Radiation risk with digital mammography in breast screening
Updated 29 July 2025
Applies to England
1. Executive summary
This review estimates the risks and benefits of breast screening in terms of number of deaths due to radiation-induced cancers and the number of lives saved due to digital breast screening in the NHS Breast Screening Programme (NHSBSP) in England.
A radiation risk model, patient dose data and data from national screening statistics were used to estimate the number of deaths due to radiation induced breast cancers in the NHSBSP in England. The breast cancer mortality reduction in the invited population due to screening, and the percentage of women diagnosed with symptomatic breast cancer who die from that cancer, were collated from the literature. The number of lives saved due to screening was calculated.
The main findings are that:
- the risk of a radiation-induced cancer for a woman attending full field digital mammographic screening (2 views) by the NHSBSP is between 1 in 49,000 to 1 in 98,000 per visit
- if a woman attends all 7 screening examinations between the age of 50 up to her 71st birthday, the risk of a radiation-induced cancer is between 1 in 7,000 to 1 in 14,000
- it is estimated that about 400 to 800 cancers are detected by the NHSBSP for every cancer induced
- the mortality benefit of screening exceeds the radiation-induced detriment by about 150:1 to 300:1
- for the small proportion of women with breasts thicker than 90mm who receive higher radiation doses, the benefit will exceed the risk by about 100:1 to 200:1
2. Introduction
The NHSBSP in England invites women for screening every 3 years from 50 up to their 71st birthday. During the screening examination, 2 views of both breasts are acquired using digital mammography.
During a mammography screening examination, the breast is exposed to ionising radiation. A review of the radiation risks of breast screening using analogue technology was published in NHSBSP Report 54[footnote 1]. There are several differences between the assumptions made in that report and current practice. At the time of the report two-view mammography was only performed at the prevalent screen and one-view mammography was performed thereafter at most services.
Two-view mammography is performed at all screening rounds in the current screening regime in the NHSBSP. Mammography systems have transitioned from using film-screen mammography to digital mammography, and use different X-ray target and filter materials, resulting in changes to the average breast dose per examination. Three international advisory bodies[footnote 2] [footnote 3] [footnote 4] have provided updated radiation risk coefficients from those used in NHSBSP Report 54. Finally, assumptions about mortality due to breast cancer outside screening have also changed due to improved treatments.
In this report, the radiation risk has been compared to the benefit of screening for the current imaging protocol used in the breast screening programme in England, and taking account of the new information discussed above. This report is based on (and parts directly taken from) an article published in the British Journal of Radiology[footnote 5].
3. Methods
This report provides an update of results from NHSBSP Report 54[footnote 1]. Therefore detailed methods and derivations of equations are not given. Greater details regarding the assumptions used and calculations performed can be found in Warren et al[footnote 5].
3.1 Assumptions
Screening regime
It was assumed that all screening examinations included 2 views. The attendance rate and the number of women in each screening round were calculated using data from NHSBSP statistics for the year April 2013 to March 2014 for England[footnote 6]. The age range 50 up to 71st birthday (current regime in the NHSBSP) was considered and also the age extension randomised control trial of women aged 47 to 73[footnote 7]. The number of women who would be screened in the screening rounds at ages 47 to 49 and 71 to 73 was estimated by extrapolating the number of women in the standard age range to this wider age range.
Radiation risk factors
The data used for the lifetime risk of cancer incidence over the range of ages seen in breast screening have been taken from the Health Protection Agency Centre for Radiation, Chemicals and Environmental Hazards Report 28 (HPA Report CRCE-028)[footnote 8] which provides the International Commission of Radiological Protection (ICRP) 103 model[footnote 2] risk factors for radiation-induced breast cancer for age bands of 10 years, between the ages of 0 to 99. The risk factors at the average age for each screening round (Table 1) were determined by using a Gaussian curve fitted to the data and interpolation.
The ICRP 103 model[footnote 2] is based on data from epidemiology studies. The dose and dose rate effectiveness factor (DDREF) is the ratio of the radiation risk at high dose and dose rates (as found in the epidemiological studies), to the risk at low dose and low dose rates (as found in screening). It is a topic of much discussion and research. In this report, results are given for DDREFs of 1.0 and 2.0, since the appropriate choice of DDREF is uncertain.
