[Withdrawn] Prostate cancer risk management programme (PCRMP): benefits and risks of PSA testing
Published 29 March 2016
1. Background
The purpose of this guidance is to help primary care teams give asymptomatic men information about the potential benefits, limitations and implications of having a prostate specific antigen (PSA) test for prostate cancer. It provides background information about the diagnosis and treatment of prostate cancer and outlines the issues surrounding the use of the PSA test.
In 2002, more than 100 GPs and primary care cancer leads and an expert multidisciplinary group set up by the Department of Health were consulted before the publication of the first edition of this guidance. The second edition was subsequently published in 2010 [footnote 1].
The Prostate Cancer Risk Management Programme (PCRMP) commissioned this third edition in 2015 based on the recent UK National Screening Committee (UK NSC) evidence review for prostate cancer screening[footnote 2] which incorporates information from research developments and the recommendations of the National Institute for Health and Care Excellence (NICE) in the Prostate cancer: diagnosis and treatment guidelines[footnote 3]. It was reviewed by GPs and members of the PCRMP scientific reference group prior to publication.
Accompanying summary information sheets for men and GPs can also be downloaded and printed out.
The overall benefits of prostate cancer screening remain uncertain but there have been considerable advances in our understanding while reviewing the emerging evidence.
2. Introduction
Prostate cancer is the most common cancer in men in the UK[footnote 4]. The PSA test is the most commonly used test that can lead to the diagnosis of localised prostate cancer for which potentially curative treatment can be offered. Increased PSA levels may be associated with a raised probability of prostate cancer.
Not all men with increased PSA levels have cancer. Some will have low-risk disease (a relatively indolent tumour) that is unlikely to progress or require treatment. The low specificity of the PSA test has led to harms of overdiagnosis and overtreatment in up to 50% of men[footnote 5]. There have been considerable advances in our understanding of prostate cancer but currently there is no evidence that the overall benefits of a PSA-based screening programme would outweigh the harms[footnote 2].
3. Informed choice
The PCRMP aims to help primary care give clear and balanced information to asymptomatic men who ask about testing for prostate cancer. The PSA test is available free to any man aged 50 or over who requests it, after careful consideration of the implications. GPs should not proactively raise the issue of PSA testing with asymptomatic men.
Before men who are concerned about their risk of prostate cancer make a decision about whether or not to have a PSA test, they should be given information about the advantages and disadvantages of the PSA test, biopsy and treatments for prostate cancer. This means men can make informed decisions about whether or not to have a PSA test.
Potential advantages of having a PSA test for an individual include knowing his PSA level and finding cancer at an early stage. There are also potential disadvantages to being tested. The man’s personal preferences are an important factor in the decision.
Decisions about whether or not to have a PSA test will vary between individuals, based on:
-
being at higher than average risk of prostate cancer (having a family history of prostate cancer or being of black ethnicity)
-
fear of cancer
-
consequences, such as anxiety of being diagnosed with a disease that is unlikely to become symptomatic
-
potential impact of treatment complications on quality of life
-
understanding of the scientific evidence
Despite the uncertainties, many men still want the PSA test because it is seen as “just another blood test” or as responsible health behaviour to prevent prostate cancer[footnote 6]. Some men may accept PSA testing without clearly understanding the potential harms[footnote 6] [footnote 7].
A study of GPs showed variation in the amount of information given to the patient. A full and balanced view of potential harms and benefits may not always be conveyed[footnote 8]. Men who need further investigation after a PSA test may often experience increased anxiety, regret and uncertainty, even if they receive a “normal” result[footnote 7].
In a randomised controlled trial, approximately 1,000 men aged 40 to 75 in selected practices in England and Wales were randomised to receive either a patient decision aid that provided balanced information about the potential benefits and limitations of the PSA test (intervention) or no patient decision aid (controls). The men who received the decision aid had improved knowledge of the PSA test and less positive attitudes towards the test[footnote 9].
4. Incidence and mortality
Prostate cancer is the most common cancer in men, representing about a quarter of all new male cancer diagnoses in the UK[footnote 4]. It is the second most common cause of cancer-related deaths in men in the UK. In 2013, 47,300 men were diagnosed with prostate cancer[footnote 4] and, in 2012, 10,837 men died from the disease [footnote 10]. The age-standardised incidence rate is 182 new prostate cancer diagnoses per 100,000 men in the UK population[footnote 4]. The mortality rate is substantially lower at about 23 deaths per 100,000 men[footnote 10].
Incidence and mortality are significantly higher in:
-
black men[footnote 11]
-
men with a family history of prostate cancer[footnote 12] [footnote 13]
-
men who are overweight or obese (specifically for advanced prostate cancer)[footnote 14]
Prostate cancer is strongly associated with increasing age[footnote 15].
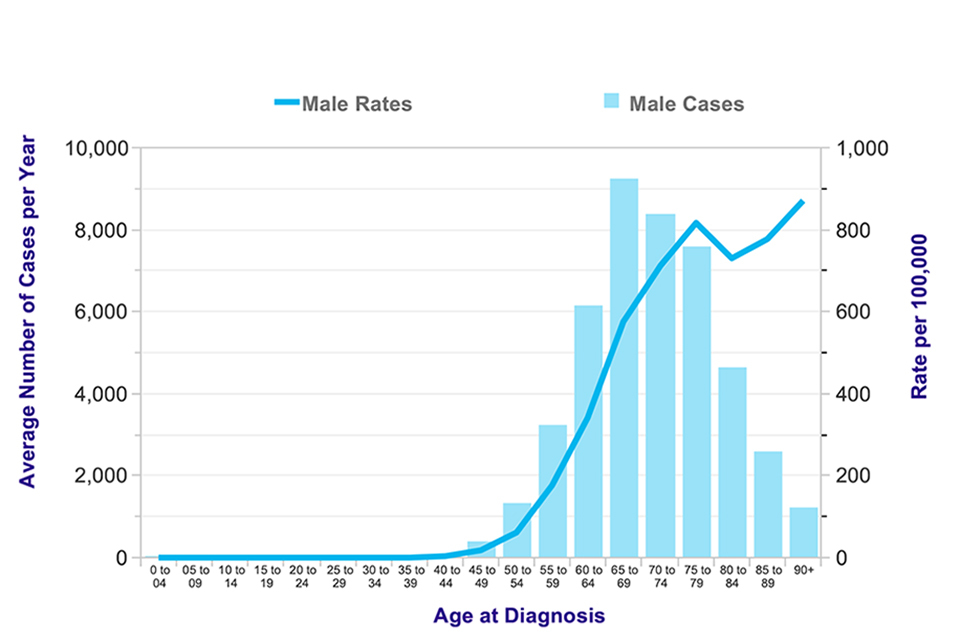
Figure 1. Average number of new cases of prostate cancer per year and age-specific incidence rates for men in the UK, 2011-2013
Diagnosis is less common below the age of 50 (Figure 1 [footnote 4]). The most common age to be diagnosed is 65 to 69 years and the highest mortality rate is seen in the 85 and older age group.
The number of deaths by age in 2012 is shown in Figure 2 [footnote 10].
