Regulation 1223/2009 and the Cosmetic Products Enforcement Regulations 2013: Great Britain
Updated 4 May 2023
Guidance on the regulations as they apply to cosmetic products being supplied in or into Great Britain.
May 2023
Introduction
1) This Guide is for businesses placing cosmetics products on the market in Great Britain (GB). Great Britain is England, Scotland and Wales. If you are placing cosmetic products on the market in Northern Ireland, you should read the separate guidance for placing cosmetic products on the market in Northern Ireland. This guide is designed to help you understand Regulation (EC) No 1223/2009 on Cosmetic Products, as amended by the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019. The Regulation sets out requirements that must be met before cosmetics products can be placed on the GB market. The purpose of the Regulation is to safeguard public health and establish a fully competitive market.
Main changes to Regulations
2) The main changes to note that took effect at 11pm on 31 December 2020 are:
- Placing on the market: the term ‘placing on the market’ means the first making available of a product on the market of Great Britain after the end of the transition period. “Making available” refers to supply on the GB market; [Article 2]
- Responsible Person: There must be a Responsible Person based in the UK under the new regime; [Articles 4, 5 and 5A]
- Product Information File: An up to date Product Information File (PIF) must be maintained in English, and made available to market surveillance and enforcement authorities at the UK address provided when asked to do so; [Article 11]
- Labelling: There will be a seven year see footnote 1 transition period from 1 January 2021 before businesses have to include the UK Responsible Person details on product labels, provided the EU responsible person details are included. This will enable existing stocks to make their way through the supply chain and reflects the typical shelf-life of a cosmetic and business’ labelling cycles; [Article 19]
- UK Submit Cosmetic Product Notification (SCPN) service: The UK Government has established a cosmetic product notification service to replace the EU’s Cosmetics Products Notification Portal (the ‘CPNP’) in Great Britain; [Article 13]
-
Notification of cosmetic products to Secretary of State (via UK SCPN service):
- For products that have not previously been notified to the Commission via the CPNP or have not been placed on the EEA market, or are placed on the GB market after 31 December 2020, UK Responsible Persons will need to provide full information to the Secretary of State via the UK SCPN service before they can be placed on the market. [Article 13]
- Serious Undesirable Effects (SUEs): SUEs should be notified on the new UK SUE form. Information on any SUE should be notified to OPSS at seriousundesirableeffects@businessandtrade.gov.uk; [Article 23]
- Products with nanomaterials: Where the inclusion in a cosmetic product of relevant nanomaterials has not been notified to the Commission prior to the end of the transition period on 31 December 2020, a cosmetic product containing novel nanomaterials not used for the purposes of colourant, preservative, or UV-filter must be notified to the Secretary of State, via the UK’s Submit Cosmetic Product Notification Portal by the Responsible Person 6 months prior to it being placed on the GB market; [Article 16]
- Importers: Since 1 January 2021 UK Businesses who bring cosmetic products into GB from an EU Member State are ‘importers’ where they would previously have been ‘distributors’. The importer of a cosmetic product, whether from the EU or another country, becomes a Responsible Person by default, although they may appoint an agent to act as the Responsible Person for them. There are particular provisions for Northern Ireland businesses who place products from countries outside of the UK (including the EEA) on the market in Great Britain; [Articles 2 and 4]
- Unfettered access: The government has committed to deliver unfettered access for qualifying Northern Ireland goods to the rest of the UK market. Cosmetic products that are placed on the market in Northern Ireland (in accordance with the European Union Regulation (EC) No 1223/2009 on Cosmetic Products, as it applies in Northern Ireland) can be sold in the rest of the UK with no additional approvals. To protect consumers across the UK, Northern Ireland businesses who are Responsible Persons, must notify the GB regulator via the UK Submit Cosmetic Product Notification (SCPN) service with the cosmetics’ ingredients and alert the GB regulator if their product is found to be unsafe. This will not require any new information to be collated: only that the same information provided to the EU via the CPNP service is also provided to the Secretary of State. This only applies to products that are placed on the GB market after the transition period. This approach applies to qualifying Northern Ireland goods. Further detail is available in Appendix 7.
To Note: for cosmetic products also contained in aerosol dispensers, the aerosol dispensers will need to meet the requirements of the Aerosol Dispensers Regulations 2009, which includes the requirement to mark the dispenser with UKCA compliance mark, which is replacing the reversed epsilon mark. There is dual recognition of the reversed epsilon (“3”), until 31 December 2021. The switch to the UKCA marking is compulsory from 1 January 2022. Until 31 December 2027, the UKCA marking may be affixed to a label affixed to or a document accompanying the dispenser. See footnote 1
The Regulation
Introduction
3) The Regulation (EC) No 1223/2009 on Cosmetic Products as amended by the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 (hereafter ‘the Regulation’) is grouped into 9 main subject Chapters.
4) This guide seeks to provide practical advice with respect to the Regulation as it applies in Great Britain. If you require further advice or information, you should contact the Trading Standards department of your Local Authority or Unitary Authority.
Chapter I – Scope and Definitions
Article 1 – Scope and Objective
5) The Regulation applies to all cosmetic products made available on the GB market. There is separate guidance available for placing cosmetic products on the market in Northern Ireland. Because of the way ‘making available on the market’ is defined, the Regulation applies to any supply of cosmetic products in the course of a commercial activity, including situations where products are given away for free. The Regulation has two aims: to ensure the functioning of the GB market, and to ensure a high level of protection for human health.
Article 2 – Definitions
Cosmetic Product
6) The definition of a cosmetic product comprises three parts: a function, field of application, and product composition. All parts of the definition must be satisfied.
7) The Regulation specifies six functions in relation to external parts of the human body for products that may be cosmetic products, namely:
- to clean
- to perfume
- to change the appearance
- to protect
- to keep in good condition
- to correct body odours
8) The field of application of cosmetics is to the external parts of the human body; that is one or more of the following sites:
- the epidermis
- the hair system
- the nails
- the lips
- the external genital organs
- the teeth
- the mucous membranes of the oral cavity
A cosmetic product may be a substance or mixture of a number of substances, and it may come in one or more than one part to be combined by the user.
9) An illustrative list of cosmetics is given in Appendix 4 of this guide.
Borderline products
10) The classification of many products on the borderline with cosmetics can be difficult to determine. The Medicines and Healthcare Products Regulatory Agency (MHRA) see footnote 2 regulates medicinal products and has issued “A Guide to what is a Medicinal Product” see footnote 3. In case of doubt as to the status of a product, advice may be sought directly from the Borderlines Section of the MHRA. Cosmetics are generally exempted from the biocides regulation but exceptions may arise in very rare cases. Cosmetic products with a primary cosmetic function can make secondary biocidal claims without being classified as biocidal products. The classification of these products is made on a case by case basis. Read further information from the Health and Safety Executive
11) Aromatherapy products supplied to consumers may fall within the scope of the General Product Safety Regulations 2005. Where they are intended to perform a medical or cosmetic function or are presented as performing such a function they may also fall within the cosmetics or medicinal products regulations. Both Local Authorities and The Aromatherapy Trade Council offer advice on this matter.
12) Products that are intended to be ingested, inhaled, injected or implanted are not classified as cosmetic products.
Manufacturer
13) The ‘Manufacturer’ is any person or business who manufactures a cosmetic product or has the product designed or manufactured, and market it under their name or trademark.
