Use of UK plasma for the manufacture of albumins and vCJD risk
Published 15 August 2023
Executive summary
In 1999, a ban was introduced in the UK on the use of UK-sourced plasma for manufacture of plasma-derived medicinal products, as a precautionary measure due to concerns over possible transmission of variant Creutzfeldt-Jakob disease (vCJD).
In 2020, following a detailed review of the safety of plasma-derived immunoglobulin products manufactured from UK plasma with respect to vCJD by the MHRA, the use of UK-sourced plasma for the manufacture of immunoglobulins was allowed.
Two years on, the MHRA has undertaken a further review of the safety of albumins manufactured from UK plasma with respect to vCJD. This paper discusses whether the conclusion relating to the vCJD safety of plasma-derived immunoglobulin products manufactured from UK-sourced plasma can be extended to albumins, as there are now concerns regarding the availability of albumins due to increasing demand and a decrease in the number of blood donations.
Assessment of the prion reduction factors (PRFs), obtained from pharmaceutical manufacturers of albumin medicinal products registered in the UK, was carried out. The data provided on PRFs ranged from 5.7 to 12.7 Log and overall, these PRFs are within the range of PRF data obtained previously for immunoglobulins.
Data obtained on the usage of albumin products (a measure of volume of albumin drug dispensed by prescription in UK retail and hospital pharmacies) showed that this was increasing each year from 4.3% in 2017/18 to 10.4% in 2021/22.
Additional expert opinion from the UK National CJD Research & Surveillance Unit, University of Edinburgh, suggested that previous estimations of possible risk were too high, based on the epidemiological evidence, and concluded that there is negligible risk from UK plasma being used to manufacture plasma-derived products.
In conclusion, based on the currently available evidence, it is considered that the use of plasma from UK donors for the manufacture of human albumin products would expose the target patient population to no or minimal additional risk of vCJD in the future.
1. Introduction
1.1. Background
The use of UK-sourced plasma for manufacture of plasma-derived medicinal products was banned in the UK in 1999 as a precautionary measure owing to concerns over possible transmission of variant Creutzfeldt-Jakob disease (vCJD). In particular, the potential length and magnitude of the vCJD outbreak was of considerable concern, with predictions, as potential second-wave, of ten cases per year in the 2020s (Garske & Ghani, 2010). However, the first year in which zero deaths due to vCJD recorded was 2012 and since then there have only been two further cases: one in 2013 and one in 2016 (NCJ DRSU, 2023). So, the actual number of observed cases (shown in red, Figure 1) over the 12 years since the last major predictive modelling for vCJD cases, is clearly significantly below their lowest estimate, suggesting that previous estimations of possible risks were too high.
Figure 1: Modelling from 2010 predicting a significant second peak of infections. Black line indicates predicted number of cases, red line indicates the observed number of cases. The actual number of observed cases has been much smaller (adapted from Garske & Ghani, 2010)
Number of cases
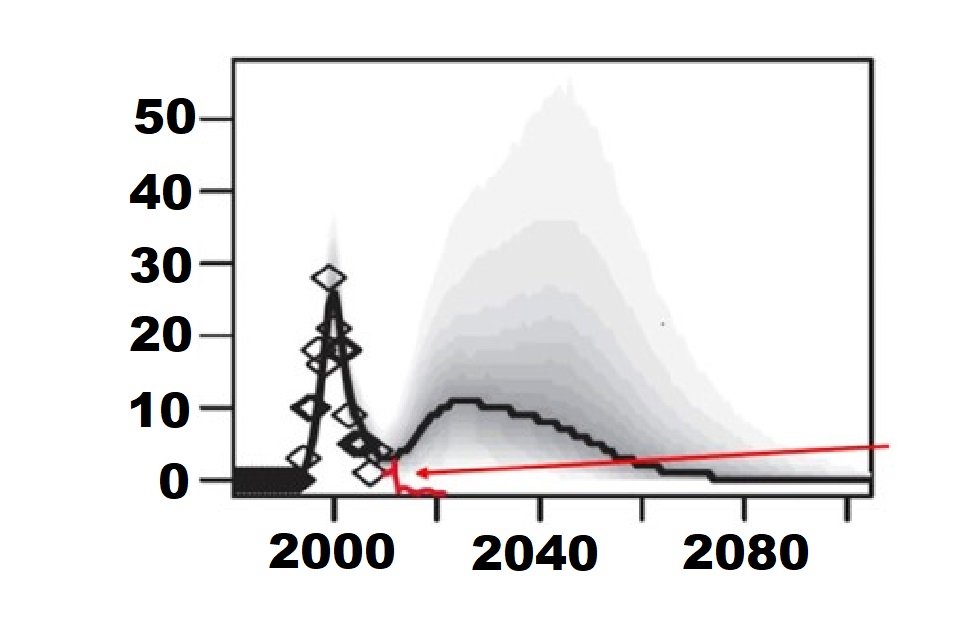 |
Diamonds: Cases identified by 2010 Black line: Median Cases Predicted Grey shades: 95% Credible intervals Red line: Observed cases (2010-2022) |
Further scientific information has become available over the years and although it has been shown that all blood components may carry some infectivity risk of vCJD, a higher level of infectivity is associated with leucocytes. Scientific publications indicate that leucodepletion is an important risk reduction measure for all blood components, although it does not remove risk entirely (McCutcheon et al, 2011; Douet et al, 2014) and recent modelling performed by MHRA, suggests that this is a minor contribution, compared to prion reduction factors (PRF) (MHRA, 2021).
