Common animal-associated infections (England) annual report: 2024
Updated 31 July 2025
Applies to England
Background
This annual report is produced by the Emerging Infections and Zoonoses team in the Travel Health, Zoonosis, Emerging Infections, Respiratory and TB Division, Clinical and Emerging Infections Directorate, UK Health Security Agency (UKHSA). As of 2023, the data presented in these reports is for England only. Data for Wales is reported separately by Public Health Wales. Previous reports presenting data for both England and Wales are also available.
The report summarises laboratory diagnosed cases of selected zoonoses reported in England from 1 January to 31 December 2024 through the Second Generation Surveillance System (SGSS) and pathogen specific reference laboratories. It is important to note that not all cases of a zoonotic infection will present to healthcare services, and some cases may be treated empirically based on clinical suspicion without the need for a confirmatory test. Therefore, the numbers in this report are expected to be an underestimate of the true burden of zoonotic disease in England, likely biased towards severe infections which are more likely to present to healthcare. Furthermore, not all zoonoses presented in this report are notifiable in England. As such, it is likely not all confirmed laboratory test results are reported to UKHSA. For a list of notifiable diseases and causative organisms in England, see the Notifiable diseases and causative organisms: how to report
The impacts of the public health measures implemented in England due to the COVID-19 pandemic during 2020 and 2021 should be considered when making comparisons with this time period.
In 2024 there were no additional enhanced surveillance activities for any zoonotic pathogens reported here, therefore data is limited to information included through routine surveillance reporting. We include additional information collected from public health records where available. The regional data presented is based on the location of the patient postcode where available. If patient postcode is not available, then location of the referring hospital or laboratory is used. This means the regional data presented does not necessarily reflect the geographic area where the cases are located or where the infection is acquired. It is also worth noting that some infections will have been contracted while abroad; however travel history is not systematically collected. Where a travel history has been given, this also does not confirm this as the source of infection.
Q-fever data for 2024 is not presented in this annual report. As part of an internal review, the data is being validated and will be published as soon as possible.
This report is produced using a finalised dataset and thus data presented here is not “provisional” as in previously published quarterly reports. However, changes can still occur to numbers reported due to late notifications and updates to records as more information becomes available. Therefore, data presented in the most recent report supersedes those previously published in quarterly reports.
Executive summary and main messages for 2024
Main messages from this annual report are as follows:
-
following a change in diagnostic testing for Brucella canis, and review of brucellosis data since 2022, case definitions for brucellosis have been updated from 2022 onwards. Brucellosis cases in 2024 have increased compared with previous years. This increase is primarily in travel-related B. melitensis or B. abortus cases
-
hepatitis E virus cases have been steadily increasing annually since 2019; infections remain more common in older men, with higher numbers of cases reported in the South East, South West and West Midlands (as over the past 5 years); no outbreaks were reported in 2024. Whilst information on exposures is not routinely gathered for cases, previous investigations have identified associations with pork and pork related products in non-travel related cases. A retrospective study between 2008 and 2017 showed that the number of infections acquired in the UK were increasing whereas the number of travel-associated infections remained stable over this period (1)
-
there was an increase in the number of confirmed leptospirosis cases in 2024 compared to previous years. Total leptospirosis case numbers (confirmed and probable) have fluctuated annually since 2021; there was an 11.6% increase in overall cases between 2023 and 2024
-
there has been a small reduction in total laboratory-confirmed Lyme disease cases in 2024 compared with 2023. Most confirmed cases in 2024 were reported from the South West, South East and London regions, which has remained consistent over the last 5 years
-
psittacosis case numbers have increased in 2024 compared to previous years. The UKHSA Respiratory and Vaccine Preventable Bacteria Reference Unit (RVPBRU) has reported that requests for testing for Chlamydia psittaci and/or Chlamydia abortus increased in 2024, but with a reduction in positivity rates of samples. The increase in case numbers could be attributed to increased awareness and testing following a temporary increase in human cases in Northern Europe reported by the World Health Organization (WHO) in March 2024.
-
this report also summarises laboratory diagnosed cases of other key zoonotic infections, including pasteurellosis, most commonly caused by pasteurella multocida, which can be transmitted by animal bites, scratches and licks on broken skin. There were 890 cases of pasteurellosis reported in 2024, compared with 815 in 2023
-
there were 10 cases of toxigenic Corynebacterium ulcerans reported in 2024, which can cause diphtheria in humans; contact with companion animals is the most frequently reported exposure
-
toxoplasmosis is reported from the Toxoplasma Reference Unit, which receives a small proportion of potential toxoplasma cases, primarily to confirm congenital toxoplasmosis or for investigation of complex clinical scenarios. There was a 13.2% decrease in toxoplasmosis cases reported in 2024 (198 cases) compared with 2023 (228 cases)
-
other less common zoonotic pathogens reported include capnocytophaga spp., mycobacterium marinum, erysipelothrix rhusiopathiae, taenia spp. and toxocara spp.
Overview
Table 1. Animal-associated infections in England: quarterly confirmed laboratory reports by specimen date, Q1 2022 to Q4 2024 (note 1)
| Disease (Organism) | Q1 2022 | Q2 2022 | Q3 2022 | Q4 2022 | Total 2022 | Q1 2023 | Q2 2023 | Q3 2023 | Q4 2023 | Total 2023 | Q1 2024 | Q2 2024 | Q3 2024 | Q4 2024 | Total 2024 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthrax (Bacillus anthracis) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brucellosis (Brucella spp.) | 1 | 4 | 4 | 2 | 11 | 1 | 6 | 3 | 3 | 13 | 3 | 8 | 11 | 6 | 28 |
| Hepatitis E | 322 | 397 | 343 | 457 | 1,519 | 380 | 385 | 409 | 356 | 1,530 | 372 | 395 | 456 | 449 | 1,672 |
| Leptospirosis (Leptospira spp.) | 4 | 10 | 26 | 12 | 52 | 5 | 8 | 34 | 23 | 70 | 15 | 15 | 32 | 40 | 102 |
| Lyme disease (Borrelia burgdorferi) | |||||||||||||||
| - all cases | 157 | 243 | 577 | 269 | 1,246 | 158 | 253 | 905 | 351 | 1,667 | 190 | 308 | 689 | 395 | 1,581 |
| - acute infections | 101 | 192 | 441 | 182 | 916 | 87 | 183 | 701 | 180 | 1,151 | 73 | 163 | 461 | 262 | 959 |
| Pasteurellosis (Pasteurella spp.) | 173 | 214 | 230 | 184 | 801 | 151 | 222 | 255 | 187 | 815 | 219 | 206 | 239 | 226 | 891 |
| Chlamydia psittaci or Chlamydia abortus | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 5 | 2 | 4 | 4 | 1 | 11 |
| Toxigenic Corynebacterium ulcerans | 3 | 1 | 4 | 3 | 11 | 3 | 1 | 3 | 4 | 11 | 2 | 1 | 5 | 2 | 10 |
| Toxoplasmosis (Toxoplasma gondii) (note 2) | 54 | 37 | 57 | 45 | 193 | 63 | 54 | 56 | 55 | 228 | 48 | 41 | 51 | 58 | 198 |
Note 1: Data reported in table 1 reflects laboratory confirmed case numbers of animal associated infections only. References may be made in this report to probable case numbers; these are not included in the above table.
Note 2: Based on date specimen received.
Brucellosis
Brucella bacteria can be rough or smooth – this refers to appearance of the bacteria when grown on an agar plate. Smooth strains including Brucella melitensis (B. melitensis), Brucella abortus (B. abortus) or Brucella suis (B. suis) are typically more pathogenic than rough strains such as Brucella canis (B. canis) and are the primary cause of brucellosis in humans. Brucellosis is rare in England and human cases are usually caused by smooth strains of Brucella species acquired following travel; and often following consumption of unpasteurised dairy products in countries where brucellosis is endemic. The UK is officially free of brucellosis in cattle, sheep and goats.
The pan-Brucella species PCR test is only accredited for smooth strains of Brucella. A laboratory confirmed smooth strain brucellosis case is defined as either:
- isolation of smooth strain Brucella spp from any clinical site
- a titre of 1:160 or higher on standard agglutination testing.
B. canis is a recognised zoonotic pathogen affecting dogs and is not considered endemic in the UK dog population. Since 2020, there has been an increase in the number of reports of B. canis infection in dogs, the majority of which have been in dogs imported into the UK from Eastern Europe. Cases in humans are sporadically reported globally, but there is no available routine surveillance data for comparison in other countries. The first human case of brucellosis caused by B. canis in the UK was diagnosed in 2022 (2).
In contrast to smooth strain Brucella species (B. melitensis, B. abortus or B. suis) there are currently no specific accredited serological or molecular tests for B. canis in humans. The only way to confirm diagnosis of B. canis is through culture. In 2023, the Brucella Reference Unit (BRU) adopted canine serology tests (B. canis serum agglutination test (SAT) and a canine B. canis IgG ELISA test), alongside the pan-Brucella species PCR test to support clinical diagnosis. Following a review of PCR test results in 2024, the following case definitions have been applied for brucellosis surveillance in England from 2022 onwards:
Confirmed Brucella canis is any person with isolation of Brucella canis from a clinical specimen.
Probable Brucella canis is any person with a clinically compatible illness with:
- B. canis-specific antibody response (B. canis IgG ELISA, Serum Agglutination Test, or Rapid Slide Agglutination) with seroconversion or increase in titres on convalescent or paired serology testing OR
- other B. canis-specific antibody response AND detection of human pathogenic Brucella spp nucleic acid in clinical specimen AND negative non-canis serology
In 2024, there were 31 cases of brucellosis reported. Of these, 28 were smooth strain Brucella species (B. melitensis, B. arbortus or B. suis), and 3 were probable B. canis. No confirmed cases of B. canis were reported in 2024 (table 2). There were more brucellosis cases reported in 2024 compared with 2023, primarily due to an increase in smooth strain species (B. melitensis/B.abortus/B. suis), with numbers more than doubling between 2023 and 2024 (115.4% increase). This increase could reflect a general increase in travel-related infections, however previous brucellosis surveillance has also demonstrated annual variation in cases.
Table 2. Brucellosis cases in England by quarter, Q1 2022 to Q4 2024
| Organism | Q1 2022 | Q2 2022 | Q3 2022 | Q4 2022 | Total 2022 | Q1 2023 | Q2 2023 | Q3 2023 | Q4 2023 | Total 2023 | Q1 2024 | Q2 2024 | Q3 2024 | Q4 2024 | Total 2024 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. melitensis, B. abortus, or B. suis (note 1) |
1 |
3 |
4 |
2 |
10 |
1 |
6 |
3 |
3 |
13 |
3 |
8 |
11 |
6 |
28 |
| B. canis (note 2) | |||||||||||||||
| - confirmed | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| - probable | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| Total | 1 | 4 | 4 | 2 | 11 | 1 | 6 | 3 | 3 | 13 | 3 | 8 | 12 | 8 | 31 |
Note 1: Smooth strain Brucella
Note 2: Rough strain Brucella
Of the 28 smooth strain brucellosis cases reported in 2024, 12 (42.9%) were female, 14 (50.0%) were male and 2 (7.1%) did not have sex recorded. The median age of cases was 52 years (range 10 months to 90 years).