Importer
14) The ‘Importer’ is any person or business established in the UK who places a cosmetic product from a country outside the United Kingdom on the GB market. This includes products that are placed on the market in Great Britain from Northern Ireland, where that product was supplied to a Northern Ireland business from outside the UK, including the EU or EEA. There are different rules that apply when these Northern Ireland businesses place qualifying Northern Ireland goods on the market in Great Britain (see NI guidance).
Distributor
15) A ‘Distributor’ is a person or business, other than the Manufacturer or the Importer, that supplies a cosmetic product on the GB market. It includes what are commonly known as retailers or wholesalers. Professional outlets such as hairdressers are also classed as Distributors for those products sold or given to customers (consumers). There may be multiple Distributors of the product in the supply chain.
Making Available
16) ‘Making available’ on the market is any supply of a cosmetic product for distribution, consumption or use in the course of a commercial activity. This holds regardless of any associated payment (i.e. whether in return for payment or free of charge). “Making available” will refer to supply on the GB market.
Placing on the Market
17) ‘Placing on the market’ means the first making available (supply) of a cosmetic product on the GB market from 31 December 2020. See footnote 4
Nanomaterial
18) Nanomaterials are defined in the Regulation as materials that are ‘insoluble or biopersistent and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100nm.’
Undesirable Effect / Serious Undesirable effect
19) An ‘Undesirable Effect’ is an adverse effect to human health that occurs following the normal or reasonably foreseeable use of a cosmetic product. There should be a demonstrable link between the affected person and the product.
20) A ‘Serious Undesirable Effect” (SUE) is an undesirable effect which results in temporary or permanent functional incapacity, disability, hospitalisation, congenital anomalies or an immediate vital risk or death.
Competent Authority and Enforcement Authorities
21) The “competent authority” and the “enforcement authorities” are the Secretary of State, and local authority trading standards in England and Wales and Scotland (local weights and measures authorities). The Secretary of State may also authorise others to be the competent authority.
Chapter II – Safety, Responsibility & Free Movement
Article 3 – Safety
22) A product must be safe for human health under normal and reasonably foreseeable conditions of use. Manufacturers in the first instance should consider the conditions of use which can be reasonably foreseen prior to placing a product on the market. They must therefore look beyond what they consider to be the intended use of a product, and put themselves in the position of the average user of the product – envisaging how they would reasonably consider using it. The safety requirement does not cover misuse of a cosmetic product (except where it is a reasonably foreseeable misapplication of the product). Ultimately it is for the Responsible Person (see below) to ensure that this obligation is complied with.
23) The Article provides that the presentation of the cosmetic product must take into account the requirements of The Food Imitations (Safety) Regulations 1989 (SI 1989 No. 1291) which concern dangerous imitations. The key provision in these Regulations is that no person shall supply, expose for supply or possess for supply any manufactured goods which are ordinarily intended for private use and are not food, but which:
- have a form, odour, colour, appearance, packaging, labelling, volume or size which is likely to cause persons, in particular children, to confuse them with food and in consequence to place them in their mouths or suck them or swallow them
- where such action mentioned above is taken in relation to them, may cause death or personal injury
Article 4 – Responsible Person
24) Article 4 provides that a cosmetic product cannot be placed on the GB market unless there is a Responsible Person established in the UK in respect of that cosmetic product. This includes if you are a Northern Ireland business placing a cosmetic product on the GB market under unfettered access – paragraph 29 below provides further details.
25) Article 4 sets out when the Manufacturer or Importer is considered to be the Responsible Person. A manufacturer based in the UK for products manufactured in the UK and placed on the GB market directly after manufacture (that is, where it is not exported and imported back into the UK after manufacture and before placing on the GB market) is the Responsible Person in respect of that product. An importer (who must by definition be based in the UK) who places a product on the GB market is the Responsible Person in respect of that product. There are particular provisions for qualifying Northern Ireland goods that are supplied from the EEA and then placed on the GB market (see below). In certain circumstances, it is also possible for a Distributor to be considered the Responsible Person (see paragraph 8 in Article 4).
26) It is possible for a Manufacturer or Importer to authorise a third party to act as the Responsible Person via a written mandate. Where the manufacturer is not based in the UK but the product is manufactured in the UK and remains in the UK between manufacture and placing on the market (i.e. it is not exported and imported back into the UK after manufacture but before being first supplied on the GB market) the manufacturer must ensure via written mandate that there is a third party based in the UK who agrees to be the Responsible Person in respect of that product. The third party must be located in the UK and in order to be the Responsible Person, must accept the mandate in writing from the Manufacturer or the Importer. If no accepted mandate exists, then the Manufacturer where they are established in the UK or the Importer is the Responsible Person.
Article 5 – Obligations of Responsible Persons
27) It is the duty of the Responsible Person to ensure compliance with the Regulation. While some of the requirements may not be directly undertaken by the Responsible Person, such as the safety assessment, it is their responsibility to ensure that all the requirements of the Regulation are fulfilled. If a Responsible Person has reason to believe that a cosmetic product is non-compliant, it is the duty of the Responsible Person to take corrective actions to bring the product back into compliance, withdraw it from the market or undertake a recall. The action taken should be commensurate with the degree of non-compliance. Further advice may be sought from the Local Authority.
28) The Article gives further provision on the duties of the Responsible Person for where a cosmetic product presents a risk to human health. The Responsible Person must immediately notify and cooperate with competent authorities, providing necessary information as set out in paragraphs 2 and 3 of the Article.
Article 5A – Obligations of Responsible Persons established in Northern Ireland
29) Northern Ireland businesses seeking to sell or supply a qualifying Northern Ireland good in GB can continue to make products in line with the EU rules that apply in NI under the terms of the Windsor Framework (Regulation (EC) No 1223/2009 on Cosmetic Products) and sell the same product in the rest of the UK. There will be no additional approvals to sell qualifying Northern Ireland goods in the rest of the UK. If the Northern Ireland business is the Responsible Person under NI law and meets the obligations under NI law, then they will be treated as complying with most of the obligations under GB law (including labelling, safety assessments etc.), although they will need to send certain information to the Secretary of State. If the Responsible Person for products placed on the Northern Ireland market is in the EEA, the Northern Ireland business will be the Responsible Person for the purposes of GB law but does not have to change the contact details on the product, nor will they have to undertake separate safety assessments. They will have to make sure that the product is safe for human health (see Article 3) and will have to send certain information to the Secretary of State (see Article 13).
Article 6 – Obligations of Distributors
30) Distributors when making a cosmetic product available on the GB market have a general obligation to act with due care in relation to the applicable requirements. In particular, distributors must verify the below when making a product available on the market:
- that the labelling information specified in Article 19 (1)(a), 19 (1)(e), 19 (1)(g), 19 (3) and 19 (4) is present
- that the minimum durability specified under Article 19 (1) has not passed
31) Only compliant products should be made available on the market. Where Distributors have reason to believe the product is not compliant with the Regulation, they should not make the product available on the market until it is compliant. Where they have already made the product available, they should ensure that corrective measures are taken or that the product is withdrawn or recalled, as appropriate. In carrying out their responsibilities, Distributors should also keep the Responsible Person informed, and agree a course of action, while cooperating with competent authorities.
32) The Distributor is not responsible for ensuring that the product has been notified under Article 13 (see below) and is not entitled to check the Product Information File (PIF) of the product (unless they are also a Responsible Person – see Article 4(8) for when this applies). This is the sole responsibility of the Responsible Person.