In 2019, the UK advisory committee on the Safety of Blood, Tissues and Organs (SaBTO) evaluated whether the risk reduction measures, previously put in place to address the potential transmission of vCJD via blood and blood components (to paediatric patients), should be maintained. These risk reduction measures included the importation of Fresh Frozen Plasma (FFP) and the use of apheresis (single donor) platelets for patients born after 1996. Modelling was performed by analysts at DHSC and reviewed by independent experts on the Advisory Committee on Dangerous Pathogens - Transmissible Spongiform Encephalopathies (ACDP TSE), who found that the impact of these measures was small. As a result, these measures were withdrawn, though other risk reduction measures such as leucodepletion were to remain in place (SaBTO Report, 2019).
Subsequently, in 2020, the MHRA undertook a review of the safety of immunoglobulins manufactured from UK plasma with respect to vCJD safety. The review was performed owing to the perceived reducing risk, and the increasing need for immunoglobulins at a time when there was a diminishing global supply and an increase in demand – challenges that could be alleviated by the ability to fractionate immunoglobulins form UK-sourced donations.
Based on the evidence presented by MHRA, the Commission on Human Medicines (CHM) concluded that the use of plasma from UK donors for the manufacture of immunoglobulin products would expose the target patient population to no or minimal additional risk of vCJD in the future. As such, the CHM confirmed that “the risk of vCJD cases arising from the use of UK plasma for the manufacture of immunoglobulin medicinal products would be negligible” and in October 2020, the ban was lifted on the use of UK plasma for manufacture of immunoglobulins. The CHM advised that UK-sourced plasma is acceptably safe, from a vCJD perspective, for the manufacture of immunoglobulin medicinal products, provided that all relevant risk-mitigation measures already in place for blood components for transfusion (the use of leucodepletion, deferral of high-risk donors and traceability between donor and recipient) are applied to UK-sourced plasma for the manufacture of immunoglobulins.
1.2. New proposal
The MHRA has now made a case to consider the safety of albumins derived from UK-sourced Plasma, as there are concerns regarding the availability of albumins due to increasing demand and a global decrease in the number of blood donations. This paper examines whether the risk of possible vCJD transmission has reduced sufficiently to allow the use of UK-sourced plasma for the manufacture of human albumin products. This review has also taken into consideration all previous work performed by the agency, including the modelling and the previous external consultation with experts.
1.3 Rationale for reviewing the vCJD safety of albumin
The CHM recommended the lifting of the ban on using UK-sourced plasma for the manufacture of immunoglobulins. Immunoglobulins are extracted from pooled donations of human plasma (source or recovered). There is significant effort associated with setting up donation centres, donor selection and donor testing. In established manufacturing practices, all useful plasma components are collected from the same pool of donations to ensure best use of this scarce and valuable raw material. A number of different proteins can be isolated from human plasma, and although recombinant clotting factors are approved and available for treatment, there is no similar alternative for human albumin. Development of recombinant albumins is at an early stage, with insufficient production/yield to meet current supply demand and they have been targeted primarily for use as excipients (Angeli et al, 2010).