For cases where exposures were recorded, 16 (57.1%) reported travel to countries where brucellosis is endemic, including Iraq (6 cases), Turkey (3 cases), Somalia (3 cases), Kenya, India, Pakistan and the Philippines (1 case each). For the remaining 12 cases (42.9%), travel history was unknown.
Of the cases who reported travel to countries where brucellosis is endemic, 5 reported consuming unpasteurised dairy products during their visit and 1 reported direct contact with animals (sheep). There was 1 infant case where the mother reported consuming unpasteurised dairy products while breastfeeding. For the remaining 8 cases who reported recent travel, no additional exposure information was provided.
There were 3 probable B. canis cases reported in 2024: 2 female and 1 male (median age 68, range 54 to 70 years). All 3 probable cases experienced symptoms consistent with acute brucellosis and were owners of dogs that had been diagnosed with B. canis infection. Due to changes in testing associated with B. canis in 2023, it is not possible to draw direct comparisons between current data and data prior to 2023.
Hepatitis E
Hepatitis E virus (HEV) surveillance reports include laboratory data (Public Health Laboratory Birmingham and the UKHSA Blood Borne Virus Unit Colindale) together with additional cases reported by local laboratories through the Second Generation Surveillance System (SGSS). The combined data sets provide a more accurate reflection of the number of acute HEV cases reported in England. This data does not include cases identified through screening of blood donations by NHS Blood and Transplant (NHSBT); the annual number of donations in which HEV is identified and any transmission events can be found in the NHSBT safe supplies annual report (3).
There are 8 different HEV genotypes, 5 of which cause disease in humans. Genotype 1 (G1) and G2 viruses are predominantly found in low- and middle-income countries and are transmitted through the fecal oral route, whereas G3, G4, and G7 viruses are responsible for infections in high-income countries and are primarily foodborne (4). In the UK, HEV G3 group 2 is the predominant cause of infections; these infections are not associated with travel but are associated with consuming pork products from outside the UK (1, 4).
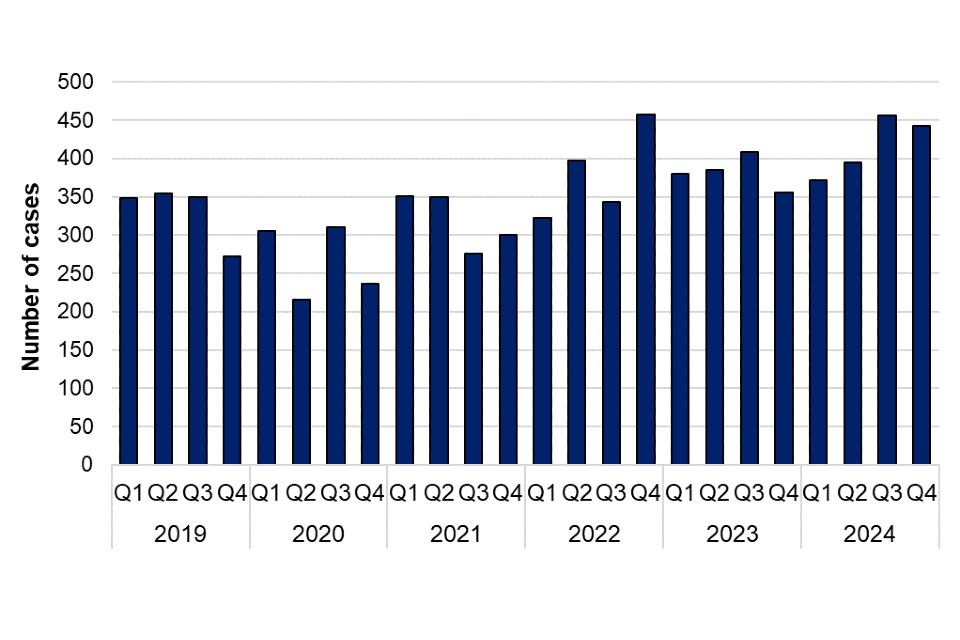
There was a 9.3% increase in laboratory confirmed cases of HEV infection reported in 2024 (1,672 cases) compared to 2023 (1,530 cases) (figure 1). HEV cases have increased annually since 2020, with no discernible seasonality (figure 2). There were no outbreaks of HEV reported in 2024.
Figure 1. Laboratory confirmed cases of hepatitis E in England by year, 2019 to 2024
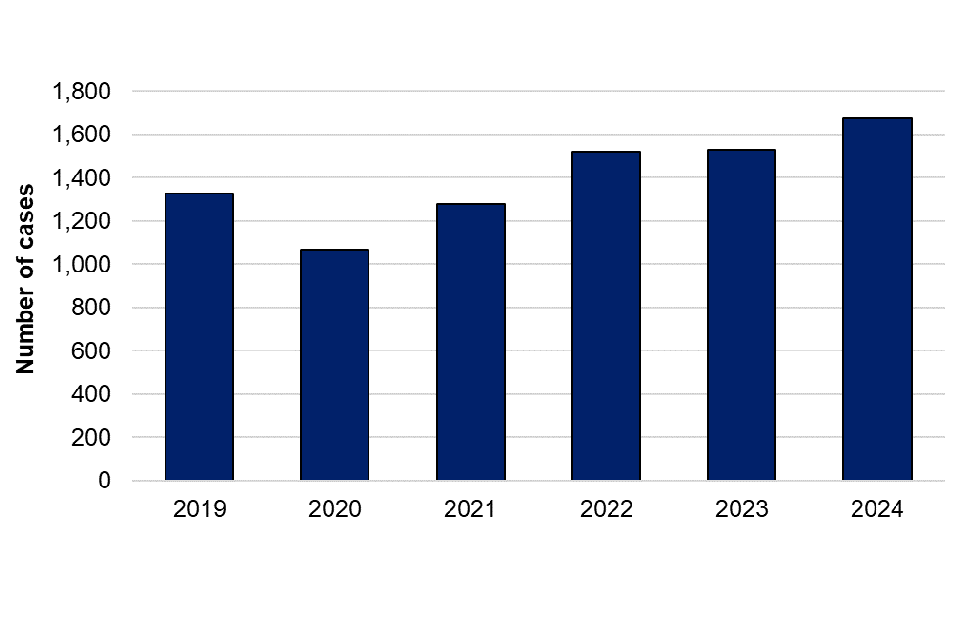
Figure 2. Laboratory confirmed cases of hepatitis E in England by quarter, Q1 2019 to Q4 2024
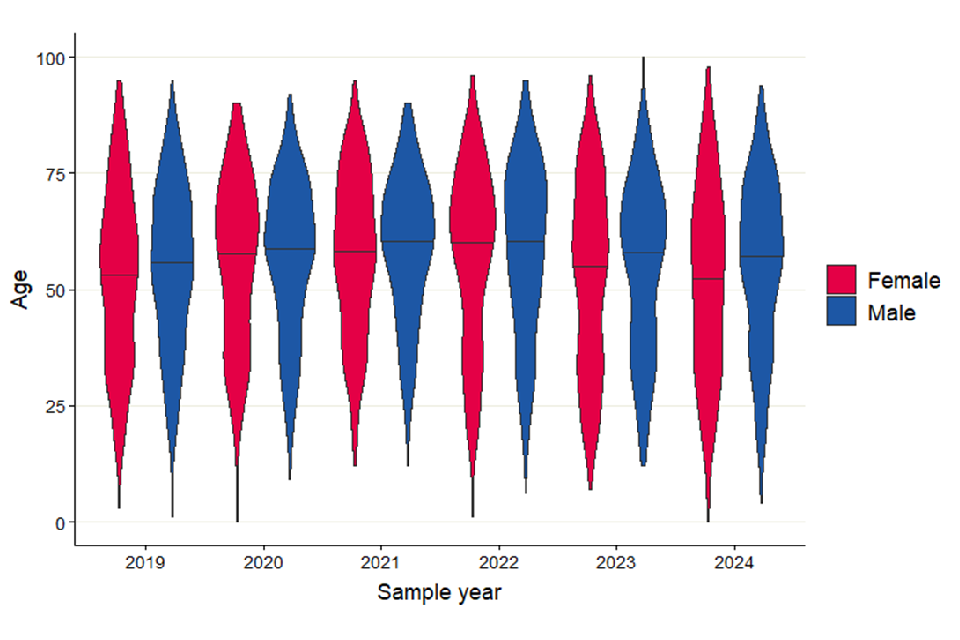
There was a total of 6,719 confirmed HEV cases between 2019 and 2023. Male cases accounted for approximately 60% of cases each year between 2019 and 2023 had a median age of 60 years, and female cases had a median age of 57 years. Over the last 5 years, the median age of males has stayed consistently higher than the median age of female cases (figure 3).
Of the 1,672 HEV cases reported in 2024, 960 (57.4%) were male (age range from 2 to 94 years, median 57 years), 710 (42.5%) were female (age range from 0 to 98 years, median 54.5 years) and 2 cases where sex was unknown (table 3). There is a persisting predominance of middle aged and older men (aged 45 years and over), with 69.1% of total cases in 2024 in this category. This finding is consistent with previous surveillance studies of HEV infection in England and Wales, which found that non-travel associated cases were higher in older men infected with HEV (genotype 3) (1). Whilst information on exposures is not routinely gathered for cases, previous investigations have identified associations with pork and pork related products in non-travel related cases (1, 4).
Table 3. Laboratory confirmed cases of hepatitis E in England by age group and sex, 2024
| Age group (years) | Male | Female | Unknown | Total |
|---|---|---|---|---|
| 0 to 14 | 12 (0.7%) | 13 (0.8%) | 0 (0%) | 25 (1.5%) |
| 15 to 24 | 53 (3.2%) | 44 (2.6%) | 0 (0%) | 97 (5.8%) |
| 25 to 44 | 195 (11.7%) | 200 (12.0%) | 0 (0%) | 395 (23.6) |
| 45 to 64 | 364 (21.8%) | 227 (13.6%) | 1 (0.1%) | 592 (35.4%) |
| Over 64 | 336 (20.1%) | 226 (13.5%) | 1 (0.1%) | 563 (33.7%) |
| Total | 960 (57.4%) | 710 (42.5%) | 2 (0.1%) | 1,672 (100%) |
Figure 3. Violin plot of laboratory confirmed cases of Hepatitis E in England by age and sex, 2019 to 2024. Line represents median age for each sex by year
The majority of HEV cases in 2024 were located in the South East (246 cases, 14.7%), West Midlands (246 cases, 14.7%) and South West (243 cases, 14.5%). These regions have consistently reported the highest number of cases of HEV annually over the past 5 years (table 4). Other regions including London and the North West have also experienced high case numbers intermittently. This data is based on the patient’s postcode where available. Where a patient’s postcode is unavailable, the location of the referring hospital or laboratory is used. As such, location of cases does not necessarily reflect the geographic area where the infection was acquired.