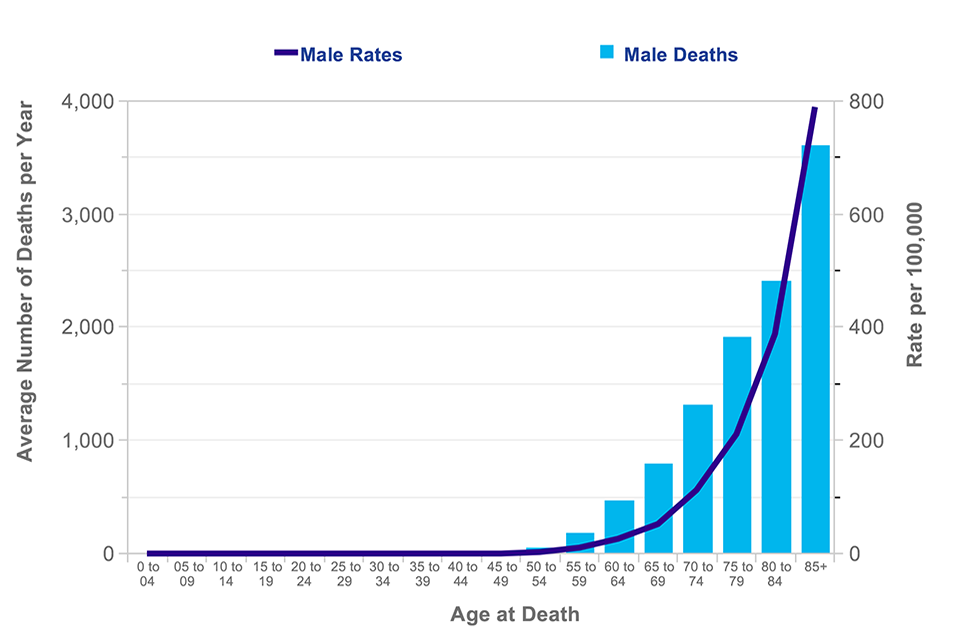
Figure 2. Average number of deaths per year and age-specific mortality rates per 100,000 population in the UK, 2010-2012 [^10]
The number of prostate cancer cases has risen steadily since 1975[footnote 4] [footnote 16].
Reasons include:
-
ageing population
-
improved ascertainment by cancer registries leading to increased diagnostic accuracy
-
additional methods of detecting prostate cancer, including an increase in the use of the PSA test
-
increase in ultrasound guided biopsies in men with raised PSA levels
Early detection and treatment may reduce mortality from prostate cancer. The European Randomized Study of Screening for Prostate Cancer (ERSPC), the largest randomised trial of PSA testing for prostate cancer, found a significant reduction in risk of death from prostate cancer by 21% after 13 years of follow-up[footnote 17].
Reasons include:
-
ageing population
-
improved ascertainment by cancer registries leading to increased diagnostic accuracy
-
additional methods of detecting prostate cancer, including an increase in the use of the PSA test
-
increase in ultrasound guided biopsies in men with raised PSA levels
5. Natural history of prostate cancer
The natural history of prostate cancer is not fully understood. The underlying mechanisms that influence cancer development are under investigation. Prostate cancer develops in the following stages:
-
initiation
-
diagnosis by screening
-
diagnosis by clinical symptoms
-
clinically detectable metastatic disease
-
death
Treatment and management of prostate cancer is difficult due to the broad spectrum of the disease, which is defined by the rate of tumour growth observed. For instance, slow-growing ‘clinically insignificant’ tumours in asymptomatic men are unlikely to progress or require treatment. Rapidly growing ‘clinically significant’ tumours have the potential to progress and metastasize. It is not known why some tumours are more aggressive.
6. Risk factors for prostate cancer
There is often increased anxiety amongst men with factors that put them at higher than average risk of prostate cancer. If these men present in primary care, it is important they receive the best available information and support to help them decide whether or not to have a PSA test.
In the UK, about 1 in 8 men will get prostate cancer at some point in their lives[footnote 11]. Factors that increase a man’s risk include:
-
increasing age
-
black ethnicity
-
family history of prostate cancer
-
being overweight or obese (specifically advanced prostate cancer)
These risk factors suggest potentially different clinical management strategies.
6.1 Age
Prostate cancer mainly affects men over the age of 50 and risk increases with age. Autopsy studies have shown that prostate cancer can have a long latent period and men show evidence of cancer cells in their prostate as early as in their 20s and 30s [footnote 18] [footnote 19]. By the age of 80, about 80% of men will have evidence of cancer cells in their prostate. However, only 2 in 50 of all men will die from prostate cancer, which supports the evidence that men are more likely to die from other causes rather than from prostate cancer[footnote 10] [footnote 18].
6.2 Ethnicity
Men of black ethnicity are at greater risk of prostate cancer than men of white ethnicity, while Asian men have a lower risk. The lifetime risk of being diagnosed with prostate cancer is 1 in 4 for black men compared to 1 in 8 for white men[footnote 11]. The UK PROCESS (Prostate Cancer in Ethnic Subgroups) Study showed black men present about 5 years younger and are more likely to have higher PSA on presentation, compared to white men[footnote 20] [footnote 21] [footnote 22]. A number of biological [footnote 23] [footnote 24] [footnote 25] or genetic factors [footnote 26] [footnote 27] [footnote 28] may explain the higher frequency of disease in black men. However, the evidence is inconclusive and further investigation is needed before understanding its usefulness in genetic screening.
To date, there is no evidence screening black men at high risk for the disease will reduce their risk of prostate cancer-related death[footnote 2].
6.3 Family history and genetics
Studies dating back to the 1950s show family history is a risk factor for prostate cancer[footnote 29]. Overall lifetime risk of prostate cancer according to family history is summarised in Table 1 [footnote 30] . A man without a family history has an absolute lifetime risk of prostate cancer of 8%. This risk increases to 12% if the father was affected at age 60 or above. The risk increases as the number of relatives affected by prostate cancer increases and if the relative was affected before age 60.
Table 1. Effect of family history of prostate cancer on lifetime risk of prostate cancer[footnote 30]
| Family history | Lifetime risk |
|---|---|
| No history | 8% |
| Father with prostate cancer at ≥ 60 years | 12% |
| One brother affected at ≥ 60 years | 15% |
| Father affected before 60 years | 20% |
| One brother affected before 60 years | 25% |
| Two male relatives with prostate cancer* | 30% |
| Three or more affected male relatives | 35 to 45% |
*Father and brother, or two brothers, or a brother and a maternal grandfather or uncle, or a father and a paternal grandfather or uncle
About 5-10% of all prostate cancers diagnosed are associated with hereditary genetic mutations[footnote 31] [footnote 32]. There is some evidence that some men with these genetic mutations are particularly susceptible to early onset of disease (age ≤50)[footnote 33] [footnote 34]. It is unclear to what extent these genetic mutations cause disease. Data shows men with early onset of disease are more likely to die from prostate cancer than older men with similar clinical diagnoses[footnote 35], particularly those with high grade or locally advanced disease.
6.4 Obesity and diet
A 2014 review from the World Cancer Research Fund and the American Institute for Cancer Research summarises the level of evidence available linking diet, nutrition and physical activity risk factors with prostate cancer. See Table 2 [footnote 14].
There is increasing evidence linking overweight and obesity with advanced prostate cancer[footnote 14]. With a quarter of men in the UK considered obese (24%)[footnote 36] and prostate cancer being the most common cancer in men, the association raises an important public health concern. The biological mechanisms are thought to be related to lower levels of testosterone in obese men, which lower their risk of localised non-aggressive tumours but increase their risk for more aggressive tumours. As well as being at higher risk for more aggressive tumours, obese men are more prone to treatment failure and complications, and prostate cancer-related deaths[footnote 37] [footnote 38] [footnote 39]. Men have a 15 to 20% increased risk of dying from prostate cancer with every 5 kg/m2 increase in BMI[footnote 39]. Although obesity is a modifiable risk factor, little data exists on the effectiveness of weight loss or exercise interventions to reduce prostate cancer risk[footnote 40] [footnote 41] [footnote 42].
There is strong evidence to show that there is no association between consumption of beta-carotene in food or supplements and prostate cancer[footnote 14].
