FOI - Rabies Vaccines Adverse Events
Published 29 September 2025
ATI1078 Request
Could you provide me with data on the number and type of adverse events recorded for the following products:
Rabisin vaccine
Nobivac Rabies 3 year vaccine
Our reply
Rabisin and Nobivac Rabies are veterinary medicinal products (VMPs) used in dogs and cats. They are both indicated for the active immunisation against rabies to reduce clinical signs and mortality.
All medicines whether for human or animal use come with risk, and the benefits of use in wider populations need to outweigh the potential risks. Information on adverse events that have been known to occur following administration of a particular product are summarised in sections 3.6 or 4.6 of the Summary of Product Characteristics (SPC). The SPC is a document describing the properties and the officially approved conditions of use of a medicine. The SPC and associated product information are updated as new information is available, and the latest version of an SPC can be found on our publicly available Product Information Database.
Please be aware that the data we have provided below are not subject to independent verification and the Veterinary Medicines Directorate (VMD) does not guarantee their accuracy.
We have included all adverse event reports. This includes reports where more than one product was used, reports when the product was used off-label, that is using a medicine in a way that is not specified on the product’s label, or reports where, on further evaluation, there may have been other reasons for the adverse event occurring.
Evaluation is dependent on the accuracy and quality of data received from veterinary professionals and animal owners, and reporting frequency can vary over time (reporting frequency may be affected by a product being new to the market or social media interest, for example).
A summary of the clinical signs can be seen below. All adverse events submitted to the VMD have their clinical signs coded using the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA). We have provided an overview of the breakdown of adverse events by System Organ Class (SOC) which is one of the hierarchical levels of VeDDRA. The graphs below show the number of cases in which these clinical signs were reported. Please be aware that one case may have more than one clinical sign belonging to the same SOC reported, and therefore the total number of occurrences of clinical signs will be greater than the total number of reports.
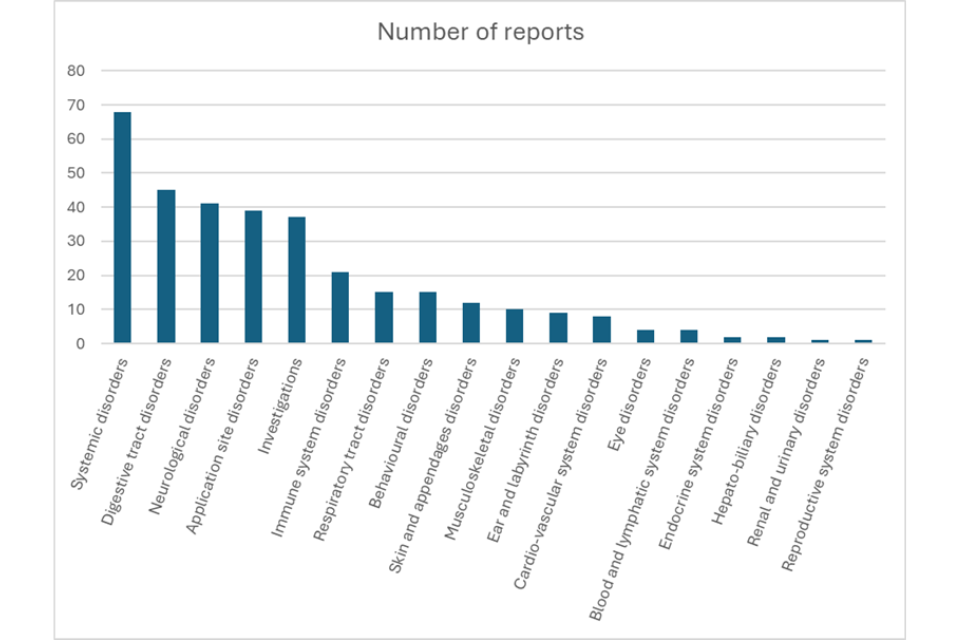
Rabisin
The VMD has received a total of 90 dog adverse event cases since this product was first authorised in 1986. Of these, there were 8 cases where lack of efficacy (where the veterinary medicinal product did not work as expected) was reported and 1 case where both lack of efficacy and an adverse reaction were reported.
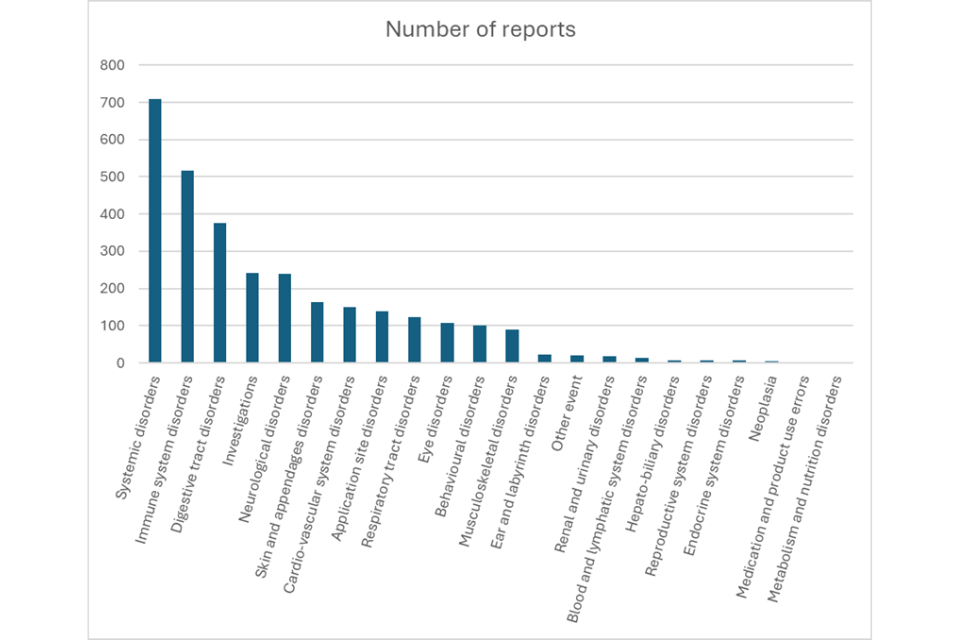
Nobivac Rabies
The VMD has received a total of 712 dog adverse events since it was first authorised in 1987. Of these, there were 66 cases where lack of efficacy was reported and 2 cases where both lack of efficacy and an adverse reaction were reported.
The number of adverse events alone cannot be used to determine the likelihood of an event occurring. This is because the numbers need to be put into context considering other factors such as differing sales volumes.
Please be aware that the VMD does not give individual clinical advice, as the specific treatment of an animal is best determined by the veterinary professional under whose care they fall. For advice on specific cases, general product advice or when considering off-label use, we advise veterinary professionals to contact the Marketing Authorisation Holder (pharmaceutical company).