Table 4. Laboratory confirmed cases of Hepatitis E in England by UKHSA region, 2019 to 2024
| UKHSA Region | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|
| East Midlands | 72 (5.4%) | 66 (6.2%) | 64 (5.0%) | 127 (8.4%) | 132 (8.6%) | 150 (9.0%) |
| East of England | 139 (10.5%) | 120 (11.2%) | 122 (9.6%) | 125 (8.2%) | 108 (7.1%) | 109 (6.5%) |
| London | 158 (11.9%) | 110 (10.3%) | 120 (9.4%) | 230 (15.1%) | 138 (9.0%) | 194 (11.6%) |
| North East | 43 (3.2%) | 37 (3.5%) | 56 (4.4%) | 42 (2.8%) | 60 (3.9%) | 57 (3.4%) |
| North West | 125 (9.4%) | 92 (8.6%) | 106 (8.3%) | 167 (11.0%) | 214 (14.0%) | 218 (13.0%) |
| South East | 245 (18.5%) | 184 (17.2%) | 152 (11.9%) | 180 (11.8%) | 212 (13.9%) | 246 (14.7%) |
| South West | 206 (15.5%) | 186 (17.4%) | 272 (21.3%) | 300 (19.7%) | 259 (16.9%) | 243 (14.5%) |
| West Midlands | 160 (12.1%) | 131 (12.3%) | 192 (15.0%) | 213 (14.0%) | 215 (14.1%) | 246 (14.7%) |
| Yorkshire and the Humber | 131 (9.9%) | 118 (11.0%) | 170 (13.3%) | 113 (7.4%) | 162 (10.6%) | 176 (10.5%) |
| Unknown | 46 (3.5%) | 24 (2.2%) | 23 (1.8%) | 22 (1.4%) | 30 (2.0%) | 33 (2.0%) |
| Total | 1,325 (100%) | 1,068 (100%) | 1,277 (100%) | 1,519 (100%) | 1,530 (100%) | 1,672 (100%) |
Leptospirosis
Data for leptospirosis was obtained from the Rare and Imported Pathogens Laboratory (RIPL, UKHSA Porton). The diagnostic tests for leptospirosis changed in August 2020, therefore data is presented from 2021 onwards. As of 1 August 2020, a laboratory confirmed case of leptospirosis is defined by a positive 16S rRNA PCR result only. An Immunoglobulin M (IgM) enzyme-linked immune-absorbent assay (EIA) continues to be performed on all samples of suspected leptospirosis cases. A case with a strongly positive IgM and compatible clinical history, but without a confirmatory PCR result, is classified as a probable case for surveillance purposes. These probable cases will usually be treated clinically on the basis of this result. National surveillance of probable cases was introduced in 2021. Since testing protocols were changed in 2020, comparisons for reporting between years for leptospirosis cases are considered from 2021 onwards.
There were 154 cases of leptospirosis (confirmed and probable) reported in 2024, compared with 138 cases reported in 2023, representing an 11.6% increase in overall cases. Annual case numbers (confirmed and probable) have fluctuated since 2021 – with a peak in overall cases in 2022 (162 cases) (figure 4). For data in 2024 it was observed that there was a marked increase in laboratory-confirmed cases. Of the 154 overall cases reported in 2024, 102 (66.2%) were confirmed and 52 (33.8%) were probable cases. This represents a 45.7% increase in confirmed cases in 2024 (102 confirmed cases) when compared with 2023 (70 confirmed cases). Conversely, there was a 23.5% decrease in probable cases reported in 2024 (52 probable cases) when compared with 2023 (68 probable cases) (figure 5).
Confirmed leptospirosis cases in 2024 (102 cases) were higher than the average number of confirmed cases between 2021 and 2023 (58 cases per year). There has been no change in the testing methods or reporting used since 2020, but there has been an increase in the numbers of leptospirosis tests performed. This could be related to a general increase in travel associated infections as seen with other zoonoses included in this report, as well as increased clinician awareness of leptospirosis. There are a higher number of confirmed cases of leptospirosis in the third and fourth quarters of each year, indicating seasonality of cases in summer and autumn months (figure 5).
Figure 4. Overall cases (confirmed and probable) of leptospirosis in England by year, 2021 to 2024
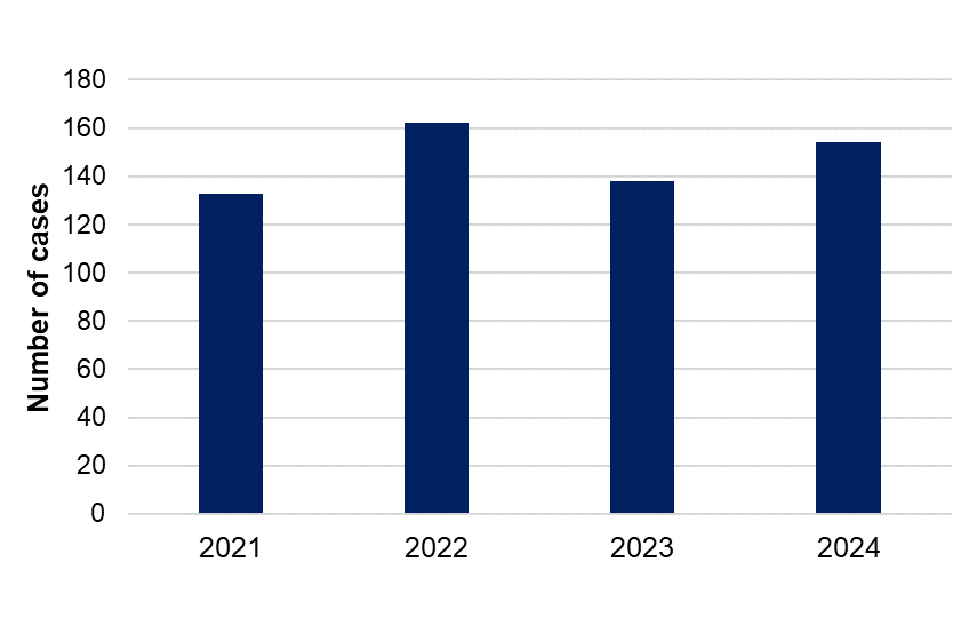
Figure 5. Laboratory confirmed and probable cases of leptospirosis in England by quarter, Q1 2021 to Q4 2024
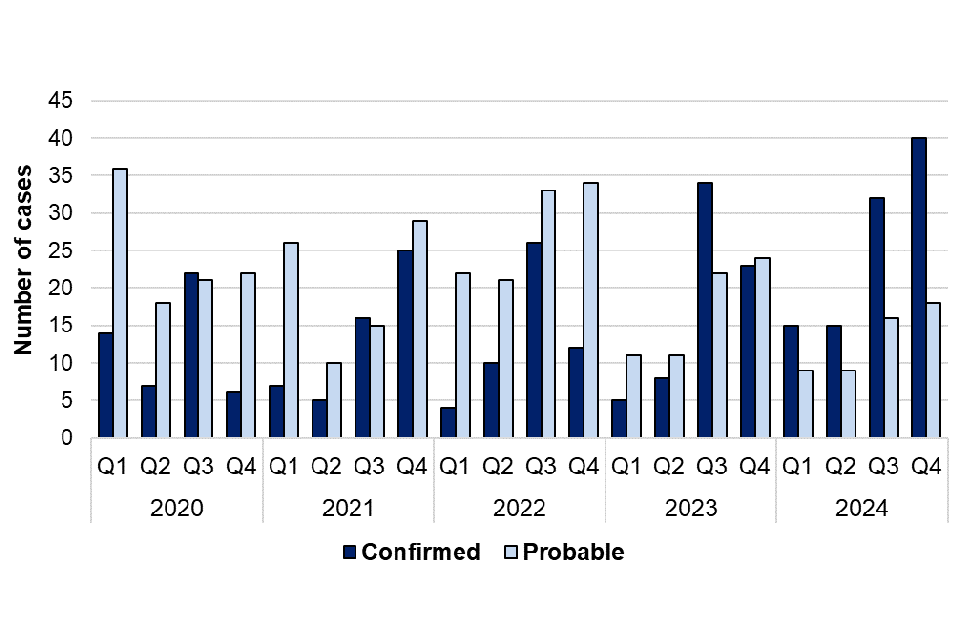
Confirmed leptospirosis cases
Of the 102 confirmed cases in 2024, 78 (76.5%) were male (age range from 16 to 82 years, median 49 years) and 24 (23.5%) were female (age range from 13 to 72 years, median 40 years) (table 5). The consistently higher incidence of leptospirosis infection in males over several years in England aligns with global surveillance studies (5, 6, 7). Groups at higher risk of contracting leptospirosis are those more likely to come into contact with the urine of infected animals through occupational exposure such as agricultural workers, abattoir workers, veterinarians or the military, or from life-style exposures such as recreational water sports or pet ownership.
In total, there were 175 confirmed leptospirosis cases between 2021 and 2023; averaging 58 cases a year, of which males accounted for 86% of these. Male and female cases between 2021 and 2023 had a median age of 47 and 46 years, respectively.
Based on patient postcode, the regions with the highest number of confirmed cases in 2024 were the South West (27 cases), the South East (20 cases), and London (16 cases) (table 6). This is a broadly consistent trend over the last 5 years. Where patient postcode is not available, the location of the referring hospital or laboratory is used. As such, location of cases does not necessarily reflect the geographic area where the infection was acquired.