There is limited evidence that consumption of dairy products and diets high in calcium are associated with an increased risk of prostate cancer. In a meta-analysis of 45 observational studies, there was no association between dairy or milk intake and risk of prostate cancer[footnote 43]. However, in a meta-analysis of 13 cohort studies, the relative risk of prostate cancer increased by 13% when comparing the highest with the lowest quintile of milk consumption[footnote 44].
There is limited evidence that consumption of high plasma alpha-tocopherol concentrations reduces the risk of prostate cancer. In a review of 17 studies, the relative risk of prostate cancer fell by 1% for any prostate cancer and 2% for aggressive prostate cancer per 1mg/ml of serum alpha-tocopherol[footnote 14].
The selenium and vitamin E cancer prevent trial (SELECT) study did not identify an association between selenium and prostate cancer risk[footnote 45] [footnote 46]. However, the US Nutritional Prevention of Cancer Trial, a randomised controlled trial of selenium (intervention) vs. yeast (placebo), found after 7.5 years of follow-up that the relative risk of prostate cancer decreased by 49%[footnote 47]. The summarised evidence suggests that the link between selenium and prostate cancer risk is limited.
Table 2. WCRF/AICR evaluation of endogenous prostate cancer risk factors (reproduced from the World Cancer Research Fund International and the American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014) [footnote 14]
Factors that increase prostate cancer risk
| Level of evidence | |
|---|---|
| STRONG EVIDENCE: probable | Body fatness (BMI, waist circumference and waist-hip ratio) for advanced prostate cancer only, adult attained height (likely due to genetic, environmental, hormonal and nutritional factors) |
| LIMITED EVIDENCE – suggestive | Dairy products, diets high in calcium, low plasma alpha-tocopherol concentrations, low plasma selenium concentrations |
Factors that decrease prostate cancer risk
| Level of evidence | |
|---|---|
| LIMITED EVIDENCE – no conclusion | Cereals (grains) and their products, dietary fibre, potatoes, non-starchy vegetables, fruits, pulses (legumes), processed meat, red meat, poultry, fish, eggs, total fat, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, plant oils, sugar (sucrose), sugary foods and drinks, coffee, tea, alcoholic drinks, carbohydrate, protein, vitamin A, retinol, alpha carotene, lycopene, folate, thiamine, riboflavin, niacin, vitamin C, vitamin D, vitamin E supplements, gamma-tocopherol, multivitamins, selenium supplements, iron, phosphorous, calcium supplements, zinc, physical activity, energy expenditure, vegetarian diets, Seventh-day Adventist diets, individual dietary patterns, body fatness (non-advanced prostate cancer), birth weight, energy intake |
| STRONG EVIDENCE – substantial effect on risk unlikely | Beta-carotene |
7. Clinical features
7.1 Localised prostate cancer
Localised prostate cancer (confined within the capsule) is usually asymptomatic. Prostate cancers, unlike benign prostatic enlargement (BPE), tend to develop in the outer part of the prostate gland. It is unusual for these early cancers to cause any symptoms, but they may be palpable by digital rectal examination (DRE). Localised cancers range from just a few cells to more extensive disease that is considered ‘clinically important’.
7.2 Locally advanced prostate cancer
These cancers have extended outside the prostatic capsule and are also frequently asymptomatic.
7.3 Metastatic prostate cancer
Metastases may be the first sign of prostate cancer, which frequently metastasises to the bones, causing pain. Appearance on x-ray is usually a sclerotic lesion. Although the majority of men with metastatic prostate cancer die from the disease, it does respond well to hormonal therapy, which often keeps it controlled for several years. About 1 in 3 men (30%) with metastatic disease will live for at least 5 years after diagnosis[footnote 48].
7.4 Lower urinary tract symptoms and prostate cancer
Lower urinary tract symptoms (LUTS) are common in older men. It is important to realise that early prostate cancer itself will not usually produce symptoms and that LUTS (frequency, urgency, hesitancy, terminal dribbling and/or overactive bladder) are usually related to the presence of BPE rather than prostate cancer[footnote 49]. Between 70% and 80% of prostate tumours originate in the peripheral zone of the gland distant from the urethra[footnote 50] [footnote 51]. As a result, by the time prostate cancer itself causes LUTS, it may have reached an advanced and incurable stage. Due to the high coincidence of BPE and prostate cancer in the older age group, some men will have a benign pathology and a co-existing early prostate cancer [footnote 52] [footnote 53]. When a man seeks advice about LUTS this can lead to investigations which diagnose what is a coincidental prostate cancer.
7.5 Referral guidance for prostate cancer
According to the NICE Suspected Cancer: recognition and referral guideline (NG12)[footnote 54]:
-
refer men using a suspected cancer pathway referral (for an appointment within 2 weeks) for prostate cancer if their prostate feels malignant on DRE.
-
consider a PSA test and DRE to assess for prostate cancer in men with any of the following:
-
any LUTS, such as nocturia, urinary frequency, hesitancy, urgency or retention
-
erectile dysfunction
-
visible haematuria
-
-
refer men using a suspected cancer pathway referral (for an appointment within 2 weeks) for prostate cancer if their PSA levels are ≥3 ng/mL[footnote 2]. More details on the new recommended value for referring asymptomatic men can be found in section 9.4 below.
8. Prostate cancer diagnosis
The NICE clinical guideline 175 [footnote 3] outlines the recommended guidelines for diagnosis and treatment of prostate cancer.
The following procedures are commonly used as diagnostic tests for prostate cancer:
-
prostate-specific antigen (PSA) test
-
digital rectal examination (DRE)
-
transrectal ultrasound (TRUS)
-
TRUS-guided prostate biopsy and histology
-
magnetic resonance imaging (MRI) scan
9. The PSA test
The PSA test should not be added to a list of investigations without a careful explanation of why it is being performed and its implications.
PSA is a glycoprotein responsible for liquefying semen and allowing sperm to swim freely. It is expressed in both benign and malignant processes involving epithelial cells of the prostate. Due to an alteration in the architecture of the prostate in conditions such as prostatitis and BPE, as well as prostate cancer, PSA leaks out, leading to increased levels in the bloodstream.
Men who have a PSA test increase their chance of a prostate cancer diagnosis. The PSA test provides the opportunity for clinically relevant prostate cancer to be diagnosed at a stage when treatment options and outcome may be improved. However, the PSA test may lead to investigations which can diagnose clinically insignificant cancers which would not have become evident in a man’s lifetime.
The most commonly used PSA test measures the total amount of prostate-specific antigen. The PSA test is currently the best method of identifying an increased risk of localised prostate cancer. However, PSA is an enzyme also found in men without prostate cancer.
9.1 Test benefits
Potential benefits of having a PSA test include:
-
it may lead to the detection of cancer before symptoms develop
-
it may lead to the detection of cancer at an early stage when the cancer could be cured or treatment could extend life
-
repeat PSA tests may provide valuable information, aiding a prostate cancer diagnosis
9.2 Test limitations or risks
Potential limitations or risks of having a PSA test include:
-
the PSA test is not diagnostic: those with an elevated PSA level may require further investigation, possibly a TRUS-guided prostate biopsy (see section 9.7) and histology to confirm the presence of prostate cancer
-
PSA is not tumour specific in the prostate [footnote 55]. PSA levels can increase due to a number of other factors:
-
BPE
-
older age – PSA levels normally increase with age
-
prostatitis – infection or inflammation of the prostate gland
-
ejaculation – may increase PSA levels for a short time
-
-
lower PSA levels can be influenced by factors such as drugs containing 5-alpha-reductase inhibitors (such as finasteride or dutasteride)[footnote 56], aspirin, statin, thiazide diuretics[footnote 57]. Obese men also have lower levels of PSA[footnote 58]
-
the PSA test may give false-positive results. A man may have an elevated PSA level but have no cancer. About 75 out of every 100 men who have an elevated PSA level have a false-positive result [footnote 59]
-
the PSA test result may not be elevated and provide false reassurance. One study has shown that approximately 15% (1 in 7) of all men with a ‘normal’ PSA level may have prostate cancer, and 2% (1 in 50) will have high-grade cancer, although it is not known how many of these would have become clinically evident in a man’s lifetime [footnote 60]. This is due to the poor sensitivity and specificity of the PSA test [footnote 61]. A one-off test is therefore not reliable enough to provide reassurance
-
the PSA test may lead to the identification of prostate cancers which might not have become clinically significant in the man’s lifetime
-
a single PSA test will not distinguish between aggressive tumours which are at an early stage but will develop quickly and those which are not, but further tests may provide valuable information
9.3 Test practicalities
Before having a PSA test, men should not have:
-
an active urinary infection (PSA may remain raised for many months)
-
ejaculated in the previous 48 hours
-
exercised vigorously in the previous 48 hours
-
had a prostate biopsy in the previous 6 weeks
Before performing a PSA test, the conditions above should be met in order to ensure that, where possible, a raised PSA result is the result of prostate cancer, not a confounding physical condition[footnote 62].