Table 1. Lifetime risk of radiation-induced breast cancer for women for DDREFs of 1 and 2. Taken from Warren et al[footnote 5].
| Age (years) | Radiation risk factor (per million per mGy): DDREF = 1.0 | Radiation risk factor (per million per mGy): DDREF = 2.0 |
|---|---|---|
| 48 | 13.8 | 6.9 |
| 51 | 11.4 | 5.7 |
| 54 | 9.3 | 4.7 |
| 57 | 7.5 | 3.8 |
| 60 | 6.0 | 3.0 |
| 63 | 4.7 | 2.4 |
| 66 | 3.6 | 1.8 |
| 69 | 2.8 | 1.4 |
| 72 | 2.1 | 1.0 |
Radiation dose
The dose was calculated for the whole screening population, and 2 subgroups of the screening population. First, the whole screening population was considered. The mean glandular dose (MGD) was assumed to be equal to 3mGy for a two-view examination. This is based on average doses of 1.5mGy per view for digital mammography systems in the NHSBSP between 2010 and 2012[footnote 9].
The second situation considered was for a sub-group of the population with larger breasts, who are therefore likely to receive higher doses without an increase in cancer detection. From a dose survey of breast screening centres in the UK over the period of 2010 to 2012[footnote 9], for breasts with thickness above 90mm imaged on digital radiography (DR) systems, the average MGD was 2.3mGy for the craniocaudal view and 2.7mGy for the mediolateral oblique view. Therefore, an average MGD of 5.0mGy for a two-view examination has been assumed. Only a small proportion of women have breasts thicker than 90mm on compression (1.8%).
The final dose situation assumed was that the women with the largest breasts may have multiple images per view. For the scenario that the women with the largest breasts have 2 or more images per view, so that the entire breast is imaged twice, the resultant mean glandular dose would be 10mGy. However, it is likely women would actually receive an MGD between 5mGy and 10mGy, since usually only part of the breast is exposed twice. In a dose survey of breast screening centres in the UK over the period of 2010 to 2012, less than 0.1% of women had 2 images per view and received an MGD of greater than 5mGy (personal communication, Kenneth Young, 2016).
3.2 Calculations
Number of radiation-induced and screen detected breast cancers
The number of radiation-induced cancers (CR) for one year of screening was calculated by multiplying the lifetime risk of radiation induced cancer (per million women per mGy) for the age of women in each screening round, by the mean glandular dose (mGy) of a screening examination and the number of women screened in each screening round per year (expressed in millions), and then summing over all screening rounds.
The total number of screen-detected cancers, was calculated using the average detection rate of 8.4 per 1000 women from NHSBSP statistics for the year April 2013 to March 2014 for England[footnote 6]. Over-diagnosed cancers are not detected in the absence of screening, so the number of cancers was reduced accordingly, before calculating the number of lives saved due to screening. There is no uniform method of estimation of over-diagnosis, and estimates vary considerably from less than 5% to around 50%[footnote 10]. An independent review of the breast screening programme[footnote 11] suggests that 19% of diagnosed cancers in the screened population (screen detected and interval) are over-diagnosed. This value has been used in this work.
Using the number of radiation-induced and screen detected breast cancers, it was possible to calculate the:
- number of cancers detected in the NHSBSP per radiation-induced cancer
- risk of radiation-induced cancer per screening examination
The total number of lives saved for one year of screening (Ls) was found from the following equation, where Tsc and TI are the number of screen-detected cancers which are not over-diagnosed and the number of interval cancers, r is the breast cancer mortality reduction in the invited population, Mns is the probability of a female with a symptomatic cancer dying of the disease and f is the attendance rate for breast screening (taken from Warren et al[footnote 5]).
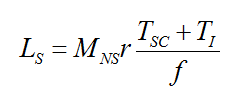
There are several publications[footnote 11] [footnote 12] [footnote 13] [footnote 14] [footnote 15] [footnote 16] [footnote 17] [footnote 18] [footnote 19] [footnote 20] [footnote 21], which have estimated the mortality reduction due to breast screening in the invited population. The mortality reduction in the population invited to screening found in the literature was mainly 20% with a range from 15% to 30%. Therefore this value and range have been used in this work.