1.4 Committee advice
Following the CHM endorsement in 2020 that UK-sourced plasma is acceptably safe, from a vCJD perspective, for the manufacture of immunoglobulin medicinal products, the CHM were further asked to advise whether this conclusion can be extended to the manufacture of albumin medicinal products.
The Clinical Trials, Biologicals and Vaccines Expert Advisory Group (CTBVEAG) and CHM were first consulted as to whether a review is needed and what additional evidence would be considered necessary to examine the vCJD safety of albumin medicinal products derived from UK-sourced human plasma.
Following the CHM meeting in June 2022, the Commission agreed that a review of the CJD safety of albumin medicinal products manufactured from UK-sourced plasma should be performed and the Commission felt that additional mathematical modelling, over that already performed for immunoglobulin products, was not required, and a decision could be based on extrapolating previously collected information and further research into the prion reduction factors of various currently registered albumin products. The Commission requested the collection of the following information:
- The prion reduction factors (PRFs) of albumin medicinal products registered in the UK (to be requested from pharmaceutical manufacturers).
- Additional expert opinion of a representative of the UK National CJD Research & Surveillance Unit.
- Information regarding clinical need for/usage of albumin products.
This information was collated and presented to the CHM in a second meeting in October 2022, for their review and the findings are presented in this paper.
2. Epidemiological data
Detailed epidemiological data on vCJD with respect to individual risk factors, transmission and subclinical diseases was presented in the 2021 MHRA review (MHRA, 2021). Over the two year period since:
- There have been no additional vCJD cases reported (Source: National CJD Research and Surveillance Unit - 2023).
- The FDA removed the recommendations to defer indefinitely blood donors for: 1) geographic risk of possible exposure to bovine spongiform encephalopathy (BSE) for time spent in the United Kingdom (UK) from 1980-1996 and for time spent in France and Ireland from 1980-2001, and 2) receipt of a blood transfusion in the UK, France, and Ireland from 1980-present (FDA-CBER 2022).
- McManus et al, 2022 (including representatives of the Australian Red Cross and the US FDA) published a risk evaluation for transmission of vCJD by blood transfusion in Australia.
Overall, epidemiological data suggest that the risk of transmission of variant Creutzfeldt-Jakob disease associated with UK plasma is acceptably small to permit use of UK plasma for the manufacture of plasma derived products, such as immunoglobulins and albumins.
3. Description of human albumin products
Albumin is a multi-functional protein with many and varied roles as an osmotically active, transport/depot protein incorporating a wide range of physiological and redox properties. It has a widespread use as a therapeutic volume expander involving global manufacture of hundreds of tonnes of purified protein each year and also its progressive use as a carrier or fusion protein deriving from its well-established molecular structure (More & Bulmer, 2013; Matejtschuk et al, 2000).
Albumins are a subset of medicinal products that can be derived from human (and animal) blood. Blood is composed of four main components that generally serve different functions in the body: plasma (including fresh frozen plasma and cryoprecipitate), red blood cells, white blood cells and platelets. Plasma can also be further processed to human albumin by “fractionating” pools of many thousands of donations.
Human albumin solution does not contain clotting factors or blood group antibodies and so crossmatching is not required before administration: Blood products -Transfusion Guidelines. Current clinical practice is summarised as follows (though appropriate use of human albumin solution is much debated):
- Isotonic solutions (4.5 and 5.0% in volumes of 50 to 500 mL) are used to replace subacute plasma volume loss caused by burns, pancreatitis or trauma and as a replacement fluid in plasma exchange.
- Concentrated solutions (20% in volumes of 50 and 100 mL) are used to start diuresis in hypo-albuminaemic patients with liver cirrhosis or nephrotic syndrome, removal of large volumes of ascites in patients with portal hypertension and to assist the reduction of high bilirubin levels by exchange transfusion in the new-born (unconjugated bilirubin binds to albumin).
