Acceptable analytical protocols
Published 24 November 2021
Applies to England
The analytical procedures employed must be capable of detecting haemoglobins S, C, DPunjab, E, and OArab, in addition to fetal haemoglobin (HbF) and HbA. Newborn samples are typically composed of HbF (75%) with approximately 25% HbA and small quantities of acetylated HbF.
The procedures used must therefore be sensitive, reliable and reproducible in terms of detecting small quantities of HbA and the abnormal haemoglobin fractions listed, in the presence of large amounts of HbF.
The methods used must be validated for newborn specimens. The use of procedures or instruments designed for adult specimens is not recommended.
Four types of analysis are in use for newborn screening for sickle cell disease using dried blood spot samples:
- High performance liquid chromatography (HPLC)
- Capillary electrophoresis (CE)
- Tandem mass spectrometry (MSMS)
- Isoelectric focusing (IEF)
All these methods are suitable for first-line screening. The second-line test, when required, must also be selected from this list but must have a different scientific principle from the first-line test. For MSMS, all samples with results outside the designated action values must be sent for second-line testing. Where the results obtained for second-line testing do not confirm the MSMS findings, samples must be further investigated if the MSMS result suggests Hb S, sickle cell disease and/or beta thalassaemia. IEF is not recommended as a second-line test for MSMS when confirming beta thalassaemia either alone or in conjunction with a haemoglobin variant[footnote 1].
See minimum requirements for quality control material for these techniques. When using HPLC or CE, the haemoglobin separations (traces) must be checked due to the possibility of misclassification of fractions. All results must be read and then checked by different suitably competent individuals, one of whom must be a registered scientist.
1. High performance liquid chromatography
HPLC uses an ion exchange resin, held in a column cartridge, in conjunction with a buffer gradient. As the ionic strength and/or pH of the buffer changes, certain haemoglobins are eluted from the column and the presence of haemoglobin is detected using a spectrophotometric technique. The time from injection to the point at which the haemoglobin fraction elutes is known as the retention time of the haemoglobin and is a reproducible measurement for a particular column, buffer, exchange resin and temperature.
It is quite common for different haemoglobins to elute at the same retention time. Therefore, the retention time is not a unique identifier. HbF is eluted separately from HbA. Haemoglobins S, C, D, E and OArab also have separate retention times and characteristic chromatographic profiles. In addition, the relative proportions of the different haemoglobins are recorded. It is therefore possible to detect the difference between carriers and affected babies and also to differentiate some types of compound heterozygosity of HbS with β+ thalassaemia.
2. Capillary electrophoresis
CE uses a combination of ion migration and electro-osmotic flow to separate protein molecules. When a voltage is applied across the capillary tube filled with an electrolyte solution, the solution begins to move towards one of the electrodes due to electro-osmotic flow. This drives the bulk flow of materials past the detector in the same way that a pump pushes the liquid in HPLC. The haemoglobin molecules move towards the detector at different speeds depending on their ionic charge and electrophoretic mobility. Both electro-osmotic flow and electrophoretic mobility are occurring at the same time, working in opposite directions to provide greater resolution.
This method of separation should not be confused with simple electrophoretic mobility as seen in cellulose acetate electrophoresis. Combining electro-osmotic flow and electrophoretic mobility is a separate phenomenon and is exploited in CE for maximum separation power. Even so, it is quite common for different haemoglobins to migrate at the same rate and appear at the same position. Thus, position is not a unique identifier.
HbF is separated from HbA. Haemoglobins S, C, D, E and OArab also have different mobility rates and characteristic profiles. In addition, the relative proportions of the different haemoglobins are recorded. It is therefore possible to detect the difference between carriers and affected infants and also to differentiate some types of compound heterozygosity of HbS with β+ thalassaemia.
3. Tandem mass spectrometry
Mass spectrometry generates charged molecules or molecular fragments and measures their mass-to-charge ratio (m/z). Differences in m/z can be used to separate molecules or atoms and to determine chemical and structural information of molecules, such as peptides. The 3 main components to the procedure are:
- ionisation of the analytical material
- determination of the m/z based on the movement of the ions through electrical and/or magnetic fields
- the detection of the ions
Electrospray ionisation employs a high voltage applied to a liquid to create an aerosol to produce ions for analysis. It has the advantage of producing multiply-charged intact ions from relatively large parent species.
MSMS employs 3 coupled quadrupole analysers in a linear arrangement. The first and third quadrupoles (Q1 and Q3) are mass analysers, with the second (Q2) operating with a fixed radio frequency voltage and used as a collision cell with ion focusing properties.
