Asymptomatic Avian Influenza Surveillance Study (AAISS) protocol
Published 29 June 2023
Summary
Study short title
Asymptomatic Avian Influenza Surveillance Study (AAISS).
Study full title
Asymptomatic avian influenza in exposed persons or persons working with known or suspected infected birds or non-human mammals enhanced surveillance study – longitudinal virological positivity surveillance.
Study details
Study sponsor and funder
UK Health Security Agency (UKHSA)
Governance
Study management group reporting to the Avian Influenza Technical Group and incident management team and through this to the incident director.
Surveillance design, participants and sample size
Surveillance design: Longitudinal, prospective cohort
Participants: Asymptomatic poultry workers and other exposed members of the public involved in culling birds on infected premises, including in domestic settings (backyard flocks), wild bird exposures and any non-human mammalian cases.
Sample size: 1,000
Primary surveillance study objectives and outcomes
Objectives:
Detection of avian influenza in nose and throat samples from potentially exposed individuals to determine:
- whether they can acquire the infection
- what the frequency of such infections is
- what the risk factors for acquiring infections are
Outcomes:
Polymerase chain reaction (PCR) positivity for avian influenza A(H5N1) clade: 2.3.4.4b or other avian influenza subtype and clade.
Timepoint(s):
Baseline sample at study entry, 2, 5 and 8 days after study entry.
Secondary surveillance study objectives and outcomes
Objectives:
To assess whether an immune response is mounted to infection, by obtaining serological samples to assess immunity to the currently circulating avian influenza clade.
Outcomes:
Serological positivity to avian influenza A(H5N1) clade: 2.3.4.4b
Timepoint(s):
Baseline sample and 21 days after study entry for asymptomatic participants. Any participant that has a positive virological sample will have further serological assessments.
Planned surveillance study procedures
Nose and throat swab samples at baseline (entry to study), 2, 5 and 8 days after exposure.
Capillary sample serological samples, for all who agree, at baseline and 21 days after enrolment. For participants who have positive virological samples, venous, capillary, and oral fluid serological samples are likely to be taken at point identified as positive for virology and 21 days later.
Questionnaire, obtaining study subject demographics, contact details and details of the exposure and personal protective equipment (PPE) used and/or antiviral medications taken, seasonal influenza vaccination status. Follow-up questions for any positive participants for outcome and symptoms.
Comparator groups
There will be no comparator group generated during the study although international and historic data will be considered.
Study investigators
Principal investigator: Richard Puleston
Investigators: Meera Chand, Katja Hoschler, Susan Hopkins, Deepti Kumar, Angie Lackenby, Nick Watkins, Oluwakemi Olufon, Paula Blomquist, Michelle Chapman, Neil Cunningham, Carmellie Inzoungou-Massanga, Maaike Pietzsch, Nick Machin, Rachel Jones, Ian Brown (Animal and Plant Health Agency (APHA)), Andre Charlett, Mark Baker, Will Welfare, Sophia Makki.
Statistician: Andre Charlett, UKHSA
1. Background to the study
There is currently an unprecedented outbreak of influenza A(H5N1) in birds in the UK, which is part of a wider global epidemic of avian influenza. The clade (virus originating from a common ancestor) present in the UK is primarily 2.3.4.4b. This has caused substantial mortality in both farmed and wild birds and a stringent animal health response is in place.
Avian influenza presents both an immediate and a potential risk to human health. The immediate risk is of the virus infecting persons exposed to birds and causing severe disease in individuals; and the potential risk is that the virus may mutate to become transmissible between humans and cause an epidemic or pandemic.
In terms of immediate risks, historically influenza H5 zoonotic infections have been associated with severe, although uncommon, infections in humans. There have been very few human cases internationally of the current A(H5N1) clade: 2.3.4.4b, so it is not possible yet to assess whether a similar severity profile will be seen as with historical cases. An asymptomatic testing programme in winter 2021 to 2022 of approximately 120 individuals in England did identify one positive case, however this case had extensive, unprotected contact with birds in a domestic setting (Oliver and colleagues, 2022). To date, few other people have been detected worldwide who were positive for this specific clade. These detections are within an unknown sampling frame and surveillance of exposed persons is generally limited. There is no known human-to-human transmission of this virus.