NHS Scotland also advises on use of human albumin in spontaneous bacterial peritonitis, hepatorenal syndrome and therapeutic apheresis.
People who are administered human albumin are usually very ill and in an unstable condition; fluid and electrolyte therapy should be adjusted according to the patient’s condition as advised by NICE.
Adverse effects include hypersensitivity reactions that may become severe.
Crystalloid solutions or synthetic colloidal plasma substitutes are alternatives for use as plasma expanders in acute blood or plasma loss.
Human albumin for use in the UK:
At present, authorised albumin preparations in the UK comply with a core SPC of 2011: Core SPC for Human Albumin Solution
The indication in the core SPC is:
4.1 Therapeutic indications
Restoration and maintenance off circulating blood volume where volume deficiency has been demonstrated, and use of a colloid is appropriate.
The posology in the core SPC is:
4.2 Posology and method of administration
The concentration of the albumin preparation, dosage and the infusion-rate should be adjusted to the patient’s individual requirements.
Human albumin is administered by the intravenous route.
4. Differences between immunoglobulin and albumin medicinal products:
4.1 The manufacturing process
Both immunoglobulins and albumins are extracted from human plasma. Recovering useful proteins from human plasma is carried out by means of a fractionation process, involving multiple sequential precipitation steps and subsequent purification of the protein of interest from the same starting material. The process utilises the ability of different proteins to separate under different conditions. Cold ethanol fractionation (used in immunoglobulin and albumin manufacture) in particular, depends on the different solubility of plasma proteins. Albumin has the highest solubility, lowest isoelectric point compared to other plasma proteins; prion proteins (PrP) have low solubility. As such, albumin will precipitate last and is likely to have the least vCJD risk - considered lower risk compared to other plasma proteins including immunoglobulins.
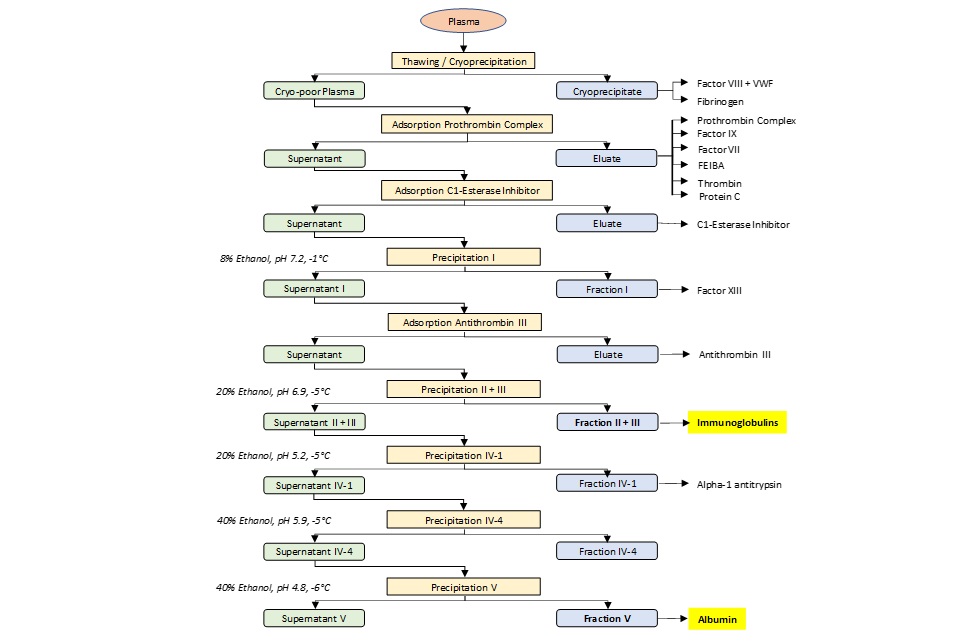
Although the starting material is the same, the specific sequence of steps yielding immunoglobulins or albumin - is not. Therefore, the capacity of processes used to obtain immunoglobulin or albumin will not have the same vCJD reduction factor (the ability to clear or inactivate PrP), which is process-specific. An example of how immunoglobulins and albumin can be derived from the same plasma pool is illustrated in Figure 2.