Table 5. Laboratory confirmed cases of leptospirosis in England by age group and sex, 2024
| Age group (years) | Male | Female | Total |
|---|---|---|---|
| 0 to 14 | 0 (0%) | 1 (1.0%) | 1 (1.0%) |
| 15 to 24 | 9 (8.8%) | 2 (2.0%) | 11 (10.8%) |
| 25 to 34 | 8 (7.8%) | 7 (6.9%) | 15 (14.7%) |
| 35 to 44 | 14 (13.7%) | 3 (2.9%) | 17 (16.7%) |
| 45 to 54 | 20 (19.6%) | 5 (4.9%) | 25 (24.5%) |
| 55 to 64 | 16 (15.7%) | 2 (2.0%) | 18 (17.6%) |
| 65 to 74 | 10 (9.8%) | 4 (3.9%) | 14 (13.7%) |
| Over 75 | 1 (1.0%) | 0 (0%) | 1 (1.0%) |
| Total | 78 (76.5%) | 24 (23.5%) | 102 (100%) |
Table 6. Laboratory confirmed cases of leptospirosis by UKHSA region, 2021 to 2024
| UKHSA Region | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|
| East Midlands | 2 (3.8%) | 3 (5.8%) | 5 (7.1%) | 5 (4.9%) |
| East of England | 4 (7.5%) | 9 (17.3%) | 4 (5.7%) | 12 (11.8%) |
| London | 4 (7.5%) | 10 (19.2%) | 10 (14.3%) | 16 (15.7%) |
| North East | 0 (0%) | 1 (1.9%) | 3 (4.3%) | 3 (2.9%) |
| North West | 6 (11.3%) | 2 (3.8%) | 10 (14.3%) | 6 (5.9%) |
| South East | 10 (18.9%) | 13 (25.0%) | 9 (12.9%) | 20 (19.6%) |
| South West | 21 (39.6%) | 9 (17.3%) | 12 (17.1%) | 27 (26.5%) |
| West Midlands | 2 (3.8%) | 3 (5.8%) | 6 (8.6%) | 7 (6.9%) |
| Yorkshire and the Humber | 4 (7.5%) | 2 (3.8%) | 11 (15.7%) | 6 (5.9%) |
| Total | 53 (100%) | 52 (100%) | 70 (100%) | 102 (100%) |
Travel history is not always available for laboratory diagnosed cases and the reported country of recent travel does not necessarily reflect where the infection was acquired. Of the 102 confirmed cases in 2024, 38 (37.3%) reported recent travel abroad. Of the cases who reported travel abroad, the most common region reported was Southeast Asia (14 cases). Cases also reported travel to South and Central America (8 cases), the Carribean (5 cases), Europe (4 cases), South Asia (4 cases) and North Africa (2 cases). One case reported travel to both North Africa and Southeast Asia.
Of the 102 confirmed cases in 2024, exposure information was available for 27 cases: 21 (20.6%) cases reported potential exposure to a water source, with the most common exposures being activities such as swimming (7 cases), kayaking (3 cases), and white-water rafting (3 cases) in freshwater. There were also 8 cases who reported exposure to an unspecified water source. Further, 6 (5.9%) confirmed cases reported contact with a rodent or rodent excreta as a potential exposure. It should be noted that exposure information was only reported for a small number of cases and may reflect potential exposures in the UK or abroad.
Probable leptospirosis cases
Of the 52 probable leptospirosis cases reported in 2024, 41 (78.8%) were male (age range 9 to 75 years, median 44 years), and 10 (19.2%) were female (age range 16 to 79 years, median 35 years). There was 1 probable case where sex was unknown. The trend of a higher proportion of leptospirosis cases being male persists with probable cases of leptospirosis, especially in the 25 to 34 and 35 to 44 age groups where 85% of probable cases were male (table 7).
Table 7. Probable cases of leptospirosis in England by age group and sex, 2024
| Age group (years) | Male | Female | Unknown | Total |
|---|---|---|---|---|
| 0 to 14 | 1 (1.9%) | 0 (0%) | 0 (0%) | 1 (1.9%) |
| 15 to 24 | 3 (5.8%) | 4 (7.7%) | 0 (0%) | 7 (13.5%) |
| 25 to 34 | 9 (17.3%) | 1 (1.9%) | 1 (1.9%) | 11 (21.2%) |
| 35 to 44 | 8 (15.4%) | 1 (1.9%) | 0 (0%) | 9 (17.3%) |
| 45 to 54 | 8 (15.4%) | 2 (3.8%) | 0 (0%) | 10 (19.2%) |
| 55 to 64 | 7 (13.5%) | 0 (0%) | 0 (0%) | 7 (13.5%) |
| 65 to 74 | 4 (7.7%) | 1 (1.9%) | 0 (0%) | 5 (9.6%) |
| Over 75 | 1 (1.9%) | 1 (1.9%) | 0 (0%) | 2 (3.8%) |
| Total | 41 (78.8%) | 10 (19.2%) | 1 (1.9%) | 52 (100%) |
Lyme disease
Data for Lyme disease was obtained from the Rare and Imported Pathogens Laboratory (RIPL, UKHSA Porton). RIPL categorise Lyme disease cases into 3 groups:
- acute Lyme disease cases are those with significant IgM response and compatible clinical details (excluding neurological symptoms) or those with positive serology and erythema migrans
- acute neurological cases are those with positive serology and any compatible neurological symptoms
- longstanding Lyme disease cases are those with a significant IgG response
A significant IgG response may reflect past exposure or past infection with Borrelia, or a non-acute presentation of Lyme disease, and does not imply chronic, active infection. Antibodies may persist for several years despite treatment and resolution of disease and this does not indicate ongoing infection.
There was a small decrease (5.2%) in confirmed Lyme disease cases reported in 2024 (1,581 cases) compared with 2023 (1,667 cases). Similarly, there was a 16.7% decrease in the total number of acute cases reported in 2024 (959 cases) compared with 2023 (1,151 cases) (figure 6). The number of cases reported in 2024 is similar to data reported in 2018 and 2019 (2018: 1,104, 2019: 928), prior to a decrease in cases reported during COVID-19. It is not possible to determine whether this reflects more cases being treated without confirmatory testing or a true reduction in cases during this period. It should be noted that diagnosis for acute Lyme disease presenting with erythema migrans (“bullseye rash”) is made clinically and testing is not recommended, as per NICE guidelines. The number of laboratory-confirmed cases presented in this report are therefore likely an underestimate of the true burden of acute Lyme disease in England. Figure 7 shows how the number of cases continues to peak during the summer months (Q3 2024), which corresponds to the peak period of exposure to ticks in the UK in the spring and summer months.
Figure 6. Laboratory confirmed cases of Lyme disease in England by year, 2019 to 2024
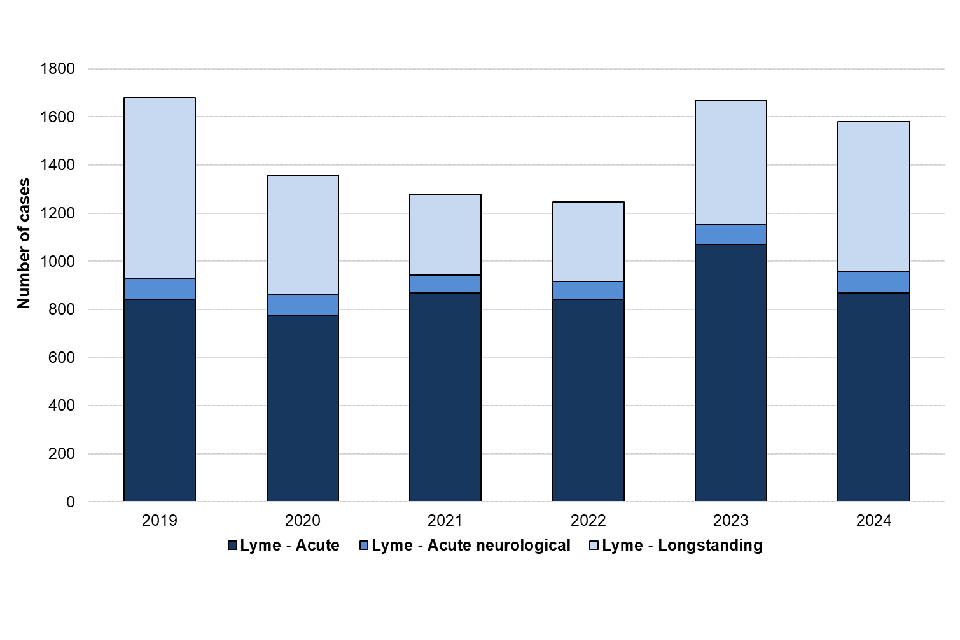
Figure 7. Laboratory confirmed cases of Lyme disease in England by quarter, Q1 2019 to Q4 2024
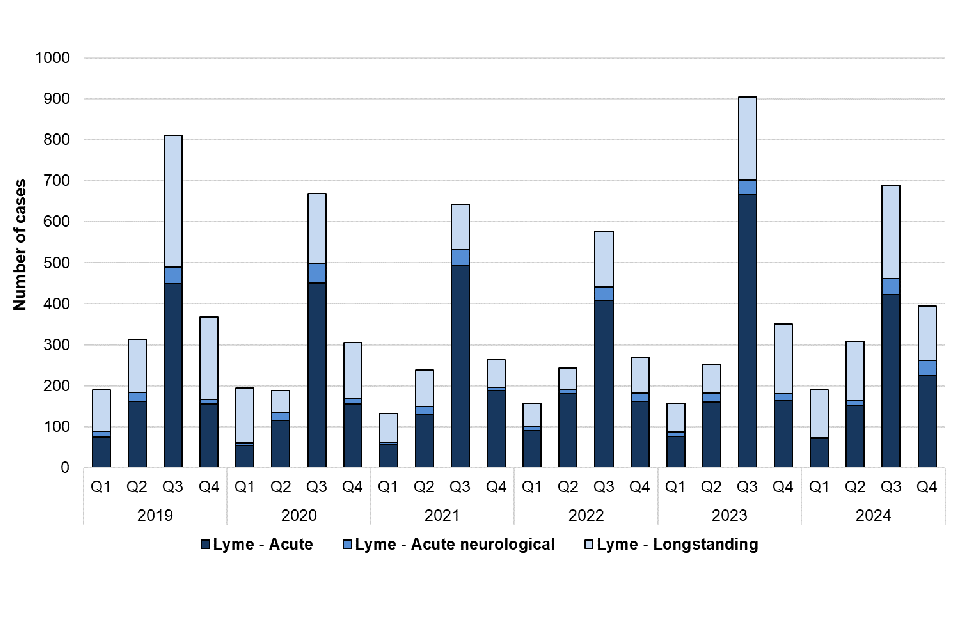
Of the 1,581 cases reported in 2024, 959 (60.7%) were acute, including 90 with neurological Lyme disease, and 622 (39.3%) were longstanding Lyme disease which may reflect past exposure or infection with the bacteria that cause Lyme disease, or a late presentation of Lyme disease, and does not imply chronic infection.
Acute Lyme disease including acute neurological Lyme disease
Of the acute Lyme disease cases in 2024, including acute neurological cases, 464 (48.4%) were male (age range 0 to 88 years, median 48 years), 487 (50.8%) were female (aged 1 to 91 years, median 50 years) and there were 8 (0.8%) cases where sex was unknown (aged 4 to 70, median 38 years) (table 8).
