[Withdrawn] Factsheet: medical devices overview
Updated 18 July 2024
See Regulating medical devices in the UK for guidance on placing a medical device on the Great Britain, Northern Ireland and European Union markets.
Top lines
There are approximately 600,000 medical devices available on the UK market. The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for ensuring the quality and safety of medical devices by enforcing the relevant regulations.
Medical devices in the UK are regulated by the Medical Devices Regulations 2002 (as amended) which provides for a system of medical device conformity assessment which enables the placing of compliant medical devices on the UK market.
Since 1 January 2021, the requirements in Northern Ireland are slightly different to those in Great Britain, due to the continued application of EU law to goods placed on the Northern Ireland market under the Northern Ireland Protocol. Amendments have been made to the Medical Devices Regulations 2002 to accommodate these differences.
The powers in this bill will enable us to update the regulatory system for medical devices as and when required, allowing us to deliver on our commitment to maintain patient safety and confidence in medical devices.
General medical devices – categories and classification
All general medical devices placed on the UK market have both a category and a classification. There are 5 categories of device, determined by their use.
Table 1: categories of medical devices
| Category | Definition | Examples |
|---|---|---|
| 1. Non-invasive | Devices which do not enter the body | Plasters, walking sticks, wheelchairs, artificial kidneys (external dialysis) |
| 2. Invasive | Devices inserted into the body’s orifices | Contact lenses, enemas, examination gloves |
| 3. Surgically invasive | Devices used or inserted in surgery | Needles, scalpels, cardiovascular catheters |
| 4. Active | Devices requiring an external source of power | X-ray equipment, ultrasound, TENS devices |
| 5. Implantable | Devices implanted into the body | Breast implants, orthopaedic implants, intraocular lenses |
There are 4 classes of general medical devices, determined by the inherent risk of a device. This risk assessment includes (but is not limited to) consideration of its intended length of use, its composition and whether it is implantable or active.
Table 2: classes of medical devices
| Class (low to high risk) | Examples |
|---|---|
| Class I | Wheelchairs, spectacles, stethoscopes, tongue depressors |
| Class IIa | Dental fillings, surgical clamps, tracheotomy tubes |
| Class IIb | Condoms, lung ventilators, bone fixation plates |
| Class III | Pacemakers, heart valves, implanted cerebral stimulators |
Regulations on medical devices
Medical devices in the UK are regulated by the Medical Devices Regulations 2002 (as amended) which provides for a system of medical device conformity assessment which enables the placing of compliant medical devices on the UK market.
The powers in this bill allow us to update the UK’s regulatory framework for medical devices in the future, ensuring we can maintain an effective system for regulating medical devices which supports patient safety, access to and availability of medical devices.
What a medical device is
A medical device is any apparatus, appliance, software, material or other article, whether used alone or in combination, intended by the manufacturer to be used by human beings for a medical purpose. For example, walking sticks, contact lenses and breast implants.
Medical devices include in-vitro diagnostics medical devices, which are devices used to test samples, such as blood or tissue that have been taken from the human body – such as blood glucose tests and pregnancy tests. They also include active implantable medical devices which are implanted into the patient and require an external source of power, such as cardiac pacemaker systems.
Regulatory cycle of a medical device
All medical devices to be used in the UK are required to undergo conformity assessment before being placed on the market.
Figure 1: regulatory cycle of a medical device
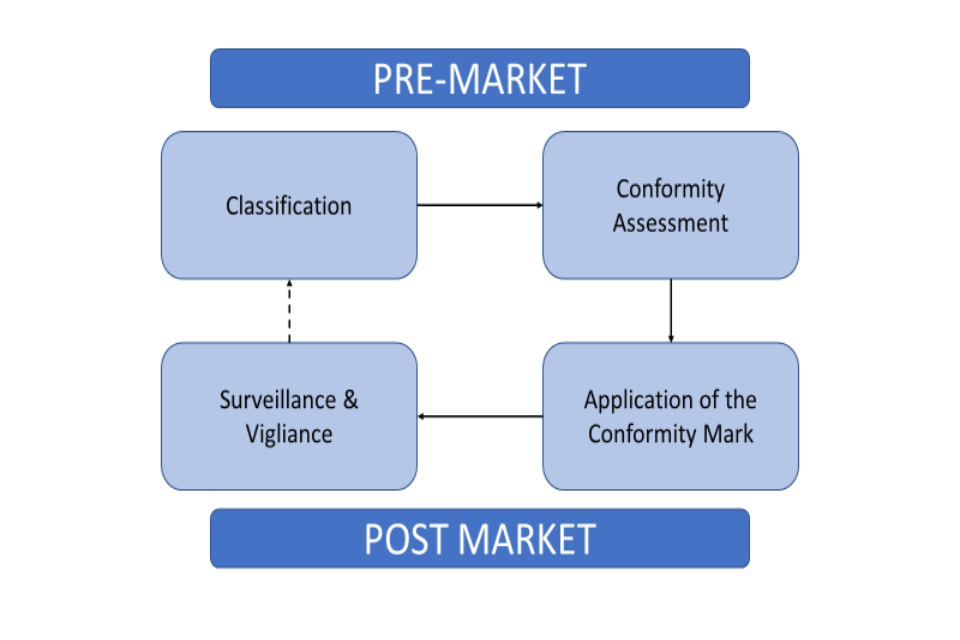
Figure 1 shows the regulatory cycle of a medical device. The pre-market assessment consists of classification of the device and then a conformity assessment. This then leads to post-market where application of the conformity mark takes place and then leads to surveillance and vigilance of the medical device.
What the bill does
The bill will allow the government to ensure that medical devices, such as pacemakers, breast implants and ultrasound imagers, continue to be subject to the highest standards of regulation, further boosting patient safety and ensuring the UK leads the way in developing pioneering health technology.
With the appropriate powers in place, regulators will be able to respond to changes in technology or patient safety concerns as soon as possible.