Evidence indicates PSA is stable in whole blood for up to 16 hours at room temperature. When taking blood you should ensure that the specimen will reach the laboratory and be separated within this time frame. The quality of PSA testing can vary between laboratories, depending on the type of PSA test employed.
9.4 Referral guidance
In 2008, the PCRMP recommended age-related referral values[footnote 1]. An updated review of the literature shows that age-specific PSA cut-offs for detecting prostate cancer are highly variable and may reflect differences in demographics and clinical characteristics in a population[footnote 2].
In addition to the wide-ranging PSA reference levels that exist in the literature, there is a wide range of referral practice throughout the UK[footnote 1]. Before a consensus can be found, the previously recommended age-related referral values by the programme have been reconsidered[footnote 1]. This is due to the concern of missing a high proportion of clinically significant cancers in older men (low sensitivity) and the potential increased rate of unnecessary biopsies in younger men (low specificity).
The two largest randomised PSA-based screening trials – ERSPC[footnote 17] and Prostate, Lung, Colorectal and Ovarian Cancer (PLCO)[footnote 63] – have evaluated PSA testing among men aged 55-69 years with biopsy indication among those with PSA ≥3.0 ng/mL. Recommended prostate biopsy referral values are being realigned to the evidence emerging from these two trials.
The new recommended referral value for men aged 50-69 is 3ng/mL[footnote 2]. Further diagnostic evaluation should consider the man’s history of comorbidities, ethnicity, family history and abnormal DRE findings prior to biopsy.
The serum PSA level alone should not automatically lead to a prostate biopsy. Other factors that should be considered in conjunction with the PSA level are:
-
prostate size
-
DRE findings
-
age
-
ethnicity
-
family history of prostate cancer
-
body weight/BMI
-
co-morbidities
-
history of any previous negative biopsy
-
any previous PSA history
The patient should be involved in any decision about referral to another healthcare provider.
9.5 Digital rectal examination (DRE) of the prostate
The DRE is a useful diagnostic test for men with lower urinary tract symptoms or symptoms suggestive of advanced disease. Symptoms for both conditions are the same (not very specific). The DRE cannot be used solely to diagnose prostate cancer. DRE should be used in combination with PSA testing.
The DRE allows assessment of the prostate for signs of prostate cancer (a hard gland, sometimes with palpable nodules) or benign enlargement (smooth, firm, enlarged gland). However, a gland that feels normal does not exclude a tumour. Most prostate cancers are located in the peripheral zone of the prostate and may be detected by DRE when the volume is about 0.2 ml or larger.
Men should be referred (for an appointment within 2 weeks) for suspected prostate cancer if their prostate feels malignant on DRE[footnote 54].
9.6 Transrectal ultrasound (TRUS)
TRUS can be used to examine the prostate and determine its size accurately but its main value is in enabling precise needle placement in the prostate during systematic prostate biopsy. It is not reliable enough to exclude prostate cancer and should not be used to screen asymptomatic men.
9.7 TRUS-guided prostate biopsy and Gleason score
Prostate cancer diagnosis is confirmed using a needle biopsy in the UK. A TRUS biopsy involves taking 10 to 12 cores of prostatic tissue through the rectum under ultrasound guidance[footnote 64]. A series of biopsies is taken in a systematic manner and additional biopsies may be taken if a lesion is seen. If a tumour is detected, histological examination reveals how well the tumour is differentiated. Tumour differentiation is graded by a Gleason score, by analysing the most common and second most common tumour patterns. Each tumour pattern is assigned a grade (1 to 5) and these grades are combined to produce the Gleason score (2 to 10). The lower the score, the better differentiated the tumour, the less likely it is to progress and the better the prognosis. Tumours can be classified into three categories on the basis of their Gleason score: low (≤6), intermediate (=7) and high grade (8 to 10). The sensitivity of detecting clinically significant prostate cancer (Gleason>6) using 12-core biopsy is 80%[footnote 65]. This suggests that about one in five prostate cancers (Gleason >6) are missed on TRUS biopsy and may require additional diagnostic evaluation if symptoms persist and PSA levels continue to increase.
As with other medical procedures, the biopsy procedure can cause significant anxiety. Most men describe the biopsy as an embarrassing, uncomfortable experience and some describe it as painful (although this should be alleviated by use of local anaesthetic).
Benefits of having a biopsy include:
-
it can find cancer before symptoms develop
-
it can identify cancerous tissue and identify the grade of tumour
-
a negative biopsy result can relieve anxiety about prostate cancer, although a second biopsy may be necessary if recommended by the multidisciplinary team, particularly if the PSA level remains elevated
-
the diagnosing capability of the biopsy procedure increases with the number of cores taken
Limitations of having a biopsy include:
-
post-biopsy complications include bleeding and infection[footnote 66]
-
up to 45% of tumours are missed at biopsy (false negatives)[footnote 67] [footnote 68] [footnote 69], although the number of tumours missed at biopsy decreases as the number of cores taken increases
-
diagnosis of prostate cancer that is not clinically significant may have a significant impact on the patient. The patient may experience increased anxiety
-
management of men with a negative biopsy but a persistently elevated PSA level is very difficult. Prolonged periods of follow-up, with the possibility of re-biopsy, may cause considerable anxiety
9.8 Magnetic resonance imaging (MRI)
False negative rates associated with TRUS-guided biopsy can be as high as 45%[footnote 67] [footnote 68] [footnote 69]. Up to half of men who are initially diagnosed with low-risk disease are under-staged and actually have a higher burden of high-risk disease[footnote 68] [footnote 70] [footnote 71] [footnote 72] [footnote 73] [footnote 74] [footnote 75]. However, diagnosis and staging of disease has the potential to improve with MRI before prostate biopsy[footnote 3]. MRI is a non-invasive test that uses a powerful magnetic field, radio frequency pulses and a computer to provide a detailed image of the prostate. Lesions seen on a pre-biopsy MRI can be used to select appropriate targets for TRUS-biopsy and may add additional information that can assess what treatment should be used to manage patients with low and intermediate risk.
Triage of men with clinical suspicion of prostate cancer (elevated PSA and abnormal DRE) to MRI prior to prostate biopsy could be more specific in selecting those with clinically significant cancer that requires treatment. This strategy could reduce the number of men with clinically insignificant disease who undergo unnecessary biopsy and treatment. This would also reduce the rate of complications that could interfere with accurate disease staging and improve disease risk stratification to manage appropriate treatment with active surveillance or radical therapies.