Figure 2. Standard plasma fractionation steps for the production of therapeutic plasma proteins (adapted from Ernst Hetzl, 2013)
4.2 Further manufacturing use
Whilst immunoglobulins are only used for patient treatment, albumins are also used as excipients in other medicinal products.
4.3 Comparison of core SPC
With reference to the core SPC, immunoglobulins are indicated for: primary immunodeficiency, primary immune thrombocytopenia, chronic inflammatory demyelinating polyradiculoneuropathy, multifocal motor neuropathy as well as Guillain Barre syndrome and Kawasaki disease (Guideline on core SPC for human normal immunoglobulin for intravenous administration (IVIg)). Immunoglobulins are used mainly for long-term maintenance in these indications that affect all ages.
The target population(s) for albumin are different; these subjects are generally ill adults with a poor prognosis who may be administered albumin for either the short- or long-term.
5. Risk evaluation
Three approaches, as recommended by CHM, have been used to address the risk of possible vCJD transmission by administering albumins manufactured from UK-sourced plasma: an evaluation of the prion reduction factors of albumin medicinal products registered in the UK (provided by pharmaceutical manufacturers), additional expert opinion from the UK National CJD Research & Surveillance Unit and a review of the usage for various human albumin products authorised in the UK.
Excluded from the review
Albumin as an excipient is excluded from this review due to the variety of medicinal products that may contain this excipient and due to the difficulties in understanding risk correlated to posology, amount used, etc.
5.1 Prion reduction factors (PRF)
Manufacturers of albumin products licensed for use in the UK provided information about the prion reduction capacity of their manufacturing processes. However, the limitations of the provided data should be noted: (1) prion reduction factors (PRFs) are determined using in vitro model systems, which may contain bias; (2) model systems used by different manufacturers may not be equivalent; (3) the whole evaluation approach is likely to be different between manufacturers; (4) the data represented should not be treated as fully exhaustive; and (5) although the presented PRFs were obtained for the products studied, other reduction factors outside of the range obtained are possible.
Manufacturer’s PRFs are summarised in the following table:
Albumin
| Min | Max | |
|---|---|---|
| Prion reduction factor (Log) | 5.7 (3.9) * | 12.7 |
The reduction factors previously obtained for immunoglobulins are provided for comparison:
Normal Immunoglobulin
| Min | Max | |
|---|---|---|
| Prion reduction factor (Log) | 4.8 | 10.5 |
Hyperimmune Immunoglobulin
| Min | Max | |
|---|---|---|
| Prion reduction factor (Log) | 7.3 | 9.4 |
The minimum PRF obtained by a manufacturer was 5.7 Log whilst the maximum was 12.7 Log. This compared well to the PRFs obtained by manufacturers of normal immunoglobulin and hyperimmune immunoglobulin products, which ranged from 4.8 - 10.5 log, and 7.3 – 9.4 log, respectively.
*The lower value, 3.9 log, represents registered dossier information, from one company, which studied experimentally the prion reduction capacity of two manufacturing steps. They applied the regulatory requirements for viral reduction to prion reduction, where at least two orthogonal reduction/inactivation steps are required by regulatory guidance, and disregarded the contribution of the second step as non-orthogonal. However, since the requirements for viral reduction/inactivation do not necessarily apply to prion safety, the company considered it scientifically appropriate to use the cumulative prion reduction factor for the purposes of the albumin vCJD safety review. Therefore, as a conservative approach, the lowest obtained reduction factors for the two manufacturing steps investigated were added, yielding a value of 2.9+2.8 = 5.7 log. The company considered that this reduction factor most accurately reflects the real capability of the process to reduce prions. The company stated further that other manufacturing steps could also contribute to the reduction factor, however, these steps had not been experimentally investigated.
Overall, the PRFs, obtained by the manufacturers for albumin products registered for use in UK, are within the range for immunoglobulins and were considered to be within the already established, previously reviewed margins of risk.
5.2 MHRA external consultation
Additional advice was sought from the UK National CJD Research & Surveillance Unit (University of Edinburgh), who previously contributed to the initial review of the vCJD safety of immunoglobulins undertaken in 2020 (MHRA, 2021). The original assessment has now been complemented with an addendum specifically addressing albumin medicinal products.