The total number of acute Lyme disease cases between 2019 and 2023 (including acute neurological cases) was 4,799. On average this is 960 cases per year, although data for 2020 should be interpreted with caution, due to the effects of the COVID-19 pandemic. Acute Lyme disease cases in 2024 (including acute neurological cases) have returned to pre-pandemic levels (2017: 1,197, 2018: 1,104, 2019: 928). Between 2019 and 2023 there were on average 491 (51.1%) male cases per year with a median age of 47 years and 465 (48.5%) female cases per year with a median age of 47 years. The median age of acute Lyme disease cases (including acute neurological cases) remained relatively stable over this period, with only a minimal difference observed between male and female cases.
Table 8. Laboratory confirmed acute Lyme disease cases (including acute neurological cases) in England by age group and sex, 2024
| Age group (years) | Male | Female | Unknown | Total |
|---|---|---|---|---|
| 0 to 14 | 38 (4.0%) | 26 (2.7%) | 1 (0.1%) | 65 (6.8%) |
| 15 to 24 | 32 (3.3%) | 33 (3.4%) | 1 (0.1%) | 66 (6.9%) |
| 25 to 34 | 57 (5.9%) | 63 (6.6%) | 2 (0.2%) | 122 (12.7%) |
| 35 to 44 | 80 (8.3%) | 85 (8.9%) | 1 (0.1%) | 166 (17.3%) |
| 45 to 54 | 81 (8.4%) | 79 (8.2%) | 1 (0.1%) | 161 (16.8%) |
| 55 to 64 | 90 (9.4%) | 98 (10.2%) | 0 (0%) | 188 (19.6%) |
| 65 to 74 | 55 (5.7%) | 71 (7.4%) | 2 (0.2%) | 128 (13.3%) |
| Over 74 | 31 (3.2%) | 32 (3.3%) | 0 (0%) | 63 (6.6%) |
| Total | 464 (48.4%) | 487 (50.8%) | 8 (0.8%) | 959 (100%) |
The regions that reported the most acute Lyme disease cases in 2024 (including acute neurological cases) were the South East (250 cases), the South West (239 cases), and London (170 cases) (table 9). Over the past 5 years, these regions have consistently reported the highest annual cases of acute Lyme disease. However, other areas such as the North West and East of England have also experienced high case numbers intermittently over the 5-year period. This data is based on the patient’s postcode where available. Where patient postcode is not available, the location of the referring hospital or laboratory is used. As such, location of cases does not necessarily reflect the geographic area where the tick bite occurred. A map of Ixodes ricinus distribution collated using the UKHSA Tick Surveillance scheme is available and further information on seasonality of Ixodes ricinus tick reports can be found on the Tick Surveillance Scheme (TSS) Dashboard.
Table 9. Laboratory confirmed acute Lyme disease cases (including acute neurological cases) in England by UKHSA region, 2019 to 2024
| UKHSA region | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|
| East Midlands | 31 (3.3%) | 20 (2.3%) | 30 (3.2%) | 23 (2.5%) | 27 (2.3%) | 29 (3.0%) |
| East of England | 66 (7.1%) | 48 (5.6%) | 64 (6.8%) | 64 (7.0%) | 46 (4.0%) | 77 (8.0%) |
| London | 165 (17.8%) | 158 (18.3%) | 167 (17.7%) | 155 (16.9%) | 206 (17.9%) | 170 (17.7%) |
| North East | 16 (1.7%) | 17 (2.0%) | 27 (2.9%) | 26 (2.8%) | 40 (3.5%) | 29 (3.0%) |
| North West | 62 (6.7%) | 53 (6.1%) | 66 (7.0%) | 69 (7.5%) | 79 (6.9%) | 80 (8.3%) |
| South East | 281 (30.3%) | 295 (34.2%) | 272 (28.9%) | 268 (29.3%) | 331 (28.8%) | 250 (26.1%) |
| South West | 228 (24.6%) | 233 (27.0%) | 237 (25.2%) | 223 (24.3%) | 306 (26.6%) | 239 (24.9%) |
| West Midlands | 32 (3.4%) | 18 (2.1%) | 27 (2.9%) | 33 (3.6%) | 51 (4.4%) | 29 (3.0%) |
| Yorkshire and the Humber | 47 (5.1%) | 20 (2.3%) | 52 (5.5%) | 54 (5.9%) | 65 (5.6%) | 56 (5.8%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.1%) | 0 (0%) | 0 (0%) |
| Total | 928 (100%) | 862 (100%) | 942 (100%) | 916 (100%) | 1,151 (100%) | 959 (100%) |
Travel history is not always available for laboratory diagnosed cases and the reported country of recent travel does not necessarily reflect where the infection was acquired. Of the 959 acute cases in 2024 (including acute neurological cases), 33 reported overseas travel to areas where Lyme disease has previously been found, including Europe (20 cases), the Americas (8 cases), Turkey (2 cases), Tanzania (1 case), and Egypt (1 case). The location of one case who reported overseas travel was not available.
Acute neurological Lyme disease
Of the 90 acute neurological cases reported in 2024, 46 (51.1%) were male (age range 1 to 88 years, median 42.5 years) and 44 (48.9%) were female (age range1 to 90 years, median 58.5 years) (table 10).
The total number of acute neurological Lyme disease cases between 2019 and 2023 was 405, with a mean of 81 cases per year. On average, there were a greater number of male compared to female cases (50 versus 31 cases, respectively). Male cases had a greater median age (48 years) compared to females (45 years). The median age of acute neurological Lyme disease cases varies slightly each year, which could be a result of the small case numbers.
Table 10. Laboratory confirmed acute neurological cases of Lyme disease in England by age group and sex, 2024
| Age group (years) | Male | Female | Total |
|---|---|---|---|
| 0 to 14 | 8 (8.9%) | 4 (4.4%) | 12 (13.3%) |
| 15 to 24 | 7 (7.8%) | 2 (2.2%) | 9 (10.0%) |
| 25 to 34 | 3 (3.3%) | 5 (5.6%) | 8 (8.9%) |
| 35 to 44 | 6 (6.7%) | 5 (5.6%) | 11 (12.2%) |
| 45 to 54 | 3 (3.3%) | 3 (3.3%) | 6 (6.7%) |
| 55 to 64 | 7 (7.8%) | 11 (12.2%) | 18 (20.0%) |
| 65 to 74 | 6 (6.7%) | 6 (6.7%) | 12 (13.3%) |
| Over 74 | 6 (6.7%) | 8 (8.9%) | 14 (15.6%) |
| Total | 46 (51.1%) | 44 (48.9%) | 90 (100%) |
Pasteurellosis
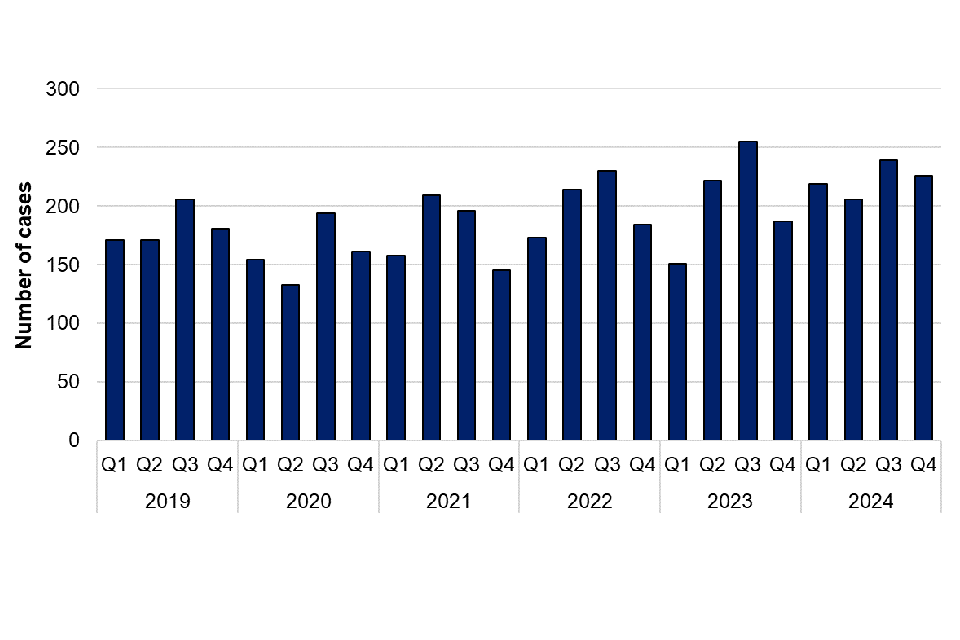
Pasteurella species are transmitted by animal bites, scratches or licks on broken skin. Animals do not have to be ill to pass the bacterium to humans, as they can carry the organism without showing symptoms. Pasteurella multocida is the most common cause of infection following a bite or scratch from domestic pets. There were 890 cases of pasteurellosis reported in 2024, which is more than the number of infections in 2023 (815 cases) (figure 8). The majority of cases were further speciated to Pasteurella multocida (552 cases) and Pasteurella canis (132 cases). Of the 890 Pasteurella cases in 2024, 521 (58.5%) were female, 367 (41.2%) were male and 2 were unknown sex. Cases were reported in the North West (178 cases), South West (157 cases), South East (138 cases), West Midlands (88 cases), East Midlands (76 cases), Yorkshire and the Humber (74 cases), London (74 cases), North East (54 cases) and East of England (51 cases).
Figure 8. Laboratory confirmed cases of Pasteurella species in England by quarter, Q1 2019 to Q4 2024
Chlamydia psittaci or Chlamydia abortus
Data for C. psittaci or C. abortus was obtained from the UKHSA Respiratory and Vaccine Preventable Bacteria Reference Unit (RVPBRU) in Colindale and from cases reported by local laboratories through SGSS. It is recognised that it is difficult to diagnose infection retrospectively through serological assays and, as such, following an external review, the surveillance case definitions for psittacosis were amended. As of 1 January 2023, a confirmed case of psittacosis is defined by a positive 16S rRNA PCR result or culture isolation only. Serological testing is no longer routinely carried out for C. psittaci and therefore less severe cases may not be identified through laboratory testing. This case definition has been applied to all retrospective data presented in this report.
It should be noted that PCR testing is unable to differentiate between C. psittaci and C. abortus. There are currently no other reliable methods to specifically identify C. psittaci as the causative agent of a patient with suspected psittacosis disease. As such, the numbers presented in this report will reflect laboratory confirmed cases with a positive PCR result for C. psittaci or C. abortus.