NICE guidelines[footnote 3] recommend that men who are negative on TRUS 10 to 12 cores biopsy should be further evaluated with multiparametric MRI (mpMRI) to consider whether a repeat biopsy with targeted biopsy is needed. mpMRI provides better visualisation of the prostate to detect clinically significant prostate cancer[footnote 76]. However, it has lower accuracy in detecting smaller tumours with low grade disease[footnote 77]. If the man is negative on mpMRI, then another biopsy should not be offered unless they are positive for other risk factors. Evidence suggests this strategy will reduce the number of repeat biopsies required compared to routine systematic TRUS re-biopsy.
10. The future of prostate cancer detection
The PSA test is the best test currently available for prostate cancer, but there are concerns about its accuracy. There has been much debate about how it can be improved to provide a more reliable detection procedure, as well as a method of differentiating between indolent and aggressive cancers. A number of additional PSA tests, risk prediction models, and screening and triage biomarkers are under investigation to improve prostate cancer detection.
10.1 Reflex testing with PSA isoforms
A total PSA test has poor specificity. Studies have suggested that reflex testing with PSA isoforms, such as ratio of free to total PSA (f/tPSA) or complex PSA (cPSA), for men with PSA values <10 ng/mL (known as the diagnostic “grey zone”) could improve specificity and reduce the number of unnecessary biopsies. Studies suggest that triage of men in the “grey zone” with tPSA 2 to 10 ng/ml using PSA isoforms could potentially reduce overdiagnosis and maintain a high cancer detection rate[footnote 78].
10.2 Risk prediction models
Risk prediction models have developed in recent years to help clinicians and patients predict prostate cancer diagnosis, stage and prognosis. These models aim to improve the accuracy of screening to detect prostate cancer. Besides PSA testing, models consider age, ethnicity, DRE result and other risk factors to predict a man’s risk of having detectable prostate cancer. A number of these risk assessment tools are readily available online as a decision aid for an individual man to evaluate his own risk for prostate cancer. These include the Prostate Cancer Prevention Trial (PCPT) Risk Calculator[footnote 79] and the European Randomized Study of Screening for Prostate Cancer (ERSPC) Risk Calculator[footnote 80]. A recent review[footnote 81] identified more than 120 unique risk prediction models. However, only six models to detect any prostate cancer and only one model, PCPT, to detect clinically significant prostate cancer have been evaluated in ≥5 study populations. This suggests that many poorly validated models exist. The review found that, in general, compared to PSA testing, prediction models have a higher predictive accuracy to detect any prostate cancer compared to PSA testing only. Although risk prediction models have the potential to improve the accuracy of PSA screening, further investigation is needed to evaluate the effect of these predictive risk models to detect clinically significant prostate cancers.
It is not clear whether or not these publicly available online risk models help men make informed decisions about the need for a prostate biopsy or a repeat biopsy after PSA screening. It is also unclear whether they help men understand their risk of clinically significant prostate cancer compared to their overall risk of prostate cancer.
The effect of these predictive risk models on reducing mortality and side effects related to overdiagnosis and overtreatment is unknown. Additional evaluations of the clinical effectiveness of these risk prediction models in clinical practice are needed before they are recommended for use.
10.3 New screening and triage markers
Recent research advances have focussed on identifying biomarkers to stratify men with low-risk and high-risk aggressive disease so that men can be managed appropriately, minimising potential harms of overdiagnosis and overtreatment[footnote 82].
Two promising urinary RNA biomarkers are prostate cancer antigen, PCA3 and fusion gene TMPRSS2:ERG, to identify men with low-risk (indolent) and aggressive (clinically significant) cancers[footnote 83].
PCA3 is highly overexpressed in over 95% of prostate cancer tumours, or up to 100 times greater in men with cancer than in those with a normal prostate[footnote 84] [footnote 85]. A review found that the sensitivity of PCA3 test (54-82%) was found to be less than PSA testing (81-98%) but the specificity for PCA3 was much better than PSA (66-89% vs. 5-28%)[footnote 86]. Therefore, the higher specificity would reduce overdiagnosis and overtreatment cases. A weakness of these studies was that none of them used PCA3 scores as a screening test to indicate prostate biopsy, making it difficult to understand its clinical value. However, PCA3 has also been considered as a reflex test for diagnosing prostate cancer. A recent NICE review[footnote 87] which investigated the clinical effectiveness of the test found no additional clinical benefit in adding PCA3 as a reflex test in diagnosing prostate cancer.
Prostate gene fusion between TMPRSS2 and ERG, an ETS (e-twenty-six) transcription factor is also overexpressed in about 50% of prostate cancers from PSA-screened cohorts[footnote 88] [footnote 89]. However, population-based cohorts have shown a much lower prevalence of 15%[footnote 89]. The reasons for these differences in prevalence are not well understood but the prevalence of TMPRSS2:ERG was found to be lowest in men with early stages tumours (T1), suggesting this marker may be useful in identifying men at risk of more aggressive disease. Further research is still needed to fully understand its clinical utility in screening and its potential use in prostate cancer management (prognosis).
Results from the prospective population-based Stockholm 3 (STHLM3) screening study suggest a combination of plasma protein biomarkers (PSA, free PSA, intact PSA, hk2, MSMB, M1C1), genetic polymorphisms (232 SNPS), clinical variables (age, family, history, previous prostate biopsy, prostate exam) and PSA concentration would increase the specificity of screening without decreasing the sensitivity of PSA testing using a cut-off of at least 3 ng/ml to diagnose high-risk prostate cancers[footnote 90]. The model performed significantly better than PSA alone for detection of clinically significant cancers (Gleason score ≥7). The clinical usefulness of these data suggests that the STHLM3 model could reduce the number of biopsies by 32% and avoid 44% of benign biopsies. Despite these promising results, the study was only carried out in Stockholm, Sweden, where the population is relatively homogenous and men were mainly of northern European descent. Further investigations are needed to validate the STHLM3 model in other populations and in ethnic groups.
11. Management of prostate cancer
The management of localised prostate cancer is central to the controversy surrounding screening. Men considering a PSA test should understand that:
-
early detection and treatment of prostate cancer may be beneficial
-
there is uncertainty about how to distinguish between aggressive and non-aggressive cancers
-
there is no strong evidence to indicate which treatment option is most suitable for which man
-
active treatments have significant side-effects, although improvements to treatment regimens and their side-effects are being made
12. Management options of localised prostate cancer
To date, there is no data from randomised controlled trials giving clear evidence about the optimum treatment for localised prostate cancer.
In the UK, the Prostate Testing for Cancer and Treatment (ProtecT) trial[footnote 91] [footnote 92] screened more than 82,000 population-based men aged 50-69 years and randomised more than 1,600 men with PSA-detected localised prostate cancer to receive one of three frequently used treatments – active monitoring, radical prostatectomy, or radical radiotherapy – in order to evaluate its impact on 10-year survival . The recruitment phase of the trial has been completed but data is not yet available as follow-up has not been completed. The first outcome results will be reported in 2016.
Results will provide key information needed to manage localised prostate cancer as well as quantifying the potential harms of over-detection and overtreatment compared with the survival gains in PSA-detected prostate cancer.
Full NICE guidance on treatment options is available [footnote 3].
Evidence suggests that any benefit to a man from undergoing radical treatment for prostate cancer may be best for those whose comorbidities and age suggests a life expectancy of >10 years. Men with advanced prostate cancers are less likely to benefit from radical treatment alone.
The main treatment options for localised prostate cancer are:
-
active surveillance
-
radical prostatectomy (open, laparoscopic or robotically assisted laparoscopic)
-
external beam radiotherapy (EBRT)
-
brachytherapy (low and high dose rate)
-
watchful waiting
-
high intensity focused ultrasound (HIFU)
-
cryotherapy
Men with low-risk localised prostate cancer will normally be offered active surveillance (monitoring of PSA kinetics, DRE, and prostate rebiopsy) or treatment with radical prostatectomy or radical radiotherapy. If a man is under active surveillance, a decision to proceed with radical treatment should be made based on his preferences, comorbidities and life expectancy. Radical treatment may also be offered if there is evidence of disease progression.