The addendum since the last review in 2020 (MHRA, 2021) emphasises two important general points:
- No cases of vCJD have been identified in this period (2020 - 2022).
- No further cases of vCJD infection due to blood/blood products have been identified, since 2020.
These facts suggest that previous estimations of possible risk were too high-in relation to both primary dietary cases and secondary blood-related transmissions.
Further comments made in the addendum have been summarised below:
- Current evidence suggests that previous risk assessments were too high and there is negligible risk from UK plasma being used to manufacture plasma-derived products.
- The UK CJD Surveillance System was enhanced to identify potential missed cases of vCJD in the elderly focussing on the Lothian region of Scotland; no cases were identified.
- The USA, Ireland and Australia removed deferral restrictions based on cumulative time spent in the UK, in May 2022 (FDA, 2022), September 2019 (IBTS, 2019), April 2022 (McManus et al, 2022), respectively.
- The paper acknowledges that different manufacturing processes may result in different prion reduction factors.
- The paper revises the initially provided view that the “dilution” of infectivity was not of significance; considering recent risk considerations that have included a “dilution” effect, the view currently being expressed is that when pathogen prevalence is very low, the larger pool size may be considered to contribute a dilution effect that reduces the likelihood of an infectious dose being present in the final products.
- The addendum concludes that regardless of any particular points that can be made about albumin production, the background epidemiological evidence suggests negligible risk in using UK plasma for the manufacture of plasma-derived products in general.
5.3 Patient need and albumin usage data in the UK
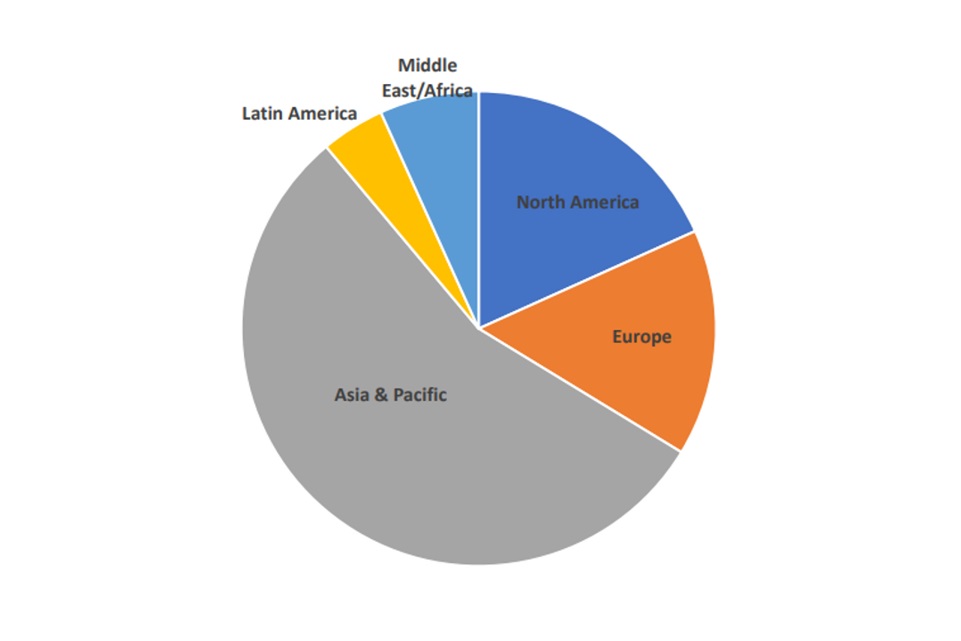
The use of albumins has been steadily increasing with the global albumin market size projected to exhibit an annual growth rate (CAGR) of 6.0% during the forecast period between 2018 to 2026, Global Albumin Market Size, Share & Growth. 2022. Fortune Business Insights. This is primarily due to surge in prevalence of chronic diseases and increasing patient demand, particularly in the Asia-Pacific market, see Figure 3.
Figure 3: Global clinical need for Albumin (adapted from The Market Research Bureau Inc. 2018)
2018 Albumin usage by region (~1000 metric tons)
UK albumin drug usage data was derived from the IQVIA MIDAS® data Q2 2017-Q1 2022. This data captures the volume of drugs dispensed by prescription in UK retail and hospital pharmacies. Retail dispensing data is based on volume products dispensed against a prescription in retail pharmacies and wholesaler sell-in data to dispensing doctors (a proxy for dispensed product). Hospital dispensing data covers usage/consumption levels of medicinal products within hospitals (irrespective of their source of supply).
