Freedom of Information request on the 1.62 million doses of the Moderna COVID-19 vaccine in Japan withdrawn due to contamination (FOI 21/997)
Published 25 February 2022
23rd September 2021
FOI 21/997
Dear Requestor,
Thank you for your request relating to the 1.62 million doses of the Moderna COVID-19 vaccine in Japan withdrawn due to contamination.
Please, see below the response (in red) to your request:
1) Please release all documentation provided to yourselves from Japanese Government departments, Takeda and Moderna relating to this recall. Affected batches have not been distributed in UK.
2) Have any doses of batch numbers 3004667, 3004734 and 3004956 been delivered in the UK? No
If yes:
a) How many were delivered?
b) How many were administered?
c) Are they subject to a recall?
d) Have recipients been informed of the incident?
3) Are these batches authorised for importation and/or distribution for human use in the UK? No
4) The type of contamination found was of the form of small black particles “a few millimetres” in size, which responds to a magnetic field. This contamination should be easily observable by visual inspection and by using a magnet. This would also have been obvious if chromatography and/or mass spectrometry was performed.
a) Have any Yellow Cards (regardless if deleted) been raised relating to any magnetic effects, either of the vaccine or the injection site for any COVID-19 vaccine? If so, how many?
As of 08/09/2021 the MHRA has not received any Yellow Card (UK spontaneous Adverse Drug Reaction [ADR]) reports relating to magnetic effects, either of the vaccine or the injection site. This search included Yellow Card reports for the 3 COVID-19 vaccines currently being used in the UK (Pfizer/BioNTech, COVID-19 Vaccine AstraZeneca and COVID-19 Vaccine Moderna) as well as reports where the vaccine details were not specified.
The MHRA continuously monitors the safety of medicines and vaccines through a variety of pharmacovigilance processes including the Yellow Card scheme. As part of our signal detection processes all adverse reaction reports received by the Yellow Card scheme are individually assessed and cumulative information reviewed at regular intervals. Any emerging evidence relating to possible risks associated with vaccines and medicines, is carefully reviewed and, if appropriate, regulatory action would be taken if any serious risks were confirmed.
The MHRA publishes interactive listings of all suspected ADRs reported by healthcare professionals and members of the public for a particular drug substance, including the 3 COVID-19 vaccines currently being used in the UK (Pfizer/BioNTech, COVID-19 Vaccine AstraZeneca and COVID-19 Vaccine Moderna) as well as reports where the vaccine details were not specified. These are known as Interactive Drug Analysis Profiles (iDAPs) and they are available here: www.yellowcard.mhra.gov.uk/iDAP.
DMRC have not received any further Yellow Card reports since the issue in Japan came to light, reported as of 09/09/2021.
b) Was the Moderna vaccine visually inspected prior to the approval any form of marketing authorisation? If so, please provide batch numbers for which visual inspection was performed and the date it was performed.
Visual inspection is performed by the manufacturer on finished product vials as part of the release testing of Moderna vaccine. In addition, the SmPC contains the following instructions to the Healthcare Professional ‘If dosage is incorrect, or discolouration and other particulate matter is present, do not administer the vaccine’.
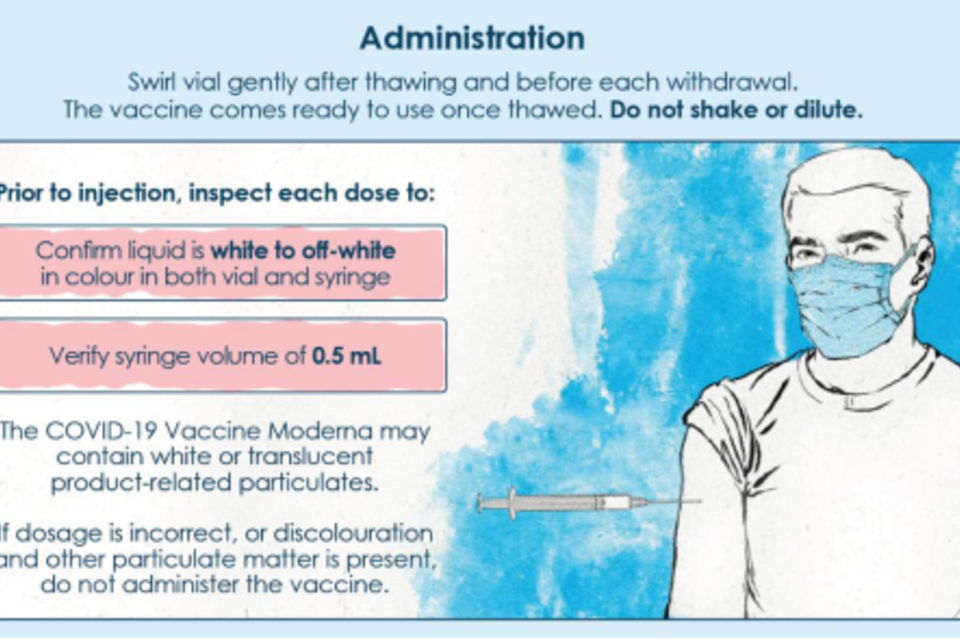
moderna_vaccine_administration
c) Was any independent chemical or physical analysis (including holding a magnet close to the material) performed on the Moderna vaccine performed prior to approval of any marketing authorisation? If so, provide the following:
-
Date of analysis
-
Place of analysis
-
Batch number(s) tested
-
Instrument(s) and/or tool(s) used
-
Experimental procedure
-
Raw data in a non-propriarary format (if it applies to the method of analysis, .mzML preferred for mass spectrometry outputs)
Representative samples from all batches of Moderna COVID-19 mRNA vaccine that are deployed in the UK undergo independent laboratory testing at NIBSC prior to their release. These tests include visual inspection and laboratory tests to measure purity, content and identity. Please find more details is the following link: https://nibsc.org/control_testing/batch-release.aspx
If you have any further questions, please do not hesitate to contact us at <IE&SFOI@mhra.gov.uk>
If you are unhappy with our decision, you may ask for it to be reviewed. That review will be undertaken by a senior member of the Agency who has not previously been involved in your request. If you wish to pursue that option please email: info@mhra.gov.uk
Due to the ongoing Covid-19 situation, we are not able to accept delivery of any documents or correspondence by post or courier to any of our offices.
After that, if you remain dissatisfied, you may write to the Information Commissioner at;
The Information Commissioner’s Office
Wycliffe House
Water Lane
Wilmslow
Cheshire
SK9 5AF
They will make a decision on whether or not we have interpreted the FOIA correctly in handling your request.
Yours sincerely
IE&S FOI Team
MHRA
Inspection, Enforcement and Standards