33) The Distributor has a duty to store and transport products properly so that compliance with the Regulation is not compromised.
Article 7 – Identification within the Supply Chain
34) This Article concerns product traceability and requires the Responsible Person to identify the relevant Distributors. The Responsible Person must make this information available to the competent authority on request.
35) The Distributor has the responsibility to identify the distributor or the responsible person from whom, and the distributors to whom, the cosmetic product was supplied. This information must be made available to the competent authority on request.
36) In both instances the data must be kept for a period of three years following the date that the batch of cosmetic product was made available to the Distributor.
Article 8 – Good Manufacturing Practice
37) Manufacturing of cosmetic products should be carried out to cosmetic Good Manufacturing Practice (GMP). ISO Standard 22716 covers GMP, but there are other ways and guidance documents are available from trade associations. See footnote 5
Chapter III – Safety Assessment, Product Information File, Notification
Article 10 & Annex I – Safety Assessment
38) The Responsible Person is to ensure that a safety assessment is completed on a cosmetic product before it is placed on the GB market in order to demonstrate that the product complies with Article 3 (that is, that it is safe for human health when used under normal or reasonably foreseeable circumstances). The intended use of the cosmetic product – and the anticipated systemic exposure to individual ingredients in a final formulation – must be taken into account in the safety assessment. An appropriate weight–of–evidence approach must be used in the safety assessment for reviewing data from all existing sources.
39) The safety assessment should take the form of a Cosmetic Product Safety Report (CPSR) signed by a qualified safety assessor. The CPSR provides evidence of how the product is safe for its intended cosmetic use and takes account of reasonably foreseeable use. In addition, a specific safety assessment is required for cosmetic products intended for use on children under the age of three, and for cosmetic products intended exclusively for use in external intimate hygiene.
40) The format for the CPSR is detailed in Annex I of the Regulation and is divided into two parts: Part A (the cosmetic product safety information), and Part B (the cosmetic product safety assessment). Northern Ireland businesses, where a safety assessment has been done under the applicable law in Northern Ireland (Regulation (EC) No 1223/2009 on Cosmetic Products), do not have to take any further steps in relation to this Article to sell a qualifying Northern Ireland good in GB.
Part A – Cosmetic Product Safety Information
41) As a minimum, there are ten points that need to be considered:
- quantitative & qualitative composition
- physical/chemical characteristics and stability
- microbial quality
- impurities, traces and the packaging material
- normal and reasonably foreseeable use
- exposure to the product
- exposure to the substances
- toxicological profile of the substances
- undesirable effects and seriously undesirable effects
- other relevant information on the product
Part B – Cosmetic Product Safety Assessment
42) The cosmetic product safety assessment consists of four key sections:
a. an overall conclusion concerning the cosmetic product. This should indicate if the product is either safe for use or safe for use with restrictions. The conclusion should be based on the data presented in Part A of the Assessment.
b. Any mandatory labelling requirements should be listed. It is the task of the safety assessor to determine which warnings or instructions of use, in addition to those listed in Annexes III to VI, need to be labelled to ensure the safe use of the product.
c. Detailed reasoning for the final safety assessment should comprise a risk–based analysis, using ‘an appropriate weight of evidence approach for reviewing data from all existing sources’.
d. The safety assessment should include the name and address of the safety assessor including proof of qualifications; it should be signed and dated by the safety assessor.
43) The safety assessment should be reviewed and revised on a regular basis. In particular, this should be done when new data is available that might alter the safety conclusion outlined above.
Article 10 (2) – The Safety Assessor
44) The person responsible for the safety assessment is called a safety assessor. The safety assessor should be in possession of a diploma or other evidence of formal qualifications awarded on completion of a university course of theoretical and practical study in pharmacy, toxicology, medicine or a similar discipline, or a course recognised as equivalent by the Secretary of State.
Article 11 – Product Information File
45) Article 11 sets out the requirements relating to the Product Information File (PIF), and the detail of the information and data that should be contained concerning:
- Description of the cosmetic product
- The Cosmetic Product Safety Report
- Method of manufacture and GMP
- Nature and proof of effect of the product
- Animal testing
46) The PIF must be kept for a period of ten years after the date the last batch of the cosmetic product was placed on the market.
47) The Responsible Person must make the PIF readily accessible to a competent authority at the address notified, in accordance with Article 13.
48) The PIF should be a ‘living document’ and should be updated as necessary. For instance, it should be updated when changes are made to the CPSR, such as the addition of new test data. If a product is significantly different from a same name product previously placed on the market, an update might not be sufficient and the Responsible Person will have to consider creating a new PIF. Northern Ireland businesses where the Responsible Person has completed a PIF under Northern Ireland law do not need to take any further action in relation to Article 11 to sell a qualifying Northern Ireland good in GB. They will be required to set out the address at which the PIF is available, in accordance with Article 13.
Article 12 – Sampling and Analysis
49) Article 12 states that sampling and analysis of cosmetic products must be carried out in a reliable and reproducible way. Sampling and analysis are assumed to be reliable and reproducible if the method complies with the relevant designated standards. See footnote 6
Article 13 – Notification
50) It is the responsibility of the Responsible Person (see Article 5 and 5A) to notify the Secretary of State of any cosmetic products made available on the GB market. The UK Government has established the Submit Cosmetic Product Notification (SCPN) service for this purpose. Article 13(1) and (2) set out the specific information that must be provided. Access the SCPN service
51) Before the product is placed (supplied for the first time after 31 December 2020) on the GB market, a number of aspects must be notified. Products containing nanomaterials must as a general rule be notified six months prior to being placed on the market (see below – Article 16).
52) Responsible Persons will have 90 days beginning with 1 January 2021 to complete their notification where products were previously notified to the European Commission (EC) via their Cosmetic Product Notification Portal (CPNP), made available on the EEA or UK markets prior to the 31 December 2020, and that they will place those same products on the GB market within 90 days of 1 January 2021. They must also give notice that there is a responsible person that has complied with the obligations under Article 13 of the EU Cosmetics Regulation. There is a reduced amount of information required to be submitted for these products to simplify the process:
- the category of cosmetic product and its name or names, enabling its specific identification
- the name of the Responsible Person
- the address at which the Product Information File (PIF) in respect of the cosmetic product is kept
- the contact details of a natural person to contact in the case of urgency
- the frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
(Note: ‘urgency’ refers to the safety and compliance of cosmetic products rather than individual medical emergencies)
53) For products that you are placing on the market in GB for the first time after the end of the transition period (that is, products that have not previously been supplied in the UK / EEA and have not been notified to the CPNP) you will need to provide the information above and the following information before you place the product on the GB market:
- the name and the Chemicals Abstracts Service (CAS) or EC number of substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR) of category 1A or 1B under Regulation (EC) No 1272/2008
- the original labelling and, where reasonably legible, a photograph of the corresponding packaging
54) For products that you are placing on the market in Great Britain for the first time after 31 December 2020, that contain nanomaterials, you will also have to supply the following information (see also Article 16):
- confirmation of the presence of substances in the form of nanomaterials
- the identification of the nanomaterials including the chemical name (IUPAC) and other descriptors as specified in point 2 of the Preamble to Annexes 2 to 6 to Regulation (EC) No 1223/2009
- the reasonably foreseeable exposure conditions of the nanomaterials
55) Where a notification of products with nanomaterials has been made to the European Commission in the six–month prior to the end of the transition period on 31 December 2020, the Responsible Person must provide the Secretary of State with information about the nanomaterials within 90 days of 1 January 2021. The Secretary of State has one extra month (that is 7 months from the date the information was submitted to the Commission) to determine whether there is sufficient scientific evidence of risks to human health from these substances and therefore whether any amendment should be made to the Annexes to the Regulation to make the substances prohibited or restricted substances. Therefore, it may take a total of 7 months from the time of notifying the Commission for the product to be accepted onto the GB market, where there are no concerns about human health. (see also article 16)
56) Where a product containing novel nanomaterials has already been supplied on the EEA or UK market and the EU Responsible Person has complied with the notification requirements under EU law prior to the end of the transition period, if a UK Responsible Person is to place the product (the first supply after 31 December 2020) on the GB market within 90 days of 1 January 2021 they must provide the information on nanomaterials within 90 calendar days of 1 January 2021 as part of their notification of the product on the UK SCPN service. (see also Article 16)
57) When any of the information required by this Article changes, the Responsible Person must immediately update the Secretary of State electronically.