The data on the total number of vials/bottles of human albumin dispensed in 10 hospitals in England is summarised in Table 1.
Volume of albumin products dispensed in 10 hospitals in England
| Review Period | Q2 2017 - Q1 2018 | Q2 2018 - Q1 2019 | Q2 2019 - Q1 2020 | Q2 2020 - Q1 2021 | Q2 2021 - Q1 2022 |
|---|---|---|---|---|---|
| TOTAL no. of vials | 215,363 | 224,664 | 232,339 | 252,110 | 278,383 |
The data shows that there has been approximately 30% increase in volume of albumin usage from 2018 to 2022, with ~10% increase in the last year alone (2021-2022), indicating that albumin usage increased.
Given this is sample based on 10 hospitals in England, it is not possible to estimate the total extent of use across the UK.
It is not possible to obtain patient-specific data such as numbers of patients prescribed or patient demographics (or from which to establish indication) from this data source.
Source: Based on internal analysis by the MHRA using data from the following source: IQVIA MIDAS® Sales data for the period Q2 2017-Q1 2022. UK Retail and Hospital data in volume, Albumin products in ATC classes K03B & K03C reflecting estimates of real-world activity. Copyright IQVIA. All rights reserved.
Audits of hospital use would be required to add to information on use of albumin. There is one such audit report from Guys and Thomas’s Hospitals, London, Yazdani et al, 2017). This audit was prompted by a shortage of human albumin in 2015. Guys and Thomas’s Hospitals started a Trust-wide demand-management programme followed by a 6-month audit; the authors report that most examples of use of human albumin were ‘appropriate’ and that plasma exchange accounted for about 85% of use of human albumin.
6. Overall discussion
After over 20 years since precautionary measures were put in place following the outbreak of vCJD, there have been no documented vCJD cases due to dietary exposure in anyone in the UK born after 1989, the year major dietary protection measures were introduced), no documented transmissions of vCJD via blood components in the UK population (since universal leucodepletion was introduced in 1999) and no documented transmissions via plasma or platelets. Furthermore, there have been no cases of transfusion-related transmission anywhere else in the world even though some countries, such as France, have had cases of vCJD (SaBTO Paediatric Components Working Group Report, 2019). In addition, there has been no evidence of a second peak of infections as initially predicted.
Over this period, there has been an increase in the demand for plasma-derived medicinal products (PDMPs), in particular for immunoglobulin and albumin products.
Following a review in 2020 (MHRA, 2021), the ban on the use of UK plasma to manufacture immunoglobulins was lifted. Due to perceived diminishing risk and an increased need for albumin products, the MHRA have now undertaken a further review of the safety of albumins manufactured from UK plasma with respect to vCJD risk.
Three approaches have been taken to evaluate the risk of allowing for the use of UK plasma in the manufacture of albumins.
The prion reduction factors (PRFs) of albumin medicinal products registered in the UK were obtained from pharmaceutical manufacturers, who provided such data with PRFs ranging from 5.7 - 12.7 Log. Overall, these PRFs were within the range for immunoglobulins and were considered as acceptable from a safety perspective, in the context of current epidemiological evidence.
Additional expert opinion from the UK National CJD Research & Surveillance Unit (University of Edinburgh), emphasised that, since the ban was lifted on use of UK plasma for manufacture of immunoglobulin, there were no cases of vCJD identified and no further cases of vCJD infection due to blood/blood products have been identified. The expert opinion suggested that previous estimations of possible risk were too high and there is negligible risk from UK plasma being used to manufacture plasma-derived products.
Data on usage of albumin products was obtained from the IQVIA MIDAS database (a commercial database), which provided the volume of albumin drug dispensed by prescription in UK retail and hospital pharmacies and showed that this was increasing each year from 4.3% in 2017/18 to 10.4% in 2021/22.