There were 11 laboratory confirmed cases of C. psittaci or C. abortus in 2024, representing a 120% increase compared with 2023 (5 cases) (figure 9). Of the 11 cases in 2024, 7 were male (age range 24 to 85 years, median age 67), and 4 cases were female (age range 8 to 79 years, median age 55) (table 11). The East Midlands and East of England regions reported the largest number of cases in 2024 (3 cases each) (table 12). Exposure information was available for 8 cases in 2024, with 3 cases reporting exposure to birds, 3 cases reporting possible exposure to birds, and 2 cases reporting no discernible exposure risks. There was no observed clustering of cases in UKHSA regions in 2024. The second and third quarters of 2024 had the highest number of cases since the first quarter of 2023, although case numbers returned to seasonal norms by Q4. There were no laboratory confirmed cases of C. psittaci or C. abortus identified in 2022 (figure 9).
Between 2019 and 2021 the RVPBRU noted a decrease in requests for testing likely due to the COVID-19 pandemic with sample numbers received increasing from 2022 onwards. The larger number of cases of psittacosis in 2024, particularly in Q2 and Q3 compared with previous years, may be attributed to increased clinician awareness and testing in England following a temporary increase in human cases in Northern Europe, reported by the WHO in March 2024 (8). The RVPBRU reported an increase in requests for testing of C. psittaci or C. abortus in 2024, with positivity rates of samples decreasing from 8.2% in 2023 to 4.4% in 2024.
Figure 9. Laboratory confirmed cases of C. psittaci or C. abortus in England by quarter, Q1 2019 to Q4 2024
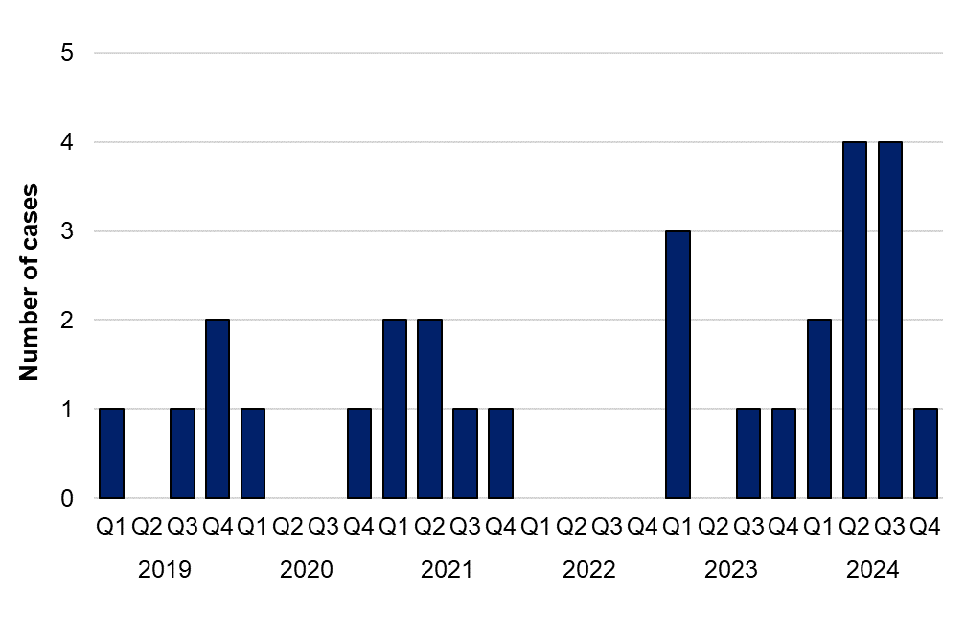
Table 11. Laboratory confirmed cases of Psittacosis in England by age group and sex in 2024
| Age group (years) | Male | Female | Total |
|---|---|---|---|
| Under 15 | 0 (0%) | 1 (9.1%) | 1 (9.1%) |
| 15 to 24 | 1 (9.1%) | 0 (0%) | 1 (9.1%) |
| 25 to 34 | 0 (0%) | 0 (0%) | 0 (0%) |
| 35 to 44 | 0 (0%) | 0 (0%) | 0 (0%) |
| 45 to 54 | 1 (9.1%) | 1 (9.1%) | 2 (18.2%) |
| 55 to 64 | 1 (9.1%) | 1 (9.1%) | 2 (18.2%) |
| 65 to 74 | 3 (27.3%) | 0 (0%) | 3 (27.3%) |
| Over 75 | 1 (9.1%) | 1 (9.1%) | 2 (18.2%) |
| Total | 7 (63.6%) | 4 (36.4%) | 11 (100%) |
Table 12. Laboratory confirmed acute cases of C. psittaci or C. abortus in England by UKHSA region, 2019 to 2024
| UKHSA Region | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|
| East Midlands | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (60%) | 3 (27.3%) |
| East of England | 1 (25%) | 2 (100%) | 2 (40%) | 0 (0%) | 0 (0%) | 3 (27.3%) |
| London | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 1 (9.1%) |
| North West | 1 (25%) | 0 (0%) | 1 (20%) | 0 (0%) | 1 (20%) | 2 (18.2%) |
| South East | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9.1%) |
| South West | 1 (25%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (9.1%) |
| Yorkshire and the Humber | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 |
| Total | 4 (100%) | 2 (100%) | 5 (100%) | 0 (0%) | 5 (100%) | 11 (100%) |
Toxigenic Corynebacterium ulcerans
Toxigenic Corynebacterium ulcerans (C. ulcerans), is a zoonotic pathogen that can cause diphtheria in humans.
Data for laboratory confirmed toxigenic C. ulcerans were obtained from the Respiratory and Vaccine Preventable Bacteria Reference Unit (RVPBRU) in Colindale. A confirmed case of toxigenic C. ulcerans infection is defined by isolation of C. ulcerans that is positive for the diphtheria toxin gene by PCR, and expresses the toxin as demonstrated using the Elek test. C. ulcerans speciation is initially established by the submitting laboratory and then confirmed in RVPBRU using a combination of species-specific PCR, additional biochemical testing and MALDI-TOF MS, as required. Further information on 2024 diphtheria cases in England can be found in the UKHSA annual diphtheria report for 2024 (9).
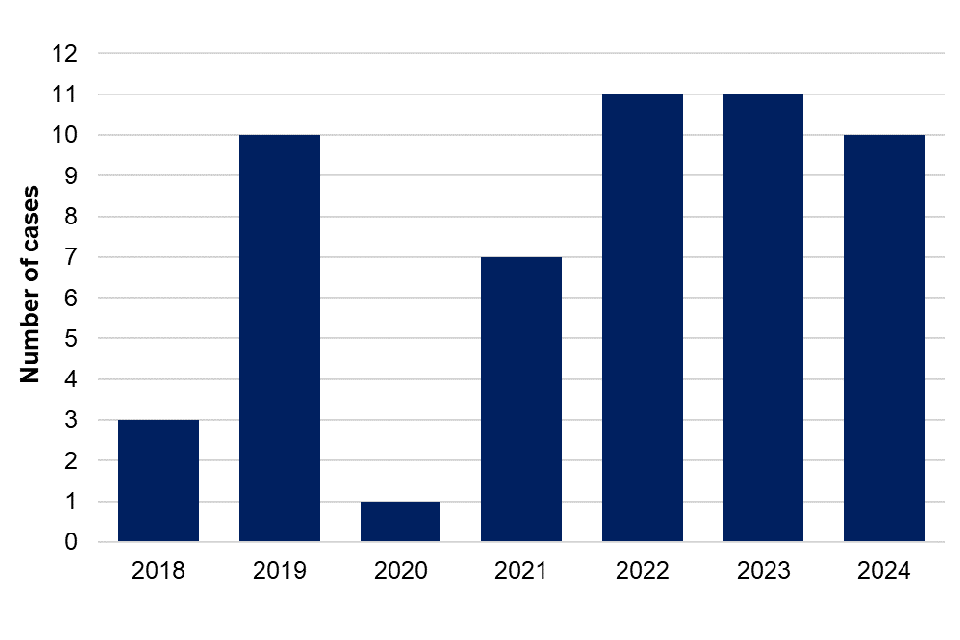
There were 10 reports of toxigenic C. ulcerans infection in 2024, of which 6 were female with a median age of 63 years, and 4 were male and had a median age of 52.5 years. This is similar to the number of cases reported in 2022 and 2023 (11 cases each) (figure 10). Of the 10 cases in 2024, 9 (90%) reported contact with animals. Companion animals of 4 cases were swabbed and 2 had a companion animal that tested positive for toxigenic C. ulcerans.
In England, contact with companion animals remains the most frequently reported exposure for individuals with confirmed toxigenic C. ulcerans infections. However, the animals may not show evidence of infection and it is not always possible to confirm the carriage of C. ulcerans in animals.
Figure 10. Laboratory confirmed cases of toxigenic Corynebacterium ulcerans in England by year, 2018 to 2024
Toxoplasmosis
Data for laboratory confirmed toxoplasmosis in England were obtained from the Toxoplasma Reference Unit (TRU, Public Health Wales, Swansea). The TRU receives only a small proportion of samples for testing, which are primarily for confirmation of congenital cases or other more complex scenarios, therefore data reported is an underestimation of the true burden of disease in England. Confirmed cases by the TRU are defined as testing positive using a Sabin-Feldman dye serological test that measures IgG and IgM. PCR assays and an in-house IgM Enzyme Immunoassay are used to test certain clinical samples to confirm active infection.
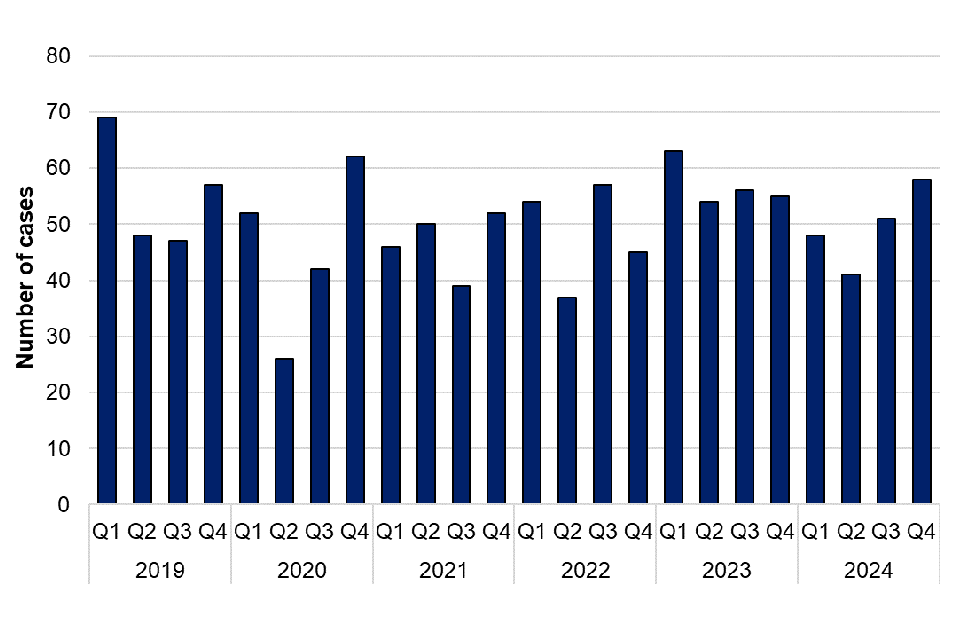
198 confirmed toxoplasmosis cases were reported by the TRU in 2024. This is a 13.2% decrease in the total number of confirmed cases compared with 2023 (228 cases). Confirmed cases in 2024 were slightly lower than the 5-year average from 2019 to 2023 (202 cases per year). A higher number of cases were reported in the third and fourth quarters of 2024, compared with first and second quarters of the year, however there is no apparent seasonality identified across the 5-year period (figure 11).