At this time the only CE-marked commercial reagent set available is produced by SpotOn Clinical Diagnostics. Methodological information relates to the use of these reagents. Any MSMS instrument used for this work must perform as well as, or better than, the instruments deemed acceptable in the NHS SCT Screening Programme tandem mass spectrometry newborn screening pilot study[footnote 2] as assessed by the action value limits and the false positive rate.
Unlike HPLC, CE and IEF, the analysis is not performed on intact haemoglobins but on fragmented globin chains (peptide fragments). The method incorporates a series of experiments performed simultaneously to detect HbS, HbC, HbDPunjab, HbE and HbOArab.
The method is mutation-specific. Therefore, all cases containing the mutation under investigation will be detected and misclassification of coeluting variants should be minimised. The signal for the wild-type beta and gamma globin is also assessed, acting as a surrogate for HbA and HbF. The gamma/beta wild type ratio represents the relative proportion of HbF to HbA. A high ratio suggests low HbA, which may be due to prematurity or β thalassaemia, and a low ratio indicates a low HbF, which may be due to age, transfusion or gamma thalassaemia.
An internal standard is also included to act as a methodological control. The material is a synthetic, stable isotope-labelled, sickle sequence peptide with an extension peptide, which requires tryptic activity to generate the stable isotope-labelled peptide to be detected in the analysis. Low values are indicative of incomplete digestion but may also be seen if the sensitivity/performance of the instrument has deteriorated.
High values indicate reduced ion suppression, which may be found in samples with low haemoglobin content or where the digestion process has not accessed all of the sample. Accidental addition of multiple shots of reagent will also result in high values. Laboratories must monitor the internal standard values for drift/trends as an indication of the MSMS performance within and between runs.
Results are expressed as a ratio of variant haemoglobin divided by corresponding wild-type signal. To determine which samples are potentially screen positive and therefore require second-line testing, common action values have been derived from the numeric ratio of the variant and wild type signal in each acquisition. See action values for tandem mass spectrometry screening
4. Isoelectric focusing
IEF gives good separation of HbF from HbA and variant haemoglobins S, C, DPunjab, E and OArab. The separation of different haemoglobins is accomplished through application of a haemoglobin sample on to a precast agarose gel containing ampholytes at pH 6 to 8. Ampholytes are low molecular weight amphoteric molecules with varying isoelectric points (pIs). When an electric current is applied, these molecules migrate through the gel to their pIs forming a stable pH gradient. The haemoglobin variants also migrate through the gel until they reach the point at which their pI equals the corresponding pH of the gel. At this point, the net charges on the variants are zero and migration ceases. The electric field counteracts diffusion and the haemoglobin variants form discrete thin bands. In UK laboratories this procedure is now only used as a second line procedure.
5. General analytical considerations
Users must be aware that the laboratory handbook highlights common analytical and diagnostic issues, but every laboratory must follow the principles of good laboratory practice including satisfying themselves that they understand the capabilities and limitations of their chosen technique. The equipment and protocol chosen must fulfil the requirements of the screening programme and UKAS/ISO 15189 and demonstrate suitable performance on external quality assurance (EQA).
The use of rules to screen samples for further action/reporting or the use of post-analytical data analysis algorithms must have software quality control procedures, including regular process audit, to ensure that quality is not compromised. It is essential that the process is risk assessed and that there are failsafe mechanisms in place. Raw data, such as HPLC chromatograms and CE plots, must always be reviewed and any post-analytical procedures including algorithms must be fully documented and traceable to ensure consistency of quality.
The application of HPLC and IEF for newborn screening has a disadvantage in that the process also separates the normally occurring adducted fractions, that is acetylated HbF (HbF1) and degraded haemoglobin fractions which can make interpretation difficult. The retention times and migration patterns of different haemoglobin variants are not unique and thus the results obtained can only be regarded as provisionally identifying the variant(s) concerned.
If HPLC is used as the screening technique it is essential to check and maintain the positions of the windows which are used as the first stage identification of any variants found. This is usually achieved by the use of retention time markers.
If CE is used, appropriate control material must be used to ensure optimal analytical performance. Optical density (OD) levels greater than 0.07 and the presence of sufficient HbF are required to determine the migration position and thus permit ‘zoning’ and a provisional identification of haemoglobins present in the sample. If failure to zone is due to low OD, this is usually related to the amount of haemoglobin in the sample. This should be corrected by increasing the spot to diluent ratio and allowing a longer elution time. Extreme care is needed if extraneous haemoglobin is added to a clinical sample to allow zoning.