7. Overall conclusion
Overall, from the perspective of vCJD safety, considering the totality of the collected evidence, such as existing manufacturing and process capability, the most recent epidemiological data, expert advice, and having regard to previous reviews examining vCJD safety performed by the MHRA, it was concluded that UK-sourced plasma can be used for the manufacture of albumin medicinal products, in addition to the already approved use for the manufacture of immunoglobulins.
8. List of references
- Albumin solution - NICE
- Angeli P, Ascione A, Bernardi L, Belondi L, et al (2010). Albumin: From a plasma volume expander to a drug (a practical guide for the therapeutic use of albumin). Aim publishing, Rome.
- Blood products - Transfusion Guidelines.
- Core SPC for Human Albumin Solution.
- Douet JY, Zafar S, Perret-Liaudet A, Lacroux C, Lugan S, Aron N, Cassard H, Ponto C, Corbière F, Torres JM, Zerr I, & Andreoletti O. Detection of Infectivity in Blood of Persons with Variant and Sporadic Creutzfeldt-Jakob Disease. Emerg Inf Dis 2014; 20(1) 114-117. PMID: 24377668, doi.
- Ernst Hetzl, (2013). The pharmaceutical manufacturing environment. In: Production of Plasma proteins for Therapeutic Use. (Bertolini, Goss, Curling Eds). John Wiley & Sons, Inc., New Jersey.
- Food and Drug Administration Center for Biologics Evaluation and Research: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Components Guidance for Industry. 2022.
- Garske T and Ghani A. Uncertainty in the tail of the variant Creutzfeldt-Jakob disease epidemic in the UK. PLoS One 2010; 23;5 (12):e15626. doi.
- Global Albumin Market Size, Share & Growth. Fortune Business Insights. 2022. https://www.fortunebusinessinsights.com/albumin-market-102637
- Guidelines on core SPC for human normal immunoglobulin for intravenous administration (IVIg). 2021
- Guidelines for the Usage of Human Albumin Solution 2018 (scot.nhs.uk)
- Irish Blood Transfusion Service (IBTS) (2019)
- Matejtschuk P, Dash CH and Gascoigne EW. Production of human albumin solution: a continually developing colloid. B. J. Haem 2000: 85 (6): 887-895.
- McCutcheon S, Alejo Blanco AR, Houston EF, de Wolf C, Tan BC, Smith A. All Clinically-Relevant Blood Components Transmit Prion Disease following a Single Blood Transfusion: A Sheep Model of vCJD. PLoS ONE 2011; 6(8): e23169.
- McManus H, Seed CR, Hoad VC, Kiely P, Kaldor JM, Styles CE, Yang H, Law M, Gosbell IB. Risk of variant Creutzfeldt-Jakob disease transmission by blood transfusion in Australia. Vox Sang 2022; 117(8):1016-1026. doi: 10.1111/vox.13290. Epub 2022 May 24. https://onlinelibrary.wiley.com/doi/10.1111/vox.13290
- Medicines & Healthcare products Regulatory Agency (MHRA). Critical risk assessment report: Use of UK plasma for the manufacture of immunoglobulins and vCJD risk. 2021.
- More M and Bulmer M. (2013). Human serum albumin: a multifunctional plasma protein. In: Production of Plasma proteins for Therapeutic Use. (Bertolini, Goss, Curling Eds). John Wiley & Sons, Inc., New Jersey.
- National CJD Research & Surveillance Unit. CREUTZFELDT-JAKOB DISEASE IN THE UK.
- SaBTO: Advisory Committee on the Safety of Blood, Tissues and Organs. Paediatric Components Working Group Report: Importation of plasma and use of apheresis platelets as risk reduction measures for variant Creutzfeldt-Jakob Disease. 2019/
- SaBTO Paediatric Components Working Group Report. Importation of Plasma and use of apheretic platelets as risk reduction measures for vCJD. March 2019.
- The Market Research Bureau Inc. The worldwide plasma protein market 2018. The Market Research Bureau Inc. CT, USA. April 2023.
- Yazdani MS, Retter A, Maggs T, Li P, Robson MG, Reid C, Holmes P, Garood T, Robinson SE. Where does the Albumin go? Human Albumin Solution usage following the implementation of a demand management programme. Transfus Med 2017; 27: 192-199