Figure 11. Laboratory confirmed cases of toxoplasmosis in England by quarter, 2019 to 2024
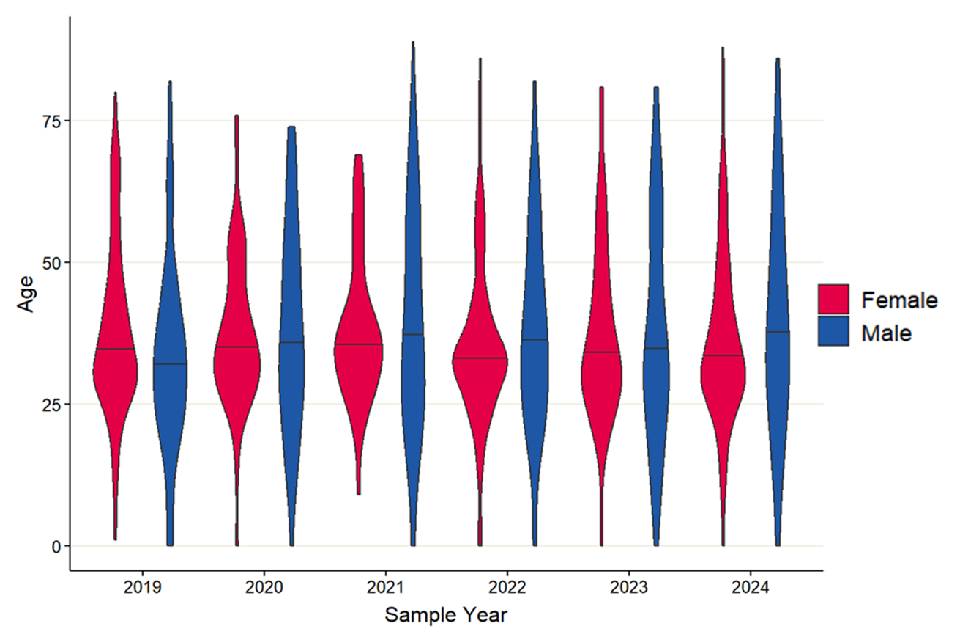
Of the 198 TRU confirmed cases in 2024, 74 (37.4%) were male (age range 0 to 86, median 37 years), 118 (59.6%) were female (age range 0 to 88, median 33 years) and there were 6 cases where sex was unknown (table 13). The largest number of cases were in the 25 to 34 age group (62 cases, 31.3%), with the majority of cases in this group being female (45 cases, 72.6%). Since 2019, females have consistently made up a greater proportion of cases each year. The TRU has reported that the majority of the samples received for testing are cases in pregnant women and women of childbearing age. Screening for toxoplasmosis during pregnancy is not routine, however testing may be prompted based on clinical suspicion if foetal abnormalities are identified in scans, as these may result from toxoplasma infection. This is likely the reason for the larger number of female toxoplasmosis cases reported across a number of years, specifically in the 25 to 34 age group.
The median age of male cases was higher than the median age of females across all years with an exception in 2019 although in 2020 and 2023 this difference was marginal (figure 12).
Table 13. Laboratory confirmed cases of toxoplasmosis in England by age group and sex, 2024
| Age group (years) | Male | Female | Unknown | Total |
|---|---|---|---|---|
| Under 14 | 8 (4.0%) | 6 (3.0%) | 1 (0.5%) | 15 (7.6%) |
| 15 to 24 | 8 (4.0%) | 13 (6.6%) | 2 (1.0%) | 23 (11.6%) |
| 25 to 34 | 17 (8.6%) | 45 (22.7%) | 0 (0%) | 62 (31.3%) |
| 35 to 44 | 15 (7.6%) | 23 (11.6%) | 0 (0%) | 38 (19.2%) |
| 45 to 54 | 10 (5.1%) | 13 (6.6%) | 0 (0%) | 23 (11.6%) |
| 55 to 64 | 6 (3.0%) | 9 (4.5%) | 1 (0.5%) | 16 (8.1%) |
| 65 to 74 | 6 (3.0%) | 4 (2.0%) | 0 (0%) | 10 (5.1%) |
| Over 75 | 4 (2.0%) | 3 (1.5%) | 0 (0%) | 7 (3.5%) |
| Unknown | 0 (0%) | 2 (1.0%) | 2 (1.0%) | 4 (2.0%) |
| Total | 74 (37.4%) | 118 (59.6%) | 6 (3.0%) | 198 (100%) |
Figure 12. Violin plot of laboratory confirmed cases of toxoplasmosis in England by age group and sex, 2019 to 2024. Excludes 19 cases where sex is unknown. Line represents median age for each sex by year.
Other zoonotic organisms
Data for other zoonotic organisms are obtained from cases reported by local laboratories through SGSS. The region is based on the location of the patient’s postcode where available. If the patient’s postcode is not available, then the location of the referring hospital or diagnostic laboratory is used.
Capnocytophaga spp
Capnocytophaga species are frequently carried in the mouths of companion animals (cats and dogs) or humans and may be associated with an animal or human bite, or opportunistic infections in those with impaired immune systems. There were 73 cases of Capnocytophaga infection in 2024, which is the same number of cases as in 2023. Of the cases reported in 2024, 51 were further speciated to Capnocytophaga canimorsus, 3 were Capnocytophaga ochracea and 6 were Capnocytophaga other. Of those speciated to Capnocytophaga canimorsus, 25 cases were male and 26 were female, and most cases were reported in the South East and South West (14 cases each). There tends to be more cases reported in Q3, with a notable peak in Q3 2023. This could be related to an increase in dog bites reported in summer months (10).
Figure 13. Laboratory confirmed cases of Capnocytophaga spp. in England by quarter, Q1 2021 to Q4 2024
Mycobacterium marinum
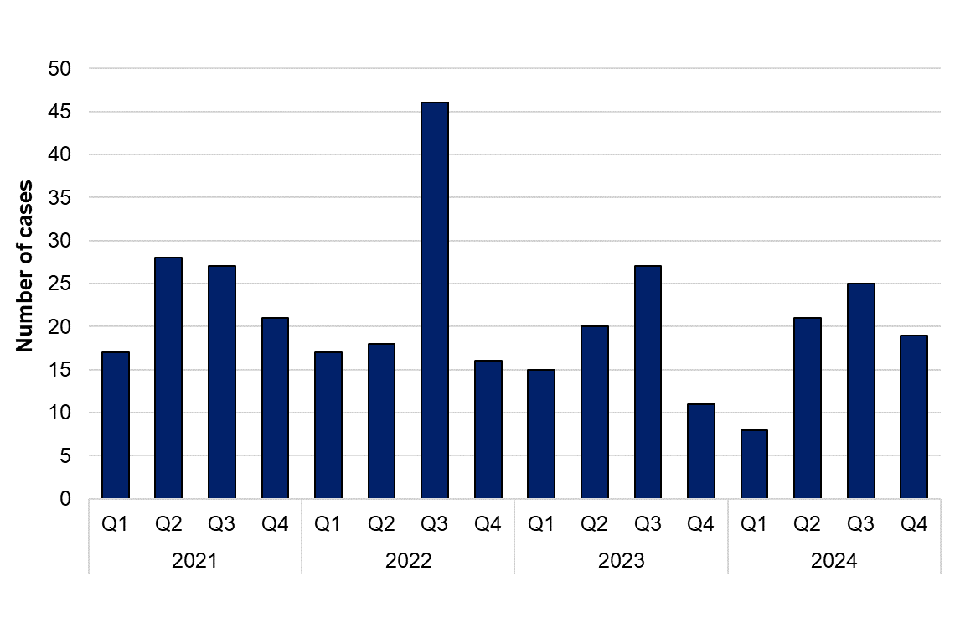
Mycobacterium marinum is a bacteria found in both salt and fresh water and can cause skin lesions in humans and fish. Humans can be exposed to Mycobacterium marinum through direct contact with contaminated water or fish. There were 17 cases of Mycobacterium marinum infection in 2024, which is similar to the number of cases in 2023 (18 cases); 13 cases were male, and 4 cases were female. Cases were reported in the South West (8 cases),Yorkshire and the Humber (4 cases), South East (2 cases), East Midlands, London and North East (1 case each). There was no exposure information available for these cases.
Figure 14. Laboratory confirmed cases of Mycobacterium marinum in England by quarter, Q1 2021 to Q4 2024
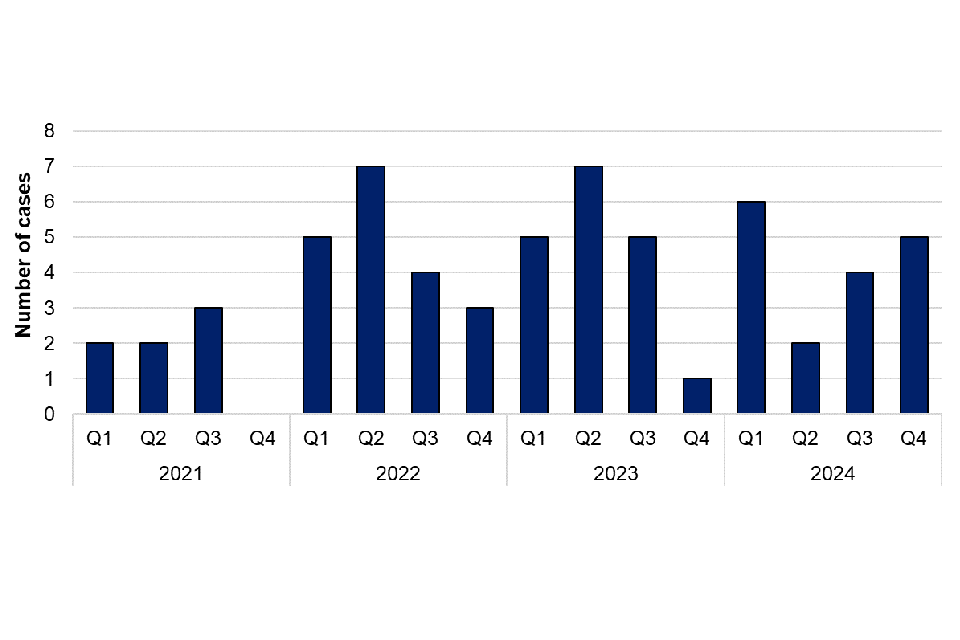
Erysipelothrix rhusiopathiae
Erysipelothrix rhusiopathiae are bacteria found in various animals including pigs, turkeys and sheep. Transmission to humans usually occurs through direct contact with infected animals or their excretions. There were 12 cases of Erysipelothrix rhusiopathiae reported in 2024, which is more cases than in 2023 (9 cases). Of the cases in 2024, 9 were male and 3 were female. Cases were located in the South West (3 cases), North West (3 cases), London (2 cases), West Midlands (2 cases), North East (1 case) and South East (1 case).