Asymptomatic infection may give the virus the opportunity to acquire changes which enable it to cause infection in and transmit between mammals including humans, increasing the risk of a human-to-human transmissible strain. It is therefore important to establish an understanding of whether the current virus can cause asymptomatic infection in humans and at what frequency and whether an immune response is mounted to infection. Understanding the total number of infections allows us to make proportionate assessments of the severity of infection and overall risk to human health. Supporting this approach, the World Health Organization (WHO) has recommended testing of asymptomatic persons with significant contact with infected poultry or mammals, or potentially infected environments when unprotected with PPE (WHO, 2022).
Currently, there is very little information on whether the current virus (A(H5N1) 2.3.4.4b) causes asymptomatic infection in humans (and how frequently) as there are few human cases reported internationally. With the outbreaks of avian influenza in birds, there are multiple instances where people have been exposed to or have been working closely with infected birds, which are opportunities for infection. We need to establish whether infection occurs as these events may be an early sign that the virus can adapt towards human infection or may give the virus the opportunity to acquire further changes which confer mammalian adaptation, increasing the risk of a human-to-human transmissible strain. Additionally, we need to determine whether these people develop immunity to avian influenza. Understanding the total number of infections and immunity status allows us to make proportionate assessments of the severity of infection and overall risk to human health.
This enhanced surveillance study therefore aims to investigate whether persons working with birds (or non-human mammals) known or suspected to have avian influenza acquire asymptomatic infection, through serial swabbing post involvement in the incident and whether they develop an immune response to the virus through serological testing. The proposed enhanced surveillance study participants will primarily be persons employed to depopulate infected farms; however, other farm workers or members of the public (for example with backyard flocks) who are also working with, or have had contact with, birds or animals infected or suspected to be infected with avian influenza can be included.
2. Surveillance study design and procedures
2.1 Study inclusion
Inclusion criteria
a) Any person who has been exposed to or has been working with birds or non-human mammals known to be or suspected of being infected with avian influenza (with or without wearing PPE) defined as:
- persons who are exposed prior to the identification of an incident, who were not
wearing appropriate PPE (and using antivirals, if applicable) at all times of exposure, for example farm workers, owners of backyard flocks and veterinary staff - persons working with the birds or animals during the response to the incident, whilst wearing appropriate PPE (and using antivirals, if applicable), for example workers involved in the culling, disposal and clean-up operations at infected premises
- non-occupational exposures, for example members of the public (or others) inadvertently handling sick or dead birds or animals, or their faecal matter that is confirmed to be infected with avian influenza
with the following exposures (regardless of the use or not of PPE or antiviral prophylaxis):
- working on an infected premises with direct involvement in the culling of birds or non-human mammals, depopulating, clearing up dead or sick birds, sick animals, bird feathers, excreta, cleaning the barns or facility after depopulation
- working on an infected premises directly providing animal husbandry to the birds or non-human mammals, for example entering the barns or facility to feed the birds, or clear up excreta or handling birds
- directly handling captive or wild birds or non-human mammals – dead or alive – where avian influenza is suspected or confirmed or being close to them (less than 2 metres)
Contacts (household and prolonged close contact) of any positive individuals from the study identified (or positive individuals outside of the surveillance study), will also be eligible.
b) Aged 18 years or over
c) Will be resident in England in the 21 days from enrolment
d) Able to understand the information about the surveillance study and provide informed consent
e) Willing and able to undertake self-administered swabbing of nose and throat and if they are willing also the capillary blood serological samples
Exclusion criteria
a) Influenza symptoms at baseline – entry to surveillance study (for example, fever, recent onset of respiratory symptoms (within last 2 weeks), conjunctivitis). These individuals will require a clinical diagnostic test including for avian influenza and will be the responsibility of the local health protection team (HPT) to manage according to established protocols.
b) Unable to provide informed consent or unable or unwilling to consent to self-sample for the surveillance study period.
Individuals working at multiple premises sequentially
Contractors may move between infected premises. This does not preclude recruitment repeatedly, however information on exposures at all relevant premises should be included in the questionnaire. If a participant is already in the virological sampling phase of the surveillance study but is now working at a different infected premises that is being included in the study, they would not be re-recruited. However, if they have completed their virological sampling from a former exposure, they would be eligible for re-recruitment.