Men with intermediate-risk localised prostate cancer can consider active surveillance (if the man does not wish to undergo radical treatment immediately) or be offered radical treatment. They may also be offered a combination of radical radiotherapy and androgen deprivation therapy, rather than radical radiotherapy or androgen deprivation therapy alone. High-dose rate brachytherapy in combination with external beam radiotherapy may also be considered.
Men with high-risk localised prostate cancer are offered radical treatment if there is a realistic probability of long-term disease control. They should not be offered active surveillance. Men will also be offered 6 months of androgen deprivation therapy before, during or after radical treatment. High-dose rate brachytherapy in combination with external beam radiotherapy may also be considered. High-intensity focussed ultrasound and cryotherapy would not be offered to men with localised prostate cancer outside the context of a controlled clinical trial.
Watchful waiting may be elected by asymptomatic men with localised prostate cancer, since prostate cancers are often slow growing and treatment can cause side effects, including sexual dysfunction and urinary incontinence, and impact on a man’s daily life. A man under watchful waiting will be monitored for disease progression by a member of the urological MDT (multidisciplinary team) over the long term in order to avoid treatment unless symptoms appear.
Before choosing a treatment regimen, men should be appropriately counselled about the important quality of life differences between the options. For example, radical treatment for prostate cancer can result in loss of sexual potency, ejaculation, fertility and urinary continence.
12.1 Active surveillance and active monitoring
During active surveillance or monitoring the patient is followed up regularly by an oncologist or urologist. This option is offered to men who are generally younger and fitter and who wish to avoid the possibility of unnecessary treatment of indolent cancers. The downside is that disease may spread locally and advanced disease may develop, which may be more difficult to treat. The aim is to monitor those with stable disease and identify where radical treatment may be appropriate for those whose cancer progresses. Men on active monitoring will be monitored by serial PSA tests. Men on active surveillance will be monitored by serial PSA tests and repeat prostate biopsies. Radical treatment with curative intent is offered if there are signs of disease progression.
12.2 Radical prostatectomy (open, laparoscopic and robotic)
The aim of radical prostatectomy is to remove the entire prostate gland and to cure the disease. Complete tumour clearance is not always achieved and approximately 20% of men go on to develop biochemical or clinical recurrence of the disease[footnote 1]. Recurrence does not necessarily equate with death from prostate cancer. Complications of surgery include operative mortality, sexual dysfunction and urinary problems. This treatment is uncommon in men over 70.
12.3 Radiotherapy (external beam and brachytherapy)
Radiotherapy such as external beam radiotherapy (EBRT) and brachytherapy aims to cure the disease. EBRT involves an external source of radiation targeted at the tumour. Short-term side-effects relate mainly to bowel and bladder problems from the radiation. Longer-term complications include sexual dysfunction and urinary problems. This treatment is not usually recommended for men with less than 10 years’ life expectancy.
Brachytherapy may be given by two very different techniques. Low dose rate (LDR) brachytherapy involves the permanent implantation of tiny radioactive seeds into the prostate to deliver a high radiation dose into the gland. High dose rate (HDR) brachytherapy requires fine catheters to be inserted into the prostate, through which a radioactive source is temporarily passed. Although the isotope used has a higher dose rate, the overall dose is lower than that given by LDR brachytherapy so it is usually given in conjunction with EBRT. This latter technique is much more recent, with limited clinical data, and is usually reserved for patients with high-risk disease. Possible side-effects include urinary symptoms and sexual dysfunction.
12.4 Watchful waiting
During watchful waiting the patient is followed up regularly in primary care. The approach is non-invasive and avoids unpleasant side-effects. Watchful waiting is offered to men who, on the grounds of their age or co-morbidity or on the basis of having slowly progressing tumours, are likely to die from other causes and will not suffer significant morbidity from their prostate cancer. These men will be offered palliative treatment only if and when symptoms of prostate cancer develop. Such treatment will not be curative, but will aim to slow the cancer growth sufficiently to prevent the man dying from it.
12.5 High-intensity focussed ultrasound and cryotherapy
High-intensity focused ultrasound (HIFU) and cryotherapy are newer radical therapies for the treatment of localised prostate cancer and are not currently recommended other than in the context of controlled clinical trials. HIFU aims to cure the disease by heating the prostate gland using ultrasound waves to cause tissue damage by mechanical and thermal effects as well as by cavitation. Cryotherapy aims to cure the disease by freezing the prostate gland.
12.6 Hormone therapy
Adjuvant hormone therapy is being used following surgery and in conjunction with radiotherapy for localised disease with the aim of improving survival[footnote 3]. Hormone therapies (luteinising hormone-releasing hormone [LHRH] analogues or anti-androgens) attempt to suppress growth of the cancer by reducing circulating androgen levels. They can be used as adjuvant treatments to those outlined above and are also widely used in the control of metastatic disease. Side-effects include sexual dysfunction, loss of libido, breast swelling, hot flushes and osteoporosis. Men on the watchful waiting regimen who develop symptoms of progressive disease are usually managed with hormone therapy.
13. Locally advanced and metastatic prostate cancer
Surgery alone cannot normally eradicate clinically advanced localised cancer. The rate of progression of the disease varies considerably. Patients with locally advanced disease mainly receive radiotherapy or hormone therapy. Some men live for many years with few symptoms, while others develop extensive disease quite rapidly.
14. Monitoring effectiveness of treatment with PSA
PSA levels are used to monitor disease activity in those with established prostate cancer, giving an indication of response to treatments. They may also give an early indication of the progression of a cancer either after treatment or as part of an active surveillance or monitoring protocol.
15. Population screening for prostate cancer
To date there is no UK data from randomised controlled trials to show the benefits outweigh the harms of using the PSA test for prostate cancer screening. Evidence from the ERSPC shows that the PSA test can reduce the risk of death from prostate cancer[footnote 17]. However, the benefits of PSA screening remain unresolved on issues of overdiagnosis and overtreatment of clinically insignificant prostate cancers as well as identifying the optimum treatment for localised prostate cancer.
The UK Cluster randomised trial of PSA testing for Prostate Cancer (CAP) Study[footnote 93] aims to address these unanswered questions by evaluating whether PSA testing of men aged 50-69 years will reduce prostate cancer mortality and be cost-effective. The study design is a cluster randomisation controlled trial of primary care centres to either PSA screening (intervention arm) or to standard clinical care (comparison arm). More than 400,000 men in England and Wales have been randomised. The ProtecT trial is the randomised controlled trial (intervention arm of CAP) that is evaluating active surveillance, conformal external beam radiotherapy and radical prostatectomy treatments for men with localised prostate cancers who attend a GP practice, randomised to PSA testing in the CAP trial. Both trials are measuring outcomes of prostate cancer-specific mortality as well as overall survival, costs and quality of life.
Long-awaited results from the CAP[footnote 93] and ProtecT[footnote 91] trials will be reported in 2016 which will provide key information needed to manage localised prostate cancer as well as quantifying the potential harms of over-detection and overtreatment compared to the survival gains in PSA-detected prostate cancer after 10 years of follow-up. There have been calls for a national screening programme for prostate cancer, just as there are for bowel, breast and cervical cancers. However, there is insufficient evidence to support PSA-based screening as recently reviewed by the UKNSC[footnote 2]. The current published data is insufficient to recommend the adoption of population screening for prostate cancer as a public health policy due to the large overtreatment effect. None of the published randomised controlled trial studies (ERSPC[footnote 17], PLCO[footnote 63], The Norrkoping[footnote 94] and Stockholm[footnote 95] studies in Sweden and the Quebec study in Canada[footnote 96]) used UK-comparable screening protocols, nor have the impacts on men’s symptoms and quality of life or costs been published for these studies, so care must be exercised in applying these results to any decisions about a screening programme in this country.