Chapter IV – Restrictions for Certain Substances
Article 14 – Restrictions for Substances Listed in the Annexes
58) Annexes 2 to 6 of the Regulation list prohibitions and restrictions on individual ingredients and how they may be used.
59) Annex 2 lists ingredients prohibited in all cosmetics.
60) Annex 3 lists ingredients that may only be used subject to the restrictions specified (e.g. they are not to be used in products for children under 3 years old). It can also set out wording of conditions of use and warnings for such products.
61) Annex 3 also includes certain ingredients commonly but not exclusively used in fragrances. These ingredients must be labelled individually if they exceed a certain threshold level, regardless of the function they perform in the product. This labelling requirement is in addition to normal perfume labelling requirements (see paragraph 19.1(g)) and does not replace them.
62) A number of these ingredients are also found in natural essential oils. In order to check the levels of these ingredients in their products, companies need to obtain information from their suppliers of essential oils and perfume compounds.
63) Only those colourants, preservatives and UV filters listed in the corresponding Annexes 4, 5 or 6 may be used, subject to any conditions specified in the Annex in which they appear.
64) For a substance in a cosmetic product to be permitted for use as either a colourant, preservative, or UV–filter, the substance must be listed in the appropriate Annex.
Article 15 – Substances Classified as carcinogenic, mutagenic, or toxic for reproduction (CMR substances)
65) Cosmetic products must not contain substances classified as category 2 or category 1A or 1B CMR substances under Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures as it applies in Great Britain, unless they are listed in one of the Annexes allowing their use (and so have been found safe for use in cosmetic products). Specific labelling in order to avoid misuse of the cosmetic product must be provided in accordance with Article 3, taking into account possible risks linked to the presence of hazardous substances and the routes of exposure.
Article 16 – Notification of Nanomaterials
66) Article 16 lays out the requirements relating to the notification of nanomaterials in cosmetic products. It does not apply where products contain nanomaterials that are colourants, preservatives, or UV–filters and are listed in Annexes 3–6 (respectively); Article 14 covers these types of nanomaterial and these nanomaterials can only be used in accordance with the conditions laid down in the relevant Annex. All other nanomaterials must be notified in accordance with Article 16. Nanomaterials which are listed in Annexes 3–6 but are not intended to be used as colourants, preservatives, or UV–filters must also be notified in accordance with Article 16 and must comply with the conditions laid down in the relevant Annex. The Responsible Person must notify electronically a product containing nanomaterials to the Secretary of State at least 6 months prior to the product being placed on the market. Article 16 (4) details the information that is required in this notification]
67) Where the Secretary of State considers it necessary (for the purpose of reducing risk to human health), they may request a Responsible Person who has not already submitted the information to the Secretary of State, to submit any of the specific notification information (in Article 13 (1)(e) to (f)). In making this request, the Secretary of State must specify the period within which the Responsible Person must respond. This period must also be reasonable and commensurate with the nature of risk that the product presents.
68) It is the duty of the Secretary of State to share the specific notification information (in Article 13 (1)(a)–(f) (that is, everything except the frame formulation and Article 13(2) (original labelling)) with all other competent authorities. The Secretary of State must make all information available to UK National Poisons Information Service (NPIS) poison centres. The Secretary of State must also share the original labelling and the photo of the packaging (where applicable). Following this, the competent authorities are only permitted to use the information for the following purposes: market surveillance, market analysis, evaluation, and consumer information (in the context of non–compliance). The UK poison centres are only permitted to use the information for medical treatment purposes.
69) If a competent authority has concerns regarding the safety of a nanomaterial, they may ask a Responsible Person to submit to them information about the nanomaterial used in any cosmetic product, and the reasonably foreseeable exposure conditions. When a competent authority makes this request, it must specify the period within which the Responsible Person must respond. This period must be reasonable and commensurate with the nature of the competent authority’s concerns.
70) In certain circumstances, the notification information may be provided by a ‘Designated Person’ (defined in Article 16 (12)).
71) The Secretary of State may provide a reference for the toxicological profile. This reference may be provided in place of the information referred to in Article 16 (4)(d).
72) Responsible Persons will need to work closely with their ingredient supplier of nanomaterials, and contractual arrangements should ideally reflect the high level of disclosure and cooperation necessary for the Responsible Person to comply with its obligations under Article 16. Responsible Persons for the GB market that are established in Northern Ireland, under Article 5A, do not have to fulfil the requirements in Article 16 when placing qualifying Northern Ireland goods on the GB market, as long as the Responsible Person for the purposes of NI law has complied with these requirements.
Article 17 – Traces of Prohibited Substances
73) The non-intended presence of a small quantity of a prohibited substance is permitted, provided that the presence is in conformity with Article 3. Article 17 provides information on the circumstances under which this situation may arise.
Chapter V – Animal Testing
Article 18 – Animal Testing
74) Cosmetic products are not permitted on the GB market if the product’s ingredients, combination of ingredients or final formulation have been the subject of animal testing used to prove their safety for the purposes of this Regulation. However, historic animal testing data from animal testing that took place before such testing was banned at EU level may still be used in order to meet the requirements of the Regulation.
Chapter VI – Consumer Information
Article 19 – Labelling
75) Article 19 sets out the labelling requirements for cosmetic products. The Regulation requires all cosmetic products (except where specific exceptions apply – see below) to have clearly and indelibly marked on their container and packaging the following information:
- name and address of the Responsible Person
- country of origin for imported products
- nominal quantity of contents
- date of minimum durability (“Best Before” date)
- or (where the minimum durability is more than 30 months) a ‘Period After Opening’ (PAO)
- warning statements and precautionary information
- batch number
- product function, when not obvious from its packaging / presentation
- list of ingredients
76) Northern Ireland businesses selling qualifying Northern Ireland goods into GB do not need to fulfil these requirements as long as the products meet the requirements in Northern Ireland, under Regulation (EC) No 1223/2009 on Cosmetic Products), as applied under the terms of the Windsor Framework. This means that where under NI law the Responsible Person is based in the EEA, a Northern Ireland business does not need to change the contact details on the packaging to sell a qualifying Northern Ireland good in GB – though the requirement to have a Responsible Person based in the UK still stands.
Article 19 (1)(a) – Name and Address
77) The name and address required is that of the (UK based) Responsible Person placing the product on the market (although see below for transitional provisions).