The addition of haemoglobins that were not present in the initial sample will make the electropherogram more difficult to interpret and may lead to the misinterpretation of the results. Such modified samples must be analysed with a unique identifier distinguishable from both the original specimen and from any other clinical sample. Experiments have shown that denatured HbF (acetylated F), which increases with age of the sample, appears in zone N9 but can appear on the borders of zone N10. When only a small amount of HbA is present the 2 may overlap, falsely increasing the measured level of HbA. Caution is needed in interpreting such results.
If MSMS is used, the instrument must be optimised using the synthetic peptide standards, with documented traceability and quality. This will ensure optimum detection of required haemoglobins. Failure to optimise target masses can result in misidentification. MSMS cleaning protocols must always be followed and appropriate controls must be run with each plate of samples. The internal standard results must be monitored and be within the limits established for the instrument/laboratory. A high internal standard result is usually indicative of low haemoglobin concentration in the blood spot. During reagent addition care must be taken to avoid splashing between wells as this may result in false positives. If using an instrument/software which can produce a value of zero for the denominator the resulting ratio will also be zero regardless of the value of the numerator and a positive result can be missed.
If IEF is used, then control haemoglobins must be run with each plate. Care must be taken to ensure that there is clear delineation between the bands for adjacent specimens. This can be achieved by firmly blotting the gel and blotting again after the template has been added before the addition of the sample.
When using the percentages of the haemoglobin fractions to interpret the results, the possibility of the presence of transfused blood must be considered (see figures below).
5.1 Figure 1: percentage of HbA in routine specimens taken from untransfused babies with gestations from 23 to 42 weeks
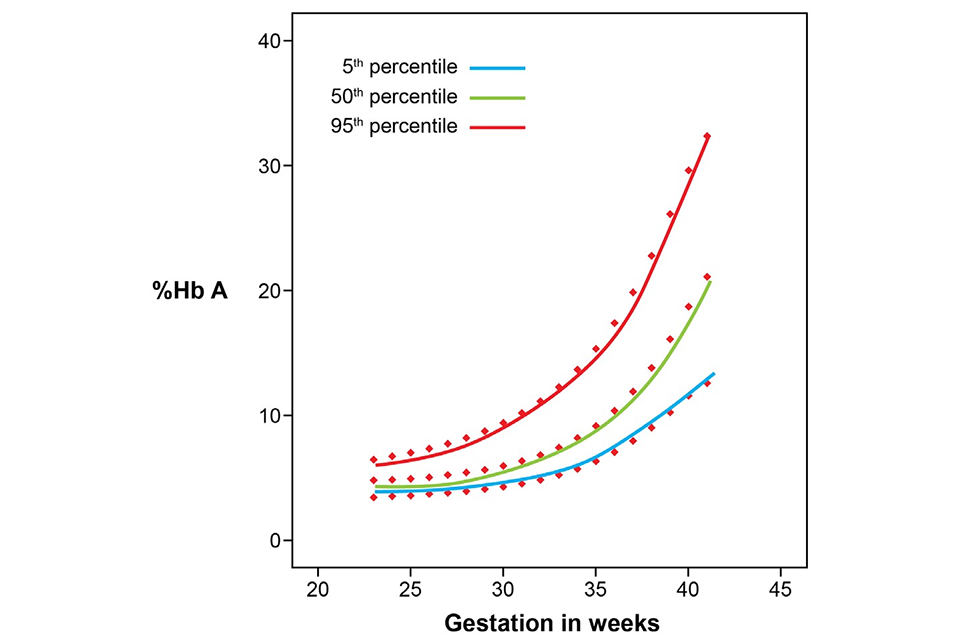
Above graph shows percentage of HbA increasing with length of gestation for babies in the 5th percentile, 50th percentile and 95th percentile.
5.2 Figure 2: percentage of HbA in untransfused babies one month to one year old

Above graph shows percentage of HbA in untransfused babies increasing rapidly up to 100 days after birth and levelling off below 80% HbA between 200 and 400 days after birth.
-
Daniel Y, Henthorn J. Newborn haemoglobinopathy screening using tandem mass spectrometry: Lessons from investigation of an unusual case. Journal of Medical Screening 2019, 26(4) pages 219 to 220. https://doi.org/10.1177/0969141319868242 ↩
-
Daniel Y, Henthorn J. Tandem Mass Spectrometry for Sickle Cell and Thalassaemia Newborn Screening Pilot Study, Public Health England. 2015. ↩
