MAVIS Hub Edition 115
Published 31 July 2020
1. Product news
Availability of Lactating Cow Intramammary Antibiotics
2. Enforcement
Animal medicine seizure notice: Border Force, Inverness Airport
3. AMR (Antimicrobial Resistance)
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met virtually on 30th June 2020 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to animal and public health. The meeting included a recap on discussions from the February meeting and ambitions for the future of AMR monitoring and surveillance. Updates on current AMR priorities from the DARC group members were also provided, including from APHA, PHE, Welsh Government, Scottish Government, DAERA, FSA and the Environment Agency.
Summary minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
3.2 Communications
EAAW/WAAW 2020 discussions have begun, kicking off with a planning meeting with PHE and other stakeholders including NHS England. The group discussed campaign ideas for 2020, which will probably be digitally focussed and the impact the week will have this year due to Covid-19.
4. Inspections
During the period from 01/03/20 to 30/05/20 the Inspections and Investigations Team carried out a total of 40 inspection visits of retail and feed business premises. Please note that due to the current pandemic, no inspections have been carried out since 17/03/20.
4.1 Retail Premises
There were 24 retail premise inspection visits (vet practice premises and SQP retailers). The compliance ratings for these inspection visits are as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage* | ||
|---|---|---|---|---|
| 5 | 6 | 25 | ||
| 4 | 13 | 54.17 | ||
| 3 | 5 | 20.83 | ||
| 2 | 0 | - | ||
| 1 | 0 | - |
The most common issues that required corrective action were:
- Controlled Drug (CD) Registers not maintained as required
- Broach dates not recorded or exceeded
- Insufficient temperature records for ambient and/or cold chain products
The requirements for retailers are published on Gov.UK
4.2 Feed Business Operator premises
There were 16 Feed Business Operator (FeBO) premise inspection visits. The compliance ratings for these inspection visits are as follows (5 being the highest, 1 being the lowest):
| Rating | No. of premises | Percentage* | ||
|---|---|---|---|---|
| 5 | 5 | 31.25% | ||
| 4 | 8 | 50% | ||
| 3 | 3 | 18.75% | ||
| 2 | 0 | - | ||
| 1 | 0 | - |
- discrepancy of 0.1% in total due to rounding
The most common issues that required corrective action were:
- HACCP plan not documented/implemented/maintained as required
- Recall procedure not tested/ recorded.
- Maintenance and/or cleaning records not of the required standard
The requirements for Feed Business Operator (FeBO) premises are published on Gov.UK
5. Guidance Updates
Veterinary practice premises: temporary change of premises
Veterinary surgeons: temporary changes to the retail supply of veterinary medicines
SQPs temporarily allowed to prescribe and authorise supply of veterinary medicines remotely
Extemporaneous products: temporary change of supply
SQP retailer premises: temporary change of premises
Changes to exemptions from the Veterinary Medicines Regulations (VMR) for small pet animals
6. Research and Statistics
Top ten imported veterinary medicines - Quarterly report 1 April 2020 - 30 June 2020
Residues of veterinary medicines in food 2020 surveillance results.
7. Corporate News
VMD’s Annual Report & Accounts 2019/20 Published
Extramural Studies (EMS) Placements at the VMD
VMD incoming postal deliveries – Request to not send post to the VMD office.
Veterinary Medicines Directorate office closure over May Bank Holiday period
The Special Imports System will be unavailable 20 and 21 June 2020
8. Organogram as at 31 July 2020
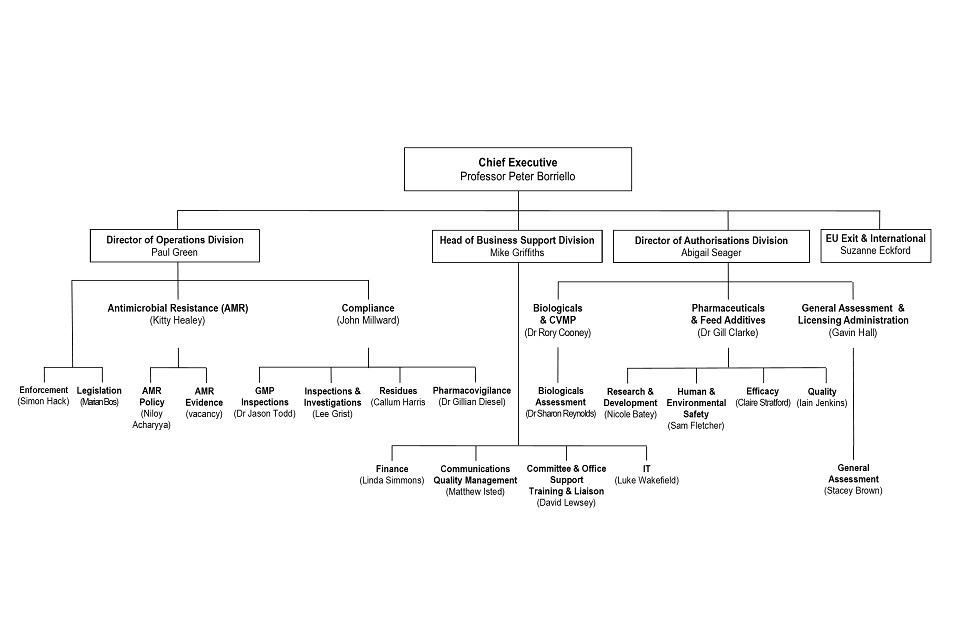
VMD Organogram as at 31 July 2020