78) The name and address allow identification of the Responsible Person. The details may be abbreviated, as long as the Responsible Person and their address can be identified. The information must be given on both the container and the outer packaging.
79) If several addresses are listed the address through which the Product Information File (PIF) is readily accessible must be highlighted.
Article 19 (1)(a) – Country of Origin
80) The country of origin must be specified for imported cosmetic products, including products imported from the EU. (Note: ‘Made in the EU’ is not accepted as the country of origin, as the EU is not a country).
Article 19 (1)(a) transitional arrangements
81) For a period of seven years see footnote 7 until 31 December 2027, the name, address and country of origin requirements are satisfied if there is compliance with the requirements of Article 19(1)(a) of the Regulation as it has effect in EU law (that is, where it has the name and address etc. of the Responsible Person based in the EU/EEA and meets the other requirements of Article 19(1)(a) of the EU Cosmetics Regulation).
Article 19 (1)(b) – Statement of Contents
82) The Regulation requires the labelling of the nominal content at the time of packaging, given by weight or by volume. However, the following products are exempted:
- free samples
- where packing contains less than 5g or 5 ml
- single application, for example, sachets
- packs normally sold as a number of items, for which the details of weight or volume are not relevant, for example, bath balls, where the number of items appears on the packaging or is obvious, or if the product is normally sold individually
83) In addition to the requirements of the Regulation, compliance with the UK Weights and Measures (Packaged Goods) Regulations 2006 see footnote 8 must be ensured.
84) The system of weight checking is known as ‘average quantity’. The three ‘Packers Rules’ for the average quantity system are:
- the average contents for a batch of product must not be less than the declared nominal quantity
- the proportion of packs which are short of the stated quantity by a defined amount – the ‘tolerable negative error’ or TNE – must be sufficiently small to satisfy certain specified requirements
- no pack should be short by more than twice the TNE
85) Advice on Weights and Measures matters can be obtained from local weights and measures authorities in Great Britain.
Article 19 (1)(c) – Minimum date of durability (Best Before Date)
86) A product which is likely to deteriorate up to and including 30 months from the date of manufacture so that it:
- ceases to satisfy the general safety requirement in Article 3 (being safe for human health under normal or reasonably foreseeable conditions of use), or
-
ceases to fulfil its intended function
-
must have a date of minimum durability marked on its container and packaging using either the words ‘best used before the end of’ or the ‘Hour–Glass’ symbol, given in Annex 7 (at point 3) to the Regulation, (reproduced in Appendix 3(3) of these Guidelines), immediately followed by either:
- the earliest date, in the form month, year or day, month, year, in which one of the matters set out in the bullet points above may occur, or
- an indication of where that date appears on the packaging
87) The minimum durability date must appear on both the primary container and outer packaging in English. Best before November 2010, Best before Nov 10 and Best before 11/10 are all acceptable forms.
88) Any special precautions to be observed, such as storage conditions, must also be marked in English on both the primary container and outer packaging. This is in order to keep the product in a condition that satisfies the Regulation within the minimum durability date.
Article 19 (1)(c) – “Period After Opening” (PAO)
89) Dates of minimum durability (best before date) is not mandatory for products with a minimum durability of more than 30 months. Instead, for these products a Period After Opening (PAO) symbol is required it must appear on both the primary container and outer packaging.
90) The “Period After Opening” is the time after which the cosmetic product is safe and can be used without any harm to the consumer once the product has been opened i.e. after this date it ceases to comply with the general safety requirement in Article 3.
- By requiring labelling of a “Period After Opening”, the Regulation aims to provide useful information to consumers.
- The PAO symbol must be used when, after opening, the deterioration of the product may lead to harm to the consumer.
Opening of the product may be considered as occurring when the consumer opens the product for use for the first time. The PAO symbol will not be necessary where there is no risk of harm to the consumer, as there is no risk of deterioration that could lead to damage to human health (in accordance with Article 3 of the Regulation) that is to say for single-use products, products not at risk of deterioration or products which do not open e.g. aerosols.
91) The PAO is indicated by a symbol representing an open cream jar, together with the period of time in months or years shown as a number i.e. 12 m. This is depicted in Annex 7 (2) of the Regulation and reproduced in Appendix 3 (1) of this guidance.
Article 19 (1) (d) – Warning Statements and Precautionary Information
92) Information must be provided on both the primary container and outer packaging in English. The additional presence of this information in other languages is not prohibited. Conditions of use and warnings for a range of ingredients are specified in the Annexes to the Regulation as follows:
- chemical substances – Annex 3, column i
- colours – Annex 4 column j
- preservatives – Annex 5, column i
- UV filters – Annex 6, column i
93) If the ingredients are contained in these Annexes, any associated mandatory warnings must be provided in English.
94) These requirements also apply to products intended for professional use. Careful consideration should be given as to how the product is used and whether there is increased risk due to prolonged exposure or more unusual conditions of use. If judged to be necessary, special precautionary information must be provided in English.
Article 19 (1) (e) – Batch Number
95) A code which enables the manufacturer or supplier to identify the batch in which the product was manufactured must be marked on both the primary container and outer packaging. If the product is not made in a batch, then the code should enable the date and place of manufacture to be identified.
96) Where it is impossible for reasons of size for the batch number to appear on both the primary container and outer packaging, it may appear on the outer packaging alone.
Article 19 (1) (f) – Product Function
97) Unless this is clear from the presentation, the function of the product must be clearly stated on both the primary container and outer packaging in English. For example, the function of lipstick is obvious. However, a depilatory cream should not only be labelled as a ‘cream’.
Article 19 (1) (g) – List of Ingredients
98) A full list of ingredients. This may be given on the packaging alone and must be headed or preceded by the word ingredients. Where the product is not pre-packaged, the list must appear on the container or on a notice in immediate proximity to that container (see also paragraphs 108 to 126 on Labelling Difficulties); where the product is pre–packaged for immediate sale, the information must appear on an attached label, tag, tape or card or in an enclosed leaflet (where this is impossible for practical reasons this information must appear on a notice in immediate proximity to the container). This listing must:
- show all ingredients (“ingredient” means any substance or mixture intentionally used in the cosmetic product during the manufacturing process)
- use the name given in the glossary of Common Ingredient names; the Secretary of State will publish this list
- in the absence of a common ingredient name, a term as contained in the generally accepted nomenclature listed in Appendix 1 of this guidance note may be used
- for colourants (other than those intended to colour the hair), use the common ingredient name as detailed above. Colourants may be listed in any order after the other ingredients, using the Colour Index Number or denomination shown in Annex 4 of the Regulation (where applicable)
- perfume and aromatic compositions and their raw materials shall be referred to by the terms ‘parfum’ or ‘aroma’. For example, a flavour ingredient or mixture is an aroma. See also paragraph 104 concerning certain ingredients that must be labelled individually even if they form part of a perfume composition or essential oil. The threshold levels for declaration are 0.001% for leave-on products and 0.01% for rinse-off products.
- show ingredients in descending order of weight (determined at the time the ingredients are added to the product)
- all ingredients present in the form of nanomaterials should be indicated in the list of ingredients immediately following the INCI name of the ingredient in question. This is done by adding ‘(nano)’ after the ingredient name.