The probability of a woman with symptomatic breast cancer dying of the disease was assumed to be 24%, based on Mook et al[footnote 22]. This is reduced from the mortality rate of 50% used in NHSBSP Report 54[footnote 1], taking account of the improvement in treatment over time.
The attendance rate was assumed to be 72%, based on national screening statistics for 1 April 2013 to 31 March 2014 for England[footnote 6].
The number of lives lost due to radiation-induced cancers for one year of screening (LL) was estimated by multiplying the number of radiation-induced cancers (CR) by the fraction of women with radiation-induced breast cancer, who later die from the disease (fR). This fraction was assumed to be the average of the fraction for screen-detected and symptomatic cancers.
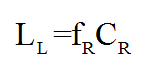
Using the number of lives saved due to screening, and lost due to radiation induced cancers, it was possible to calculate the risk of radiation-induced cancer death per screening examination. If a woman is screened regularly over her lifetime it is possible to calculate:
- the ratio of the number of lives saved due to screening to the number of lives lost due to radiation induced cancers
- the number of women that must be screened regularly over their lifetime in the English screening programme to save a life
4. Results
For the age range 50 to 70 years, with a 20% breast cancer mortality reduction in the population invited to screening and an average MGD to the screened population of 3mGy, it is estimated that, per year:
- 1,770,436 women were screened
- 14,872 breast cancers were screen-detected
- 1071 lives were saved
- 18 to 36 breast cancers were induced due to radiation
- 3 to 7 lives were lost due to radiation-induced breast cancer
The range given in the two bullets above indicates the use of DDREF of 1 and DDREF of 2. There were therefore around 400 to 800 cancers detected by NHSBSP for every cancer induced. The risk of a radiation-induced breast cancer is therefore around 1 in 49,000 for a DDREF of 1, and 1 in 98,000 for a DDREF of 2. The risk of a radiation-induced breast cancer death is around 1 in 26,000 and 1 in 52,000 for a DDREF of 1 and 2 respectively. Finally, the ratios of the number of lives saved due to screening to the number of lives lost due to radiation-induced breast cancer was between 156:1 and 312:1.
Assuming a woman attends all 7 screening rounds between the ages of 50 up to her 71st birthday:
- the risk of radiation-induced breast cancer is between 1 in 7,000 and 1 in 14,000
- the risk of a radiation induced breast cancer death is between 1 in 37,000 and 1 in 74,000
It was found that 238 women must be screened in 7 screening rounds between the ages of 50 and 70 years to save a life.
There is some uncertainty over the breast cancer mortality reduction in the population invited to screening, with the range in the literature covering 15% to 30%. For this range:
- the number of lives saved ranged from 803 to 1606
- the ratio of the number of lives saved due to screening to the number of lives lost due to radiation-induced breast cancer ranged from 110 to 268 for a DDREF of 1, and 220 to 535 for a DDREF of 2
- the number of women who must be screened in all screening rounds to save a life ranged from 158 to 318
The ratios of the number of lives saved due to screening to the number of radiation-induced breast cancer deaths have also been investigated for different sub-groups of the screening population and different age ranges (table 2):
- for the small sub-group of the population (1.8%) with compressed breasts of thickness 90mm and above – the ratio is 94:1 and 187:1 for DDREF of 1 and 2 respectively
- for the even smaller sub-group of the population (<0.1%) who receive an MGD of 10mGy – the ratio is between 47:1 and 94:1, for DDREF of 1 and 2 respectively
It was found that the calculated values of these ratios for the extended age range of 47 to 73 are very similar to the values of the ratios for the age range 50 to 70. Ratio of lives saved due to screening to number of lives lost due to radiation-induced breast cancer was calculated for each screening round age, using the detection rate at the age of each screening round (Table 3).
This differs from Warren et al[footnote 2], where average detection rate over age was used, for this calculation. The ratio of lives saved to lives lost increases with age at screening, with:
- 58:1 for the screening episode at 47 to 49
- 671:1 for the screening episode at 71 to 73
Table 2: Ratio of lives saved due to screening to number of lives lost due to radiation-induced breast cancer.