15.1 UK National Screening Committee (UK NSC) recommendation
A 2015 review by the UK NSC[footnote 2] shows advances have been made in our understanding of prostate screening but there is no updated evidence to justify introducing a national screening programme. Despite the benefit of PSA screening to reduce prostate cancer mortality by at least 21% (ERSPC trial)[footnote 17], there are still significant gaps in our knowledge of overdiagnosis and overtreatment of clinically insignificant prostate cancers as well as identifying the optimum treatment. The potentially harmful effects of prostate screening are significant. While some early cancers would be detected and lives saved, the introduction of a PSA-based screening programme at this stage would undoubtedly lead to some men with indolent disease unnecessarily experiencing the side-effects of radical treatment, including sexual dysfunction, urinary problems and, in extreme cases, death.
The UK NSC has therefore recommended against a prostate cancer screening programme in the UK at this time. Instead, the PCRMP exists so that asymptomatic men who ask about a PSA test can make an informed choice, based on good quality information from their GP or Practice Nurse, about the advantages and disadvantages of having the test. An online patient decision aid has been developed to help men make a decision which is right for them.
16. PSA testing patient decision aid option grid
| Having the PSA Test | Not having the PSA Test | |
|---|---|---|
| HEALTH | If you have the PSA test and follow-on treatment you are less likely to die of prostate cancer than men who do not have the test. Having an abnormal PSA test result means you may be offered further tests and treatments, which may harm your health. | If you do not have the PSA test you are more likely to die of prostate cancer than men who do have the PSA test. You are also more likely to experience the complications of advanced incurable prostate cancer. |
| TEST RESULTS | The PSA test may reassure you if the result is normal. But it can miss cancer and provide false reassurance. If you have prostate cancer, you are more likely to be diagnosed and treated early. But an abnormal test result may also lead to unnecessary worry and medical tests when there is no cancer. The test cannot tell the difference between fast-growing cancers and slow-growing cancers that may not cause symptoms or shorten your life. | If you do not have the PSA test you may avoid unnecessary worry and tests after an abnormal result when there is either no cancer or a slow-growing cancer. If you have prostate cancer, you are less likely to be diagnosed and treated early. |
| ACCURACY OF TEST | About 75 out of every 100 men who have an abnormal PSA test result do not have prostate cancer. This is called a false positive result. About 15 out of every 100 men who have a normal PSA test result do have prostate cancer. This is called a false negative result. | If you do not have a PSA test, you will not get a false positive or a false negative result but the chance of early detection may be missed. |
| FOLLOW-UP TESTS | About 17 out of every 100 men who are tested have an abnormal test result. About 82 out of every 100 men who have an abnormal result will have a biopsy. Some men have problems or complications after a biopsy for prostate cancer. The most common complications are bleeding and infections. | If you do not have a PSA test, it is unlikely you will need to have a biopsy unless you get symptoms of prostate cancer, at which stage the cancer might be more advanced. |
| TREATMENT | If you are diagnosed with prostate cancer, you will need to decide about treatment. Potential treatments can include surgery, radiotherapy and hormone therapy. Side effects of treatments for prostate cancer can include problems with erections, loss of fertility and incontinence. | If you do not have a PSA test, it is unlikely you will need treatment for prostate cancer, unless you get symptoms. This means you are less likely to have any complications from treatments. |
17. Conclusions
Prostate cancer is a significant health problem, mainly affecting older men. There are problems surrounding the early diagnosis and treatment options for the disease, and to date there is no evidence to say whether the introduction of a population screening programme would reduce mortality in the UK without significant numbers of men being overtreated.
Due to the uncertainties surrounding PSA testing and treatments for prostate cancer, it is imperative that men who request a PSA test receive balanced information about the pros and cons to assist them in making an informed choice about being tested.
17.1 Resources
The following organisations have more information on prostate cancer and PSA testing:
-
Cancer Research UK 0808 800 4040
-
Prostate Cancer UK 020 3310 7000
18. References
-
Burford D, Kirby M, Austoker J. Prostate Cancer Risk Management Programme information for primary care; PSA testing in asymptomatic men. Evidence document. NHS Cancer Screening Programmes; 2010 Jan. Accessed June 2014 ↩ ↩2 ↩3 ↩4 ↩5
-
Louie KS. UKNSC Screening for Prostate Cancer Review -2015 update. UK National Screening Committee, 2016. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
National Institute for Health and Care Excellence (NICE). Prostate Cancer: diagnosis and treatment. Evidence review. London: National Institute for Health and Clinical Excellence, 2014 ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
Cancer Research UK. Cancer incidence – prostate cancer. Accessed January 2016 ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
Ilic D, Neuberger MM, Djulbegovic M, et al. Screening for prostate cancer. Cochrane Database Syst Rev 2013;1:CD004720. ↩
-
Chapple A, Ziebland S, Hewitson P, et al. Why men in the United Kingdom still want the prostate specific antigen test. Qual Health Res 2008;18(1):56-64. ↩ ↩2
-
Evans R, Edwards AG, Elwyn G, et al. “It’s a maybe test”: men’s experiences of prostate specific antigen testing in primary care. Br J Gen Pract 2007;57(537):303-10. ↩ ↩2
-
Clements A, Watson E, Rai T, et al. The PSA testing dilemma: GPs’ reports of consultations with asymptomatic men: a qualitative study. BMC Fam Pract 2007;8:35. ↩
-
Watson E, Hewitson P, Brett J, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns 2006;63(3):367-79. ↩
-
Cancer Research UK. Cancer mortality – prostate cancer. Accessed January, 2016. ↩ ↩2 ↩3 ↩4
-
Lloyd T, Hounsome L, Mehay A, et al. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008-2010. BMC Med 2015;13(1):171. ↩ ↩2 ↩3
-
Bratt O. Hereditary prostate cancer: clinical aspects. J Urol 2002;168(3):906-13. ↩
-
Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One 2011;6(10):e27130. ↩
-
World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014. ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
Office for National Statistics. Cancer Statistics: Registrations Series MB1, No. 43, 2012. ↩
-
Brewster DH, Fraser LA, Harris V, et al. Rising incidence of prostate cancer in Scotland: increased risk or increased detection? BJU Int 2000;85(4):463-72; discussion 72-3. ↩
-
Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384(9959):2027-35. ↩ ↩2 ↩3 ↩4 ↩5
-
Sakr WA, Grignon DJ, Haas GP, et al. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol 1996;30(2):138-44. ↩ ↩2
-
Yatani R, Chigusa I, Akazaki K, et al. Geographic pathology of latent prostatic carcinoma. Int J Cancer 1982;29(6):611-6. ↩
-
Ben-Shlomo Y, Evans S, Ibrahim F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol 2008;53(1):99-105. ↩
-
Chinegwundoh F, Enver M, Lee A, et al. Risk and presenting features of prostate cancer amongst African-Caribbean, South Asian and European men in North-east London. BJU Int 2006;98(6):1216-20. ↩
-
Metcalfe C, Evans S, Ibrahim F, et al. Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. Br J Cancer 2008;99(7):1040-5. ↩
-
Ellis L, Nyborg H. Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids 1992;57(2):72-5. ↩
-
Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab 2006;91(4):1336-44. ↩
-
Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab 2010;95(10):E151-60. ↩
-
Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS genetics 2011;7(5):e1001387. ↩
