled drugs: recording, using, storing and disposal
The VMD web services will be unavailable from Friday 18 Sept 6pm - Sun 5pm
From 1 January 2021 Application and Authorisation Process explainer
Northern Ireland Protocol for veterinary medicines explainer
Veterinary Medicines Digital Service
Export drugs and medicines: special rules
From 1 January 2021 Import and export of veterinary medicines explainer
Veterinary medicine wholesale dealer’s authorisation (WDA)
From 1 January 2021 MAH and Authorised Personnel Location
Report a product defect: veterinary medicine
From 1 January 2021 Pharmacovigilance System and Qualified Person for Pharmacovigilance explainer
1 January 2021 Adverse Event Reporting, Periodic Safety Update Reports and concerns
Centrally Authorised Products conversion explainer
6. Research and Statistics
Top ten imported veterinary medicines - Quarterly report 1 April 2020 - 30 June 2020
Published standards for regulatory work 2020-2021
VMD Published Standards 2020 to 2021: Monitoring performance
Top ten imported veterinary medicines - Quarterly report 1 July 2020 to 30 September 2020
7. Stakeholder Engagement
FOI requests received by the VMD between 1 January to 30 June 2020
8. Organogram as at 31 October 2020
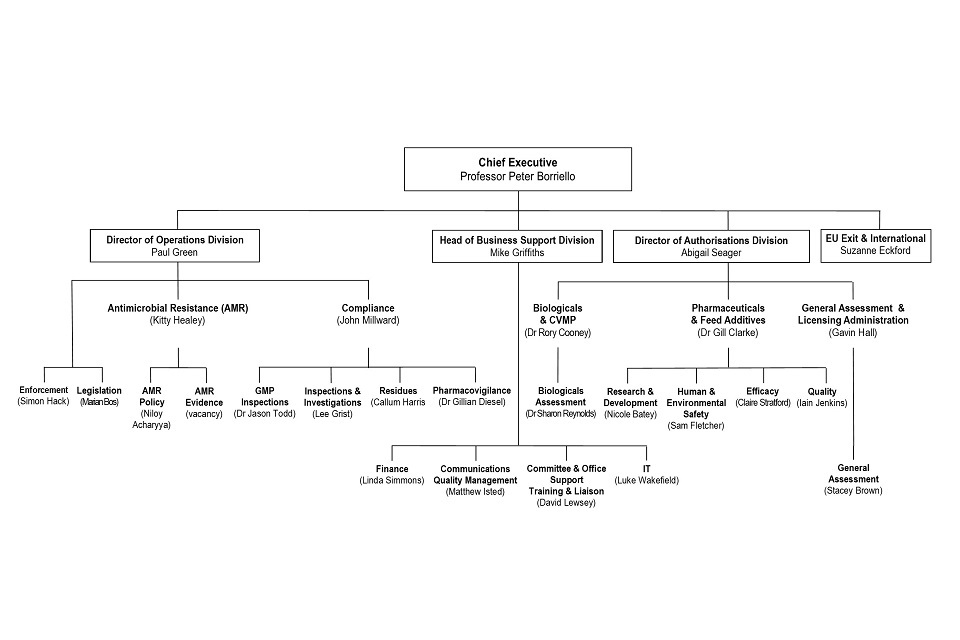
VMD Organogram