The ratios are given for the age ranges 50 to 70 and 47 to 73, DDREFs of 1 and 2, three MGDs (described in footnote) and a 20% reduction in breast cancer mortality in the invited population. The bracketed values show results for 15 to 30% reductions in breast cancer mortality. Taken from Warren et al[footnote 5].
| DDREF | MGD (mGy) | Lives saved/lives lost: Age range = 50 to 70 | Lives saved/lives lost: Age range = 47 to 73 |
|---|---|---|---|
| 1 | 3 a | 156 (110-268) | 145 (102-249) |
| 1 | 5 b | 94 (66-161) | 87 (61-149) |
| 1 | 10 c | 47 (33-80) | 43 (31-75) |
| 2 | 3 a | 312 (220-535) | 290 (205-497) |
| 2 | 5 b | 187 (132-321) | 174 (123-298) |
| 2 | 10 c | 94 (66-161) | 87 (61-149) |
a Average MGD for all thicknesses
b Average MGD for breasts of thickness 90mm or greater (1.8% of women with breasts of thickness 90mm or greater)
c MGD assuming women with breasts 90mm or greater have two images per view of the entire breast (<0.1% of women)
Table 3 Ratio of lives saved due to screening to number of lives lost due to radiation-induced breast cancer.
The ratios are given for the age at each screening round, for an MGD of 3mGy and a 20% reduction in breast cancer mortality in the invited population. The range arises from using DDREFs of 1 and 2.
| Screening Round | Age range | Number screened d | Induced cancers | Cancer deaths | Screen detected cancers | Lives saved | Lives saved/ lives lost |
|---|---|---|---|---|---|---|---|
| 1 | 47-49 | 316422 | 7-13 | 1-3 | 2013 | 145 | 58-115 |
| 2 | 50-52 | 296025 | 5-10 | 1-2 | 2297 | 165 | 85-170 |
| 3 | 53-55 | 275092 | 4-8 | 1 | 1666 | 120 | 81-163 |
| 4 | 56-58 | 255490 | 3-6 | 1 | 1654 | 119 | 108-216 |
| 5 | 59-61 | 247386 | 2-4 | 0-1 | 2021 | 146 | 170-341 |
| 6 | 62-64 | 243335 | 2-3 | 0-1 | 2204 | 159 | 241-482 |
| 7 | 65-67 | 226554 | 1-2 | 0 | 2515 | 181 | 386-772 |
| 8 | 68-70 | 226554 | 1-2 | 0 | 2515 | 181 | 496-992 |
| 9 | 71-73 | 225667 | 1 | 0 | 2540 | 183 | 671-1341 |
| All | 47-73 | 2312525 | 25-50 | 5-10 | 19425 | 1399 | 145-290 |
| All | 50-70 | 1770436 | 18-36 | 3-7 | 14872 | 1071 | 156-312 |
d The numbers screened in rounds 1 and 9 assume that the age extension has been fully adopted
5. Discussion
5.1 Comparison of results to NHSBSP Report 54
The number of cancers detected per radiation-induced cancer was found to be 5 times larger in this work compared with NHSBSP Report 541. This is due to the reduction in risk factors used and mean glandular dose assumed. The number of lives saved due to screening to the number of lives lost due to radiation-induced cancers was 300:1 in this work, compared with 113:1 in NHSBSP Report 54[footnote 2] for the same DDREF. This was a result of the combination of the reduction in risk factors and mean glandular dose with the decrease in probability that women with symptomatic cancer would later die from the disease.
5.2 Differences in radiation risk
The radiation risk factors used in this work provided in HPA CRCE-028 report[footnote 8] using the ICRP 103 model[footnote 2], are lower than those in the National Radiological Protection Board (NRPB) model[footnote 23] used in NHSBSP Report 54[footnote 1]. For the age range 50 to 70, on average the risk factors are 3 times lower in this work compared to NHSBSP Report 54[footnote 1] (for the same value of DDREF), causing a proportionate increase in the ratio of the number of cancers detected to the number of radiation-induced cancers.
5.3 Differences in cancer detection
Secondly, the cancer detection rates in this work are 1.05 times higher in this work than NHSBSP Report 54[footnote 1].
5.4 Differences in mean glandular dose
Finally, the MGD used is lower than in NHSBSP Report 54[footnote 1], causing a decrease in the number of radiation-induced cancers, and therefore an increase in the ratio of the number of detected cancers to radiation-induced cancers.