-
Haiman CA, Chen GK, Blot WJ, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nature genetics 2011;43(6):570-3. ↩
-
Han Y, Signorello LB, Strom SS, et al. Generalizability of established prostate cancer risk variants in men of African ancestry. Int J Cancer 2015;136(5):1210-7. ↩
-
Morganti G, Gianferrari L, Cresseri A, et al. [Clinico-statistical and genetic research on neoplasms of the prostate]. Acta Genet Stat Med 1956;6(2):304-5. ↩
-
Bruner DW, Moore D, Parlanti A, et al. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer 2003;107(5):797-803. ↩ ↩2
-
Carter BS, Beaty TH, Steinberg GD, et al. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A 1992;89(8):3367-71. ↩
-
Steinberg GD, Carter BS, Beaty TH, et al. Family history and the risk of prostate cancer. Prostate 1990;17(4):337-47. ↩
-
Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer 2011;105(8):1230-4. ↩
-
Lange EM, Salinas CA, Zuhlke KA, et al. Early onset prostate cancer has a significant genetic component. Prostate 2012;72(2):147-56. ↩
-
Bratt O, Damber JE, Emanuelsson M, et al. Hereditary prostate cancer: clinical characteristics and survival. J Urol 2002;167(6):2423-6. ↩
-
Office for National Statistics. Statistics on Obesity, Physical Activity and Diet: England 2014. Accessed 27/10/14. ↩
-
Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst 1997;89(5):385-9. ↩
-
Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine 2003;348(17):1625-38. ↩
-
Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4(4):486-501. ↩ ↩2
-
Allot EH, Masko EM, Freedland SJ. Obesity and Prostate Cancer: Weighing the Evidence. Eur Urol 2013; 63: 800-809. ↩
-
Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007;16(1):63-9. ↩
-
Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 2007;109(4):675-84. ↩
-
Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer 2008;60(4):421-41. ↩
-
Qin LQ, Xu JY, Wang PY, et al. Milk consumption is a risk factor for prostate cancer in Western countries: evidence from cohort studies. Asia Pac J Clin Nutr 2007;16(3):467-76. ↩
-
Dunn BK, Richmond ES, Minasian LM, et al. A nutrient approach to prostate cancer prevention: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutr Cancer 2010;62(7):896-918. ↩
-
Klein EA, Thompson IM, Jr., Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 2011;306(14):1549-56. ↩
-
Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int 2003;91(7):608-12. ↩
-
Cancer Research UK. Cancer survival – prostate cancer. Accessed January 2016. ↩
-
Orsted DD, Bojesen SE. The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol 2013;10(1):49-54. ↩
-
McNeal JE. The zonal anatomy of the prostate. Prostate 1981;2(1):35-49. ↩
-
McNeal JE, Redwine EA, Freiha FS, et al. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol 1988;12(12):897-906. ↩
-
Chang RT, Kirby R, Challacombe BJ. Is there a link between BPH and prostate cancer? The Practitioner 2012;256(1750):13-6, 2. ↩
-
Rohr LR. Incidental adenocarcinoma in transurethral resections of the prostate. Partial versus complete microscopic examination. Am J Surg Pathol 1987;11(1):53-8. ↩
-
National Institute for Health and Care Excellence (NICE). Suspected cancer: recognition and referral [NG12]. London:National Institute for Health and Clinical Excellence, 2015. ↩ ↩2
-
Hamdy FC. Prognostic and predictive factors in prostate cancer. Cancer Treat Rev 2001;27(3):143-51. ↩
-
Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. The New England journal of medicine 2003;349(3):215-24. ↩
-
Chang SL, Harshman LC, Presti JC, Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol 2010;28(25):3951-7. ↩
-
Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. Jama 2007;298(19):2275-80. ↩
-
Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360(13):1320-8. ↩
-
Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. The New England journal of medicine 2004;350(22):2239-46. ↩
-
Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA: a cancer journal for clinicians 2010;60(2):70-98. ↩
-
Price CP, Allard J, Davies G, et al. Pre- and post-analytical factors that may influence use of serum prostate specific antigen and its isoforms in a screening programme for prostate cancer. Ann Clin Biochem 2001;38(Pt 3):188-216. ↩
-
Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104(2):125-32. ↩ ↩2
-
Prostate Cancer Risk Management Programme. Guide No. 1. Undertaking a Transrectal Ultrasound Guided Biopsy of the Prostate. NHS Cancer Screening Programmes, 2006 ↩
-
Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst 2007;99(19):1484-9. ↩
-
Challacombe B, Dasgupta P, Patel U, et al. Recognizing and managing the complications of prostate biopsy. BJU Int 2011;108(8):1233-4. ↩
-
Djavan B, Ravery V, Zlotta A, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol 2001;166(5):1679-83. ↩ ↩2
-
El-Shater Bosaily A, Parker C, Brown LC, et al. PROMIS - Prostate MR imaging study: A paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemporary clinical trials 2015;42:26-40. ↩ ↩2 ↩3
-
Scattoni V, Zlotta A, Montironi R, et al. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol 2007;52(5):1309-22. ↩ ↩2
-
Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate–a 4-year experience. Urology 2007;70(6 Suppl):27-35. ↩
-
Barzell WE, Melamed MR, Cathcart P, et al. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J Urol 2012;188(3):762-7. ↩
-
Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int 2005;96(7):999-1004. ↩
-
Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urologic oncology 2008;26(5):506-10. ↩
-
Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol 2009;27(26):4321-6. ↩
-
Taira AV, Merrick GS, Galbreath RW, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer P D 2010;13(1):71-7. ↩
-
Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology 2010;255(1):89-99. ↩
-
Vargas HA, Akin O, Shukla-Dave A, et al. Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 2012;265(2):478-87. ↩
-
Roddam AW, Duffy MJ, Hamdy FC, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2-10 ng/ml: systematic review and meta-analysis. Eur Urol 2005;48(3):386-99; discussion 98-9. ↩
-
Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98(8):529-34. ↩
-
Roobol MJ, Steyerberg EW, Kranse R, et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol 2010;57(1):79-85. ↩
-
Louie KS, Seigneurin A, Cathcart P, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol 2014. ↩
-
Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol 2014;15(11):e484-e92. ↩
-
Truong M, Yang B, Jarrard DF. Toward the detection of prostate cancer in urine: a critical analysis. J Urol 2013;189(2):422-9. ↩
-
Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer research 1999;59(23):5975-9. ↩
-
de Kok JB, Verhaegh GW, Roelofs RW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer research 2002;62(9):2695-8. ↩
-
Vlaeminck-Guillem V, Ruffion A, Andre J, et al. Urinary prostate cancer 3 test: toward the age of reason? Urology 2010;75(2):447-53. ↩
-
Nicholson A, Mahon J, Boland A, et al. The clinical effectiveness and cost-effectiveness of the PROGENSA(R) prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19(87):1-192. ↩
-
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nature reviews Cancer 2008;8(7):497-511. ↩
-
Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310(5748):644-8. ↩ ↩2
-
Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2015;16(16):1667-76. ↩
-
Lane JA, Donovan JL, Davis M, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15(10):1109-18. ↩ ↩2
-
Lane JA, Hamdy FC, Martin RM, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer 2010;46(17):3095-101. ↩
-
Turner EL, Metcalfe C, Donovan JL, et al. Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). Br J Cancer 2014;110(12):2829-36. ↩ ↩2
-
Sandblom G, Varenhorst E, Rossell J, Lofman O, Carlsson P. Randomised prostate screening trial: 20 years follow-up. BMJ 2011; 342:d1539. ↩
-
Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-79 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2014; 16 (6) 1667-76. ↩
-
Labrie F, Candas B, Cusan L, et al. Screening decreases prostate cancer mortality: 11-year follow-up of the 1988 Quebec prospective randomized controlled trial. Prostate 2004; 59:311-8 ↩