99) Additionally:
- ingredients in concentrations of less than 1% may be listed in any order after those in concentrations of 1% or more
- for decorative cosmetics see footnote 9 marketed in several colour shades, all colourants (other than those intended to colour the hair) used in the range may be listed preceded by the words ‘may contain’ or the symbol ‘+/–‘
- mixtures must be broken down into their individual components
100) For the purposes of labelling, the following are not regarded as cosmetic ingredients and do not need to be shown:
- impurities in the raw materials
- subsidiary technical materials used in the preparation of the cosmetic product but not present in the final product
Ingredient listing – Labelling issues
Variable Ingredients
101) The Regulation makes provision for the listing of all colourants used in a decorative range of cosmetics, although each product would only contain a selection of those colours. The intention is to simplify manufacture by allowing all of the colourants to be listed on one label in a market where fashions and colours change frequently. However, there is no specific provision made for other ingredients which are subject to change.
102) For example, minor formulation changes of non–colour ingredients are usually necessary to accommodate the different characteristics of colour pigments used within a range of colour cosmetics.
103) The Regulation does not make specific provision for this case and a strict interpretation would require separate labelling for each formulation. However, in this type of situation, it is the accepted industry practice to include the variable ingredients in the main body of the ingredients listing. The +/– (may contain) section of the ingredients listing is reserved for colourants. Colour ingredients which do not have a CI number (as listed in Annex 4 to the Regulation), but are closely associated with colour, might only be present in some products within the decorative range. The industry interpretation is to list these items under the +/– (may contain) section of the ingredient listing.
104) An example of an ingredient listing as it might appear on a cosmetic product is given in Appendix 2.
International Nomenclatures
105) In developing the INCI system, Cosmetics Europe worked closely with the equivalent organisation in the USA, the Personal Care Product Council (PCPC). As a result, there is now a joint Cosmetics Europe / PCPC Ingredient Nomenclature Committee responsible for allocating labelling names, and recommending labelling rules for all ingredients used in cosmetics for the EU and US markets. In the interests of consumer safety, the UK fully supports the use of the INCI system, which has been adopted both in Europe and the USA and is widely accepted elsewhere.
106) Despite these efforts, there are still some geographical differences in the INCI system. These exist in the nomenclature of colours, botanicals and the so–called ‘trivial’ ingredients. This causes difficulties for importers and exporters. Where there are differences, the additional use of the alternative name, in brackets, is acceptable in the UK.
107) For example:
- water (aqua) or aqua (water)
- santalum album (sandalwood oil) or sandalwood (santalum album) oil
- CI 14700 (Red 4) or Red 4 (CI 14700)
Article 19 (2) – Labelling Difficulties
108) The variety and nature of cosmetic products and their packaging may pose difficulties when trying to include all of the information specified in the Regulation. Certain provisions have therefore been made to take into account the practical difficulties.
Warning statements and precautionary information
109) Particular precautions to be observed in use and at least those listed in Annexes 3–6 and special precautionary information will normally appear on both the primary container and outer packaging. Where this is impossible for practical reasons, the information may be given on a leaflet, label, tag, tape, or card enclosed with the cosmetic product or attached to it.
110) When the information is given in an enclosed leaflet, label, tag, tape or card, the consumer must be referred to it. This can either be done by abbreviated information, or by a special symbol given in Annex 7(1) of the Regulation (the hand and book symbol). The symbol must appear on the container or the outer packaging. This symbol is reproduced in Appendix 3(1) of this guidance.
Ingredient listing
111) An ingredient listing, as detailed in Article 19, is required on the outer packaging only, or – in its absence – on the primary container. Where it is impossible (due to practical reasons) for the list to appear on the packaging or container, it must be given on a leaflet, label, tag, tape, or card enclosed with the product or attached to it. The consumer must be referred to the text either by abbreviated information or by a special symbol, given in Annex 7 (1) of the Regulation (the hand and book symbol). This must be on the container or packaging. The symbol is reproduced in Appendix 3(1) of this guidance.
Vending Machines
112) The labelling requirements in the Regulation apply equally to products dispensed from vending machines.
Free Samples
113) Free samples, whether they are provided in–store, by direct mail or in magazines (for example shampoo samples), are considered to be within the definition of supply contained in the Regulation. Compliance with all of the applicable requirements of the Regulation is therefore required.
Article 20 – Product Claims
114) In addition to the requirements of Article 11(2)(d), Article 20 requires that in the marketing of cosmetic product, every Responsible Person must ensure that the wording of any claim in relation to a cosmetic product does not imply that the product has a characteristic or function which it does not have. This covers claims made in the form of texts, names, trademarks and figurative or other signs that say or imply that the product has characteristics or functions in the labelling or making available or marketing of the product.
115) A Responsible Person must ensure that the wording of any claim complies with the common criteria set out in the Annex to Commission Regulation (EU) No 655/2013 (as it has effect in GB law). This lays out the common criteria for the justification of claims used in relation to cosmetic products, which are as follows:
- legal compliance
- truthfulness
- evidence support
- honesty
- fairness
- allow informed decisions
116) Legal compliance: Claims must comply with all applicable legal requirements and self–regulatory regimes and should meet the reasonable expectations of the average end user of the product. Claims of compliance with legal requirements or approval by competent regulatory authority are not allowed; neither are claims that convey the idea that a product has a specific benefit when this benefit is mere compliance with the minimum legal requirements.
117) Truthfulness: Claims should not be based on false or irrelevant information. If the presence of a specific ingredient is claimed, it must be deliberately added to the product and claims relating to the properties of an ingredient must not imply the finished product has that benefit when it does not. Claims must not imply that opinions verify claims unless the opinion reflects verifiable evidence.
118) Evidence support: All claims, whether implicit or explicit, must be supported by evidence that is adequate and verifiable. Studies must follow well-designed and well-conducted methodologies, and must respect ethical considerations and should be relevant to both the product and the benefit claimed. The level of evidence must be consistent with the type of claim being made – for example where a lack of efficacy may cause a safety problem (e.g. sun protection) more evidence may be required. Best practice is the key to the quality of evidence with the level of evidence being consistent with the type of claim. Statements of hyperbole or exaggeration not taken literally or of an abstract nature will not usually require substantiation.
119) Honesty: Claims must not go beyond supporting evidence, nor imply by action or omission that the product has characteristics or functions which it does not have. Extrapolation of ingredient properties to the finished product must be supported by adequate and verifiable evidence. Claims for ‘new and improved’ must not be overstated. Claims shall not attribute to the product concerned specific (i.e. unique) characteristics if similar products possess the same characteristics. If the claimed benefit is linked to specific conditions, these must be clearly stated.
120) Fairness: Claims should be objective, and not denigrate competitors nor denigrate ingredients that can be legally and safely used in cosmetic products. Claims must not create confusion with the products of a competitor.
121) Informed decision–making: By inclusion of the necessary information on function and characteristics of the product, claims should contribute to the ability of consumers and professionals to make informed decisions. They should be clear, precise, relevant, and understandable to the average end-user in the target audience, taking into account the capacity of that end-user to understand the information.
Article 20 (3) – Reference to Animal Testing
122) The Regulation recognises that companies should be able to make claims that no animal testing was undertaken in the development of its cosmetic products. However, the Regulation is also clear that consumers must not be misled by these claims.
123) For any cosmetic product placed on the market, Article 20.3 states:
The Responsible Person may refer, on the product packaging or in any document, notice, label, ring, or collar accompanying or referring to the cosmetic product to the fact that no animal testing tests have been carried out only if;
- the manufacturer and the manufacturer’s suppliers have not carried out or commissioned any animal tests on the finished cosmetic product, or its prototype, or any of the ingredients contained in it, or
- used any ingredients that have been tested on animals by others for the purpose of developing new cosmetic products.