Figure 15. Laboratory confirmed cases of Erysipelothrix rhusiopathiae in England by quarter, Q1 2021 to Q4 2024
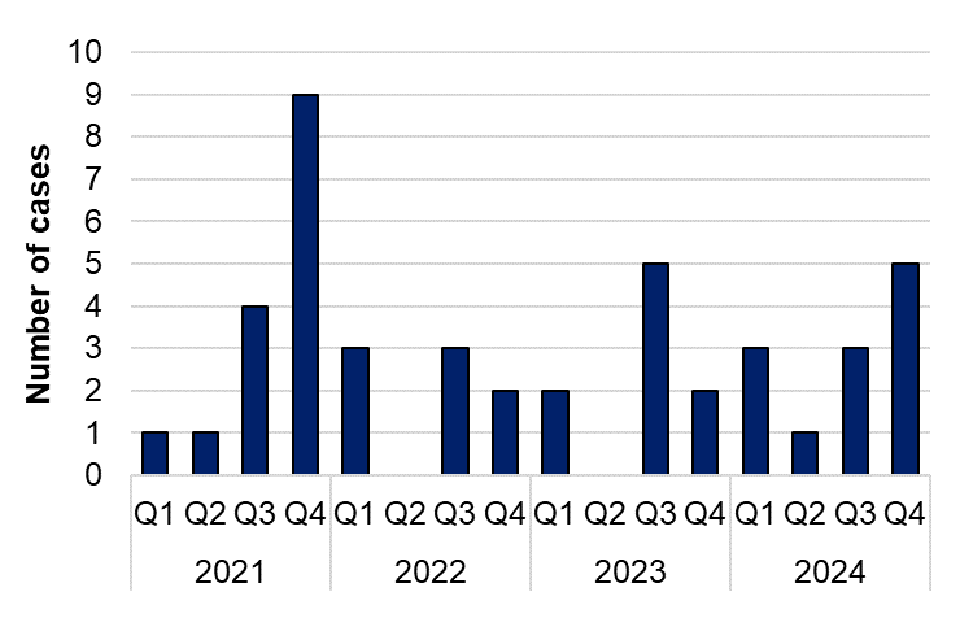
Taeniasis
Taeniasis is the infection of humans with the adult tapeworm of Taenia saginata, T. solium or T. asiatica. Humans become infected by ingesting raw or undercooked infected meat from cattle (T. saginata) and pigs (T. solium and T. asiatica). There were 35 reports of taeniasis in 2024, which is similar to the number of cases in 2023 (32 cases). Of these, 7 were further speciated to T. saginata. Of the 7 T. saginata cases, 6 of these were male and 1 was female. Ages ranged from 16 to 50 years, with a median age of 28 years.
Figure 16. Laboratory confirmed cases of Taeniasis in England by quarter, Q1 2021 to Q4 2024
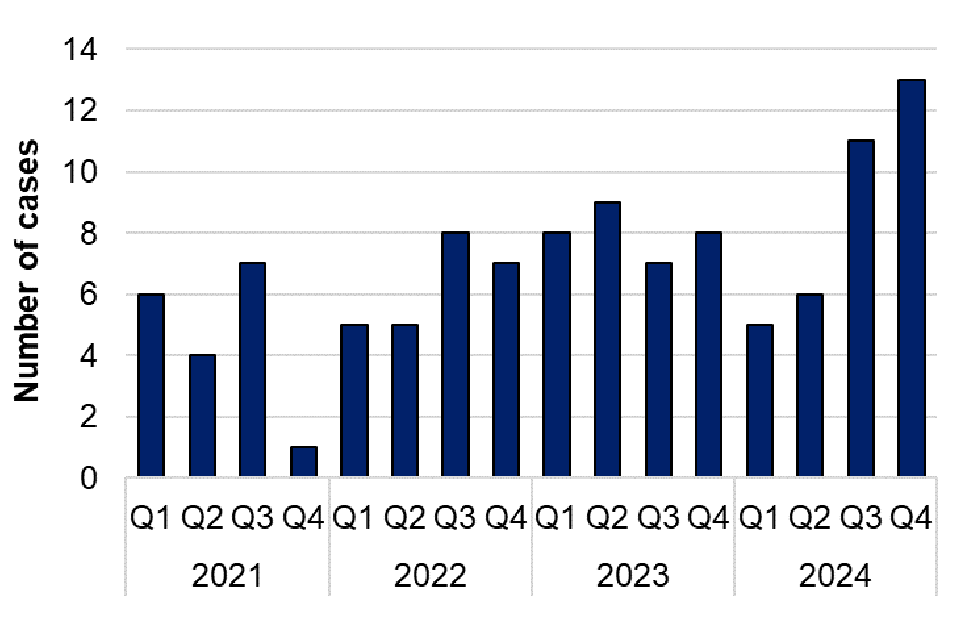
Toxocariasis
Toxocariasis, also known as round worm infection, is an infection caused by a parasite Toxocara spp that spreads to people from animals, usually dogs and cats, through exposure to infected animals faeces. In rare cases, people may get infected if they eat undercooked or raw meat like lamb or rabbit. There were 2 cases of toxocariasis in 2024, which is similar to 2023 (3 cases). Of the 2 cases in 2024, 1 was female and 1 was male.
Figure 17. Laboratory confirmed cases of Toxocariasis in England by quarter, Q1 2021 to Q4 2024
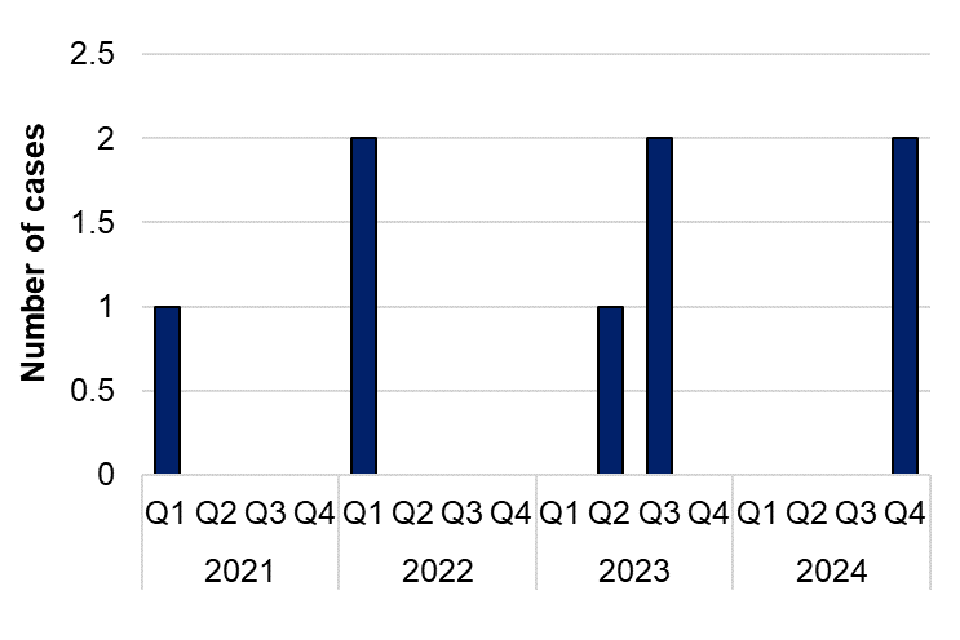
References
1. Oeser C, Vaughan A, Said B, Ijaz S, Tedder S, Haywood B, and others (2019). Epidemiology of Hepatitis E in England and Wales: A 10-Year Retrospective Surveillance Study, 2008–2017. The Journal of Infectious Diseases: volume 220, issue 5, pages 802 to 810.
2. Djokic V, Freddi L, de Massis F, Lahti E, van den Esker MH, Whatmore A, and others (2023). The emergence on Brucella canis as a public health threat in Europe: what we know and what we need to learn, Emerging Microbes & Infections: volume 12, issue 2.
3. NHS Blood and Transplant (NHSBT) and UKHSA (2023). Safe Supplies 2023: Close monitoring of blood donations.
4. Smith I, Said B, Vaughn A, Haywood B, Ijaz S, Reynolds C and others, (2021). Case-control study of risk factors for acquired hepatitis E virus infections in blood donors, United Kingdom, 2018-2019. Emerging Infectious Diseases: volume 27, issue 6, pages 1,654 to 1,661.
5. Puca E, Pipero P, Harxhi A, Abazaj E, Gega A, Puca E, and others (2018). The role of gender in the prevalence of human leptospirosis in Albania. The Journal of Infection in Developing Countries: volume 12, issue 03, pages 150 to 155.
6. Delight EA, de Carvalho Santiago DC, Palma FAG, de Oliveira D, Souza FN, Santana JO, and others (2024). Gender differences in the perception of leptospirosis severity, behaviours, and Leptospira exposure risk in urban Brazil: a cross-sectional study. medRxiv [preprint, 30 April].
7. Skufca J, and Arima Y. (2012). Sex, gender and emerging infectious disease surveillance: a leptospirosis case study. Western Pacific Surveillance and Response Journal (WPSAR): volume 3, issue 3, pages 37 to 39.
8. WHO (2024). ‘Psittacosis – European region’ (updated 5 March 2024).
9. UKHSA (2025). Diphtheria in England – 2024 Annual report. Health Protection Report: volume 19, number 4.
10. Tulloch JSP, Owczarczak-Garstecka SC, Flemming K, Vivancos R and Westgarth C (2021). English hospital episode data analysis (1998-2018) reveal that the rise in dog bite hospital admissions is driven by adult cases. Scientific Reports: volume 11, issue 1, article 1,767.