In NHSBSP Report 54[footnote 1] an MGD of 4.5mGy was assumed for the whole population and an MGD of 7mGy was assumed for the sub-group of the population with breasts of thicknesses of 90mm and above. This compares with 3mGy and 5mGy respectively for the whole population and the sub-group with the largest breasts in the present work. This is due to the switch from film-screen to digital mammography systems and the adoption of higher energy x-ray spectra.
Young et al[footnote 9] found the average MGD for a two-view examination using DR mammography systems was about 25% lower than for film-screen mammography. It should be noted that specific manufacturer’s designs can lead to consistently higher or lower doses than this average.
5.5 Differences in mortality reduction
The probability that a woman with symptomatic cancer would later die from the disease was decreased from 50% in NHSBSP Report 54 to 24% in this report, due to the improvement in treatment over time.
5.6 Women receiving higher doses
Some women require multiple images per breast. Young et al[footnote 9] estimated that 1.6% of women had one extra image per view, and 0.4% had two extra images per view. This may be due to re-positioning, re-acquisition due to the quality of the image or ‘jigsawing’ to image the entire breast. The additional dose will depend on the area of overlap of the images of the breast.
As a worst-case scenario one could assume that the women who have 2 extra images per view have the largest breasts (>90mm), and the entire breast is imaged twice, thereby doubling the dose. As seen in table 2 this causes the ratio of lives saved due to screening, to lives lost due to radiation-induced breast cancers for this group of women, to reduce from 94:1 to 47:1. It may be that only part of the breast will be imaged twice and therefore the ratio for these women will be somewhere between these 2 values.
5.7 Comparison of results to Tabar and Marmot
Our analysis found that around 240 women needed to be screened in 7 screening rounds between the ages of 50 to 70 years to save a life. Tabar et al[footnote 16] estimated that 414 women would need to be screened every 2 to 3 years for 7 years to save a life. This corresponds to 145 women screened every 2 to 3 years for 20 years between the ages of 50 to 70 to save a life.
The difference in estimates is likely to be due to the difference between the mortality rate at the time of the Swedish Two-County Trial[footnote 16] and the more recent estimate used in our calculations.
Marmot et al[footnote 11] found that inviting women aged 50 to 70 every 3 years prevents around 1300 breast cancer deaths a year. We estimated that screening prevents around 1100 breast cancer deaths a year. The difference between the value found in this work and Marmot et al[footnote 9] is likely to be due to the uncertainty associated with estimating the amount of over-diagnosis and the mortality rate for women with symptomatic cancers. If over-diagnosis were 5% rather than 19%, the number of lives saved per year would increase from 1071 to 1256. If the assumed mortality rate for symptomatic women were increased from 24% to 28% this would increase the number of lives saved per year from 1071 to 1249.
6. Limitations
A limitation of this work is that only the number of lives saved due to screening and lives lost due to radiation-induced cancers has been considered and not the number of life-years gained or lost. Estimating the number of life-years saved or gained would take into account that deaths due to induced cancers are likely on average to occur later than the deaths prevented by screening.
An additional limitation of this work is that the additional radiation exposure due to mammography at assessment was not considered. However the average percentage of women recalled for further imaging in the NHSBSP is only about 4%[footnote 6]. Since repeat imaging is usually more limited than the original screening the increase in the population dose due to assessment mammography is likely to be less than 4%.
7. Conclusion
The number of deaths caused by radiation-induced cancers is estimated to be around 150 times smaller than the number of lives saved due to screening.
8. Acknowledgements
Prepared by: LM Warren, DR Dance, K Young.
The authors would like to thank Dr Jennifer Oduko, for her assistance with patient dose data, Dr Matthew Wallis for his feedback on the detection rates and mortality rates used and Professor Sue Moss for her feedback and advice on the analysis performed.
The review was funded by Public Health England (PHE) and by Cancer Research UK.