124) Data relating to the safety assessment of the product, including details of any animal testing, must be kept in the PIF which is open to inspection by the enforcement authority (see Article 11.2(e)).
Article 21 – Public Access
125) The information listed below must be made easily accessible to the public by the Responsible Person by any appropriate means
- the qualitative and quantitative composition of the product
- the name and code number of the perfume and aromatic compositions
- the identity of the supplier
- information on existing data on undesirable or serious undesirable effects on human health (resulting from the use of the cosmetic product)
126) The qualitative information made accessible ought to be consistent with the ingredient list on the product’s package.
127) There is no obligation to provide a full declaration of the quantitative formula. However, for any cosmetic ingredients present in the product that fulfil the criteria listed in Regulation (EC) No 1272/2008 (on classification, labelling and packaging of substances and mixtures, Parts 2 to 5 of Annex I), the use concentration should be indicated.
128) Information on perfume or perfume compositions is generally subject to commercial secrecy and is part of a company’s intellectual property. They therefore do not need be made available to the public. However, the name and or code of the perfume together with the name of the supplier should be provided.
Chapter VII – Market Surveillance
Article 22 – In–Market Control
129) Article 22 outlines the responsibilities of enforcement authorities regarding market surveillance. They must monitor compliance by checking the PIF, how a company complies with GMP, and carry out physical product checks and laboratory analysis when necessary.
Article 23 – Communication of Serious Undesirable Effects
130) Information on Undesirable Effects (UEs) and Serious Undesirable Effects (SUEs) is included in the Safety Report which in turn is part of the PIF.
131) In addition, the Responsible Person or Distributor must report all incidences of SUEs to the Secretary of State. Information on any SUE should be notified to OPSS at seriousundesirableeffects@businessandtrade.gov.uk. If you would like support or advice in filling out the SUE form or any other guidance relating to SUEs, please contact your local Trading Standards or contact the relevant primary authority. The Secretary of State must immediately inform all other competent authorities of any information reported.
132) Notification should take place ‘without delay’. This is accepted in the UK to mean within 30 calendar days from when anyone in the company is informed of a possible SUE.
133) Where a Distributor reports the SUE of a cosmetic product to the Secretary of State, the Secretary of State must immediately inform the Responsible Person.
134) Consumers or health professionals may also report SUEs of a cosmetic product. If they report the SUE to any competent authority that is not the Secretary of State, then that competent authority must immediately inform the Secretary of State. The Secretary of State must then immediately inform the Responsible Person. Where consumers or health professionals report SUEs to the Secretary of State, the Secretary of State must immediately inform all other competent authorities and the Responsible Person.
135) Competent authorities may use this data for in–market surveillance purposes, market analysis, evaluation, and consumer information (in the context of Articles 25, 26 and 27 – see below).
Article 24 – Information on Substances
136) If a competent authority has serious concerns about the safety of a substance, it may request from the Responsible Person a list of all their products containing that substance and the concentration present in each product.
Chapter VIII – Non–compliance, Safeguard Clause
Article 25 – Non–compliance by the Responsible Person
137) Competent authorities are required to take action over a product that does not comply with the Regulation, primarily by requiring the Responsible Person to take corrective action.
138) A competent authority must request the Responsible Person to take all appropriate measures, proportionate to the nature of the risk, where there is certain non–compliance including corrective actions aimed at ensuring compliance, or withdrawal or recall, within an expressly mentioned time limit. If the Responsible Person does not take the measures within the time limit, or where immediate action is necessary to prevent serious risk to human health, the competent authority must take all appropriate measures itself to stop the product going on the market, or to withdraw or recall products already on the market. Competent authorities have all the powers they need to prevent any further distribution or sale of the product if the Responsible Person is not taking the necessary actions.
139) The competent authority that has taken these above measures must inform all other competent authorities of the measures taken by using the UK’s new Product Safety Database (PSD).
Article 26 – Non–Compliance by Distributors
140) Competent authorities are required to take appropriate action over a product that does not comply with the Distributors responsibilities set out in Article 6. The actions available are similar to those for the Responsible Person.
Article 27 – Safeguard Clause
141) The safeguard clause allows an enforcement authority to take direct provisional action where it ascertains there may be a serious risk to human health, or where there are reasonable grounds for concern. An enforcement authority other than the Secretary of State must obtain authorisation from the Secretary of State prior to taking any provisional measures. The Secretary of State will determine as soon as possible whether the action taken was justified. If the provisional measures are indeed justified, the Secretary of State will give authorisation to the enforcement authority to take the measures.
Article 28 – Good Administrative Practices
142) Article 28 is intended to ensure that competent authorities do not act unreasonably by taking action under Articles 25 and 27. The competent authority must follow these procedures to ensure the Responsible Person and or Distributor concerned is kept fully informed of the situation and is allowed to input into the process.
Chapter IX – Powers and Further Duties of the Secretary of State
Article 30 – Power to amend Articles
143) Article 30 sets out the amendments to Articles that the Secretary of State may make (by regulations). There are different triggers for the different amendments that can be made but they include:
- take technical progress into account
- reflect any changes in the name or structure of the recognised standardisation bodies
- extend the provisions of Article 16 to nanomaterials used as colourants, UV–filters or preservatives
- Amend article 14(1)(c) to extend its scope to hair colouring products
144) The Article further stipulates the conditions under which these amendments can take place.
Article 31 – Power to amend the annexes
145) Article 31 outlines the amendments to annexes that the Secretary of State may make (by regulation), and the conditions under which these amendments can take place.
Article 32 – Procedures for making regulations
146) The regulations to amend Articles and annexes may make different provisions for different cases. Provisions may be supplementary, transitional, transitory, consequential, or saving – as considered appropriate by the Secretary of State.
Appendix 1
Identification of Ingredients
An ingredient should be identified by its common name (INCI name).
In the absence of an INCI name, any of the following means of identification may be used:
- chemical name
- European Pharmacopoeia name
- International non–proprietary name as recommended by the World Health Organisation
- Einecs, Iupac or CAS identification reference, or
- Colour Index number
Unlisted Ingredients
Where an INCI name for an ingredient does not exist, then an application for a name should be made to the International Nomenclature Committee (INC) based in Washington, USA. Application for an INCI name should be made through PCPC (please see Appendix 6).