2.2 Approach, recruitment, and consent
We will approach all eligible individuals at the infected premises site for inclusion, subject to the inclusion and exclusion criteria being met. We will also approach other workers at the site and any other members of the public who have been working with or exposed to the birds or non-human mammals. In other settings, for example backyard flock incidents, all exposed persons will be approached for inclusion.
The participant will be spoken to about the study and given written information, will have the opportunity to ask questions and discuss the surveillance study, be advised of risks and that they may withdraw at any time.
Recognising that the potential participants may not have English as their first language and varying literacy levels, the written information will be translated into the relevant languages of the workers as far as possible. Pictorial methods, meetings and/or verbal communication with translators with potential participants will also be used as appropriate.
Potential participants will be informed that if they test positive from their nose and throat samples, they will be informed immediately and may be asked to isolate at home or go to hospital. As positive individuals are likely to be managed as a high consequence infectious disease (HCID) case, the information and consent will include that admission for affected individuals may occur and may not be to the nearest hospital, but to a specialist HCID unit. Individuals that agree to participate will provide their signature on the consent form. However, where recruitment is done remotely, the consent will be obtained verbally. They will be given written information, including surveillance study contact details, to retain. Consent materials and forms will be available in the appropriate languages and/or a telephone interpreter service may be used.
Some sites which could be potentially included in the study, are restricted in physical space for staff to deploy to do the enrolment. Where this is the case, we will attempt to recruit participants from the site remotely by phone. Verbal consent (recorded) will then be obtained over the phone and will be noted on the consent form. A copy of the consent form will be forwarded to the participant’s email address. The participant questionnaire will be completed over the phone. Test kits will be mailed or couriered to the participants. The rest of the study will be completed as per the extant protocol.
2.3 Questionnaire and sampling
All participants will be asked to fill in a short electronic questionnaire (either using the participants own smart phone or UKHSA Rapid Investigation Team (RIT) devices) confirming that they are asymptomatic and describing their level of exposure to infected birds, and use of PPE and antivirals, and seasonal influenza vaccine status.
For workers who deliberately enter the premises in response to detection of infection (for example those undertaking culling), there will be a swab (viral swab) at baseline (before work is started) and then serial viral swabs until the incubation period has completed (day 0, 2, 5 and 8). For other already exposed persons, there will be serial viral swabs after exposure (day 2, 5 and 8). For both groups, at baseline a capillary sample of blood will be obtained for serological sampling and then again at day 21 post enrolment. Sampling will all be self-administered.
The start date for the participant will be the first day they had direct onsite involvement in the incident being investigated by the surveillance study. It is recognised that some of these workers will have been involved in previous incidents; however, it would be the date of involvement in the current incident that is of interest. Data on prior exposures and/or prior working with infected birds or non-human mammals will be obtained. If the involvement in the current incident extends over multiple days, day 0, will still be the first date of direct involvement. A swab will still be taken on days 2, 5, and 8 after this initial date; and a final swab, 8 days after the last involvement in the current incident.
The choice of sample for the virological sampling is a nose and throat viral swab in Remel media or other suitable media; and for the serological sampling a capillary blood sample undertaken using Tasso self-sampling kit or equivalent. The first swab and serological sample will be a self-administered swab and capillary sample done under staff observation; and the individual will be given further kits to self-administer swabs and capillary sample and return by post. Individuals will receive text message reminders to undertake their swabbing and capillary samples. The participants will also receive information to remind them of the requirements should they become unwell at any time. Participants would still be subject to the normal HPT-led passive or active surveillance which would follow standard procedures and data would be shared with the relevant HPT to enable this.
There are other settings and scenarios that warrant particularly serological sampling of exposed workers, but for which the exposure was some time ago. There will be no value in taking sequential virological samples or paired serological samples due to the time since exposure, but a single virological sample (nose and throat) and serological samples at one point in time could be indicated.
Should an individual be PCR-positive, they will have follow-up PCR and serological sampling to confirm the presence of infection as part of the standard workup for people acquiring an HCID. Serological sampling in this instance would be to obtain a venous blood sample as soon as possible after the positive virological test and then again at 21 days post first involvement in the incident but is also likely to include capillary and oral fluid serological samples too. This is standard practice for a confirmed case and would be undertaken by the admitting or HCID team. While waiting for the follow-up PCR the participant will be required to isolate. If the follow-up PCR is positive, they will continue their isolation period as per the standard management of such individuals. A follow-up questionnaire for these positive cases will also be administered.