9. References
-
Joint working party of the NHSBSP National Coordinating Group for Physics Quality Assurance and the National Radiological Protection Board. NHSBSP Publication No 54: Review of radiation risk. 2003. ISBN: 1 871997 99 ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
The International Commission on Radiological Protection. Annuals of the ICRP. Publication 103. The 2007 Recommendations of the International Commission on Radiological Protection. 2007. ISBN: 978-0-7020-3048-2 ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radia- tion. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII— Phase 2 2006. DOI: 10.17226/11340 ↩
-
United Nations Scientific Committee on the Effects of Atomic Radiation. Effects of Ionizing Radiation. 2008. ISBN: 978-92-1-142263-4 ↩
-
Warren LM, Dance DR, Young KC. Radiation risk of breast screening in England with digital mammography. Br J Radiol 2016; 89: 20150897 ↩ ↩2 ↩3 ↩4 ↩5
-
National Statistics. Breast Screening Programme, England - 2013-14 [Internet]. 2014. Available from: http://www.hscic.gov.uk/catalogue/PUB16803 ↩ ↩2 ↩3 ↩4
-
ISRCTN registry. Nationwide cluster-randomised trial of extending the NHS breast screening age range in England [Internet]. Available from: http://www.controlled-trials.com/ISRCTN33292440 ↩
-
Wall BF, Haylock R, Jansen JTM, Hillier MC, Hart D, Scrimpton PC. HPA-CRCE-028: Radiation risks from medical X-ray examinations as a function of the age and sex of the patient. Health Protection Agency 2011. ISBN: 978-0-85951-709-6 ↩ ↩2
-
Young KC, Oduko JM. Radiation doses received in the United Kingdom Breast Screening Programme in 2010 to 2012. BJR 2016; 89: 20150831 ↩ ↩2 ↩3 ↩4 ↩5
-
Puliti D, Duffy S, Miccinesi G, de Koning H, Lynge E, Zappa M, Paci E, Overdiagnosis in mammography screening for breast cancer in Europe: a literature review, J Med Screen 2012;19:42-56 ↩
-
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG and Wilcox M. The benefits and harms of breast cancer screening: an independent review. BJC 2013;108:2205–40. ↩ ↩2 ↩3
-
Gotzsche PC, Nielsen M, Screening for breast cancer with mammography (Review), The Cochrane Collaboration, 2012; DOI:10.1002/14651858.CD001877.pub5 ↩
-
Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151:727–737 ↩
-
Canadian Task Force on Preventive Health Care, Recommendations on screening for breast cancer in average-risk women aged 40–74 years. Can Med Association J 2011;183:1991–2001 ↩
-
Demissie K, Mills OF and Rhoads GG, Empirical comparison of the results of randomised controlled trials and case-control studies in evaluating the effectiveness of screening mammography, J Clin Epidemiol 1998;51:81-91 ↩
-
Tabar L, Vitak B, Chen TH-H, Yen a. M-F, Cohen A, Tot T, et al. Swedish Two-County Trial: Impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260:658–63 ↩ ↩2 ↩3
-
Broeders M, Moss S, Nyström, Njor S, Jonsson H, Paap E, Massat N, Duffy S, Lynge E and Paci E (Euroscreen group), The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies, J Med Screen 2012;19:14-25 ↩
-
Weedon Fekjær, Romundstad PR and Vatten LJ, Modern mammography screening and breast cancer mortality: population study, BMJ 2014;348:g3701 ↩
-
Lauby-Secretan, Scoccianti C, Lommis D, Benbrahim-Tallaa, Bouvard V, Bianchini F and Straif K, Breast cancer screening – Viewpoint of the IARC working group, N Engl J Med 2015; DOI: 10.1056/NEJMsr1504363 ↩
-
Nyström L, Bjurstam N, Jonsson H, Zackrisson S and Frisell J, Reduced breast cancer mortality after 20+ years of follow-up in the Swedish randomised controlled mammography trials in Malmö, Stockholm and Göteborg, J Medi Screen, DOI: 10.1177/0969141316648987 ↩
-
Duffy SW, Yen Ming-Fang A, Chen TH, Chen SL, Chui SY, Fan JJ, Smith RA, Vitak B, Tabar L Long-term benefits of breast screening. Breast Cancer Manage 2012;1: 31–38 ↩
-
Mook S, Van ’t Veer LJ, Rutgers EJ, Ravdin PM, van de Velde AO, van Leeuwen FE et al, Independent prognostic value of screen detection in invasive breast cancer, JNCI, 2011;103;1-13 ↩
-
National Radiological Protection Board. Estimates of late radiation risks to the UK population. Doc NRPB 1993;4:15–157 ↩