Appendix 2
Ingredient labelling example

Ingredient labelling example
Appendix 3
Symbols used on Packaging / Container from Annex VII of the Cosmetics Regulation
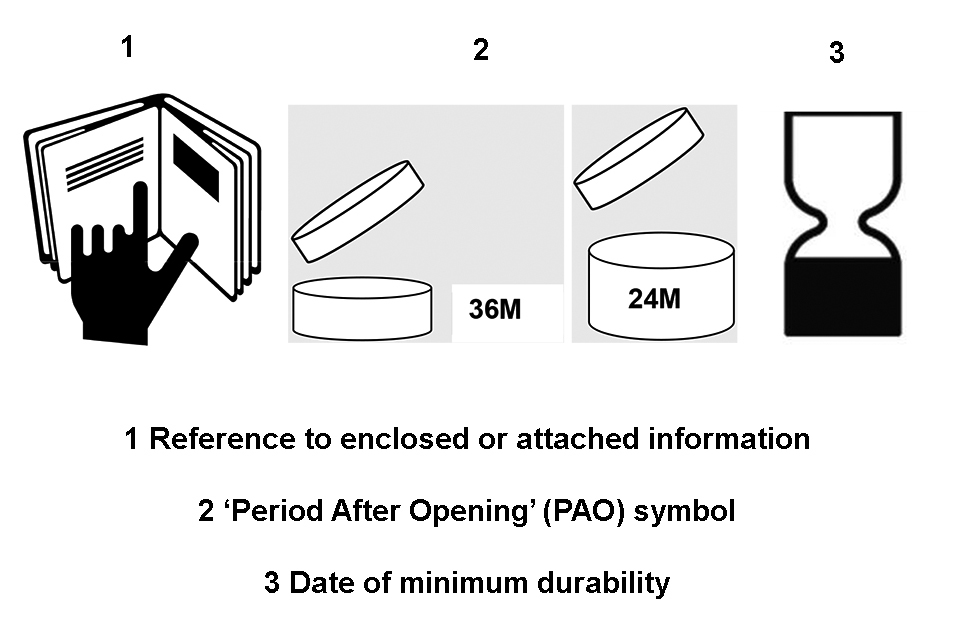
Symbols for reference to enclosed or attached information, Period After Opening (PAO), and date of minimum durability.
Appendix 4
Illustrative list of cosmetics (Recital 7 to the Regulation):
The following list is not exhaustive but is provided by way of example.
- Creams, emulsions, lotions, gels & oils for the skin
- Face masks
- Tinted bases (liquids, pastes, powders)
- Make-up powders, after-bath powders, hygienic powders etc.
- Toilet soaps, deodorant soaps, etc.
- Perfumes, toilet waters and eau de Cologne.
- Bath & shower preparations (salts, foams, oils, gels etc.)
- Depilatories
- Deodorants and anti-perspirants
- Hair colourants
- Hair products for waving, straightening, and fixing
- Hair–setting products
- Hair cleansing products (lotions, powders, shampoos)
- Hair conditioning products (lotions, creams, oils)
- Hairdressing products (lotions, lacquers, brilliantines)
- Shaving products (creams, foams, lotions)
- Products for making up and removing make-up
- Products intended for application to the lips
- Products for care of the teeth and mouth
- Products for nail care and make-up
- Products for external intimate hygiene
- Sunbathing products or sun protection products
- Products for tanning without sun
- Skin-whitening products
- Anti-wrinkle products
Appendix 5
Addresses
Aromatherapy Trade Council
PO Box 219,
Market Rasen
LN8 9BR
Tel: 01673 844 672
Office for Product Safety and Standards
4th Floor Cannon House
18 The Priory Queensway
Birmingham
B4 6BS
United Kingdom
Tel: 0121 345 1201 / Email: opss.enquiries@businessandtrade.gov.uk
Cosmetics Europe – The Personal Care Association
Avenue Herrmann Debroux 40
B–1160 Auderghem – Brussels
Belgium
Tel: + 32 2 227 66 10
Personal Care Products Council (PCPC)
1101 17th Street, NW
Suite 300
WASHINGTON
DC 20036–4702
USA
Tel: +1 202 331 1770
In the UK, PCPC publications can be obtained from:
MICELLE PRESS
12 Ullswater Crescent
Weymouth
Dorset
DT3 5HE
Tel: 01305 781574
Cosmetic, Toiletry & Perfumery Association Ltd (CTPA)
Sackville House
40 Piccadilly
London
W1J 0DR
Tel: 020 7491 8891
Guild of Craft Soap & Toiletry Makers
63 St Barnabas Road
Barnetby le Wold
North Lincolnshire
DN38 6JE
Tel: 01652 281760
LGC
Queens Road
Teddington
Middlesex
TW11 OLY
Tel: 020 8943 7000
Medicines and Healthcare products Regulatory Agency (MHRA)
10 South Colonnade
Canary Wharf
London
E14 4PU
Tel: 020 3080 6000
The Stationery Office (TSO)
St Crispins House
Duke Street
Norwich
NR3 1PD
Tel: 01603 622211
Trading Standards Institute
1 Sylvan Court Sylvan Way
Southfields Business Park
Basildon
Essex
SS15 6TH
Tel: 0808 223 1133
UK Cleaning Products Industry Association (ukcpi)
1st Floor Suite
Century House
Old Mill Place
High Street
Tattenhall
Cheshire
CH3 9RJ
Tel: 01829 770055
Appendix 6
Useful links
Read guidance on importing goods from the EU to Great Britain.
Read general European Commission guidance.
Read European Commission guidance on the Cosmetics Product Safety Report.
European Commission technical document on cosmetics claims.
European Commission cosmetic ingredients database.
Read European Commission reporting guidelines for SUEs.
Appendix 7
Northern Ireland’s unfettered access to the rest of the UK
The UK Government has been unequivocal in its commitment to unfettered access for Northern Ireland goods moving to the rest of the UK market, and to guaranteeing this in legislation. This means that you will be able to place qualifying Northern Ireland goods on the market in Great Britain without additional approvals.
As cosmetics that meet the EU’s Cosmetics Regulation are valid in Northern Ireland, these products can be placed on the market in Great Britain without additional approvals if they are a qualifying Northern Ireland good.
Further guidance on the definition of qualifying Northern Ireland goods and more details of the UK Government’s approach to unfettered access are available on GOV.UK.
Footnotes
1: The following legislative amendments and Government announcements apply:
- On 24 August 2021, the Government announced the transition periods for UKCA marking and UKCA labelling would each be extended until 31 December 2022 and 31 December 2023 respectively. The Product Safety and Metrology etc (Amendment) Regulations 2021 gave effect to this.
- On 20 June 2022, the Government announced the provisions for UKCA labelling would be extended until 31 December 2025.
- On 14 November 2022 the Government announced it would be extending the transition period for UKCA marking until 31 December 2024 and the provisions for UKCA labelling, importer information, and responsible persons’ information until 31 December 2027. The Product Safety and Metrology (Amendment and Transitional Provisions) Regulations 2022 (SI 2022/1393) give effect to this.
- On 1 August 2023, the UK Government announced its intention to extend recognition of the CE marking for the placing of most goods on the market in Great Britain, indefinitely, beyond December 2024.
2: Addresses are given in Appendix 5
3: “A Guide to What is a Medicinal Product” may be ordered from the MHRA or downloaded from its website. See Appendix 6.
4: It officially started at the end of the transition period which is also known as ‘Implementation Period (IP) completion day’ (defined as 11pm on 31 December 2020).
5: “GMP: A Practical Guide” may be obtained from The Cosmetic, Toiletry & Perfumery Association Ltd. (CTPA). See Appendix 6 for the address.
6: The UK is publishing a list of references to designated standards. These have the same function as harmonised standards. As of 1 January 2021, these designated standards will be the same as the harmonised standards.
7: On 14 November 2022 the Government announced it would be extending the existing labelling provisions for UKCA marking, importer information, and responsible persons’ information until 31 December 2027. The Product Safety and Metrology (Amendment and Transitional Provisions) Regulations 2022 (SI 2022/1393) give effect to this.
8: SI 2006 No. 659
9: Decorative cosmetics are taken to be cosmetic products intended to modify the appearance of the area to which they are applied, usually by the use of colour. Examples are lipstick, eye shadow, blusher, eye pencil, liquid.