2.4 Sampling kits
The nose and throat swab collection kit manufactured from component parts by the public health testing laboratory will be used. The components of the nose and throat sampling kits are:
- flocked nose and throat swab
- M4RT virus transport medium
- sealable plastic envelope
- green top sealable secondary plastic container
- request form
However, as highlighted elsewhere, the PCR sample collection kits will be provided to lay people who could have been exposed to the avian flu for them to self-swab. Therefore, warnings will be included in the package insert to further mitigate the fact that the kits will be used by lay people (Appendix 1).
The capillary blood sample kits to be used are manufactured by Tasso Inc or equivalent device. These are designed for self-sampling in the community and have recently acquired CE marking. There is precedent with these having been used before in a similar self-administered study during the COVID-19 pandemic (Vusirikala and colleagues, 2021).
For study participants where it is particularly important to assess immunity retrospectively several serological samples at a single point in time would be sought from the participants including venous (sera), capillary blood and oral fluid samples.
2.5 Testing and sample handling
Baseline samples from recruitment will be returned by courier to the public health laboratory for processing (7 days a week processing). The follow up samples will be posted by the participant for return directly to the public health laboratory. Appropriate biocontainment Category B, UN3373 packaging will be used and there will be pictorial instructions for the safe packaging of the samples returned by post by participants.
Virological testing will be influenza A generic PCR followed by subtyping PCR on any positive result, whole genome sequencing and viral culture if appropriate. The assay tests for influenza A (human and avian influenza), B (human influenza) and the common cold virus, respiratory syncytial virus (RSV); it is not possible to test solely for influenza A. Virological samples will be processed on receipt; however, the capillary blood serological samples will be stored in the public health laboratory storage facilities until an assay is validated for use. A rise in antibody titres will be sought from the paired sera.
Any PCR positive cases may have further serial sampling (swabs and serology) with separate consent as part of the management of confirmed cases. This is not under the governance of this protocol.
The laboratory may retain the virological samples for up to one year for public health uses (for example, repeat testing, viral isolation). Serological samples will be stored for up to 5 years to enable analysis once a serological assay is developed.
2.6 Participants who become unwell
If at any stage, staff become aware that the participant is symptomatic, they will refer the participant to the relevant public health protection team for further assessment and follow up according to standard protocols. Additionally, participants will be provided with verbal and written advice at baseline on what to do if they become symptomatic during the surveillance study or subsequently and the routine follow up that the HPT will do.
2.7 Reporting of results
Negative virology results will be relayed by the laboratory to study staff. Participants will be advised at enrolment that negative virological results will be provided to the participant at the end of their participation in the surveillance study either verbally or by text. Positive results from the nose and throat samples (A(H5N1) or other influenza) will be relayed immediately from the laboratory to study staff and the relevant public health protection team and the HCID activation cascade, including the relevant infectious diseases unit. A case in which A(H5N1) is detected will be managed according to the public health guidelines and HCID protocols. An operational plan for asymptomatic or symptomatic person infection management will be implemented. Other relevant stakeholders will also be notified as relevant for example, APHA.
As it is likely to be some time before the serological analysis can be undertaken, the serological results will not be returned to the participants as the results will be unlikely to influence any public health actions, however the data will be linked back to the participant’s records for further analysis.
3. Results analysis
The surveillance study data will be analysed using simple descriptive analysis, for example, counts and proportions. Plots of serological parameters may be presented. As there will be no comparator group, analytical statistics are unlikely to be required. There will be no statistical threshold for the study as there will be no comparator group.
3.1 Sample size
Of prime importance in the consideration of the size of the surveillance study is that of how precisely we wish to measure the estimated prevalence of asymptomatic infection. From testing undertaken previously we would anticipate that the prevalence will be low. The cluster sampling design needs to be accounted for in such designs by inflating the required sample size for a simple random sampling design of individual exposed farm worker by the design effect.
An approximate estimate of required numbers (sample size) can be obtained using the formula: 1.96 squared, multiplied by the prevalence (p), multiplied by one minus the prevalence (1-p), and divided by the desired precision (d) squared; where the prevalence (p) is one that might be anticipated to be observed, and the precision (d) is the desired half-width of the 95% confidence interval (CI) of the estimated prevalence.
This can also be expressed as the following equation:
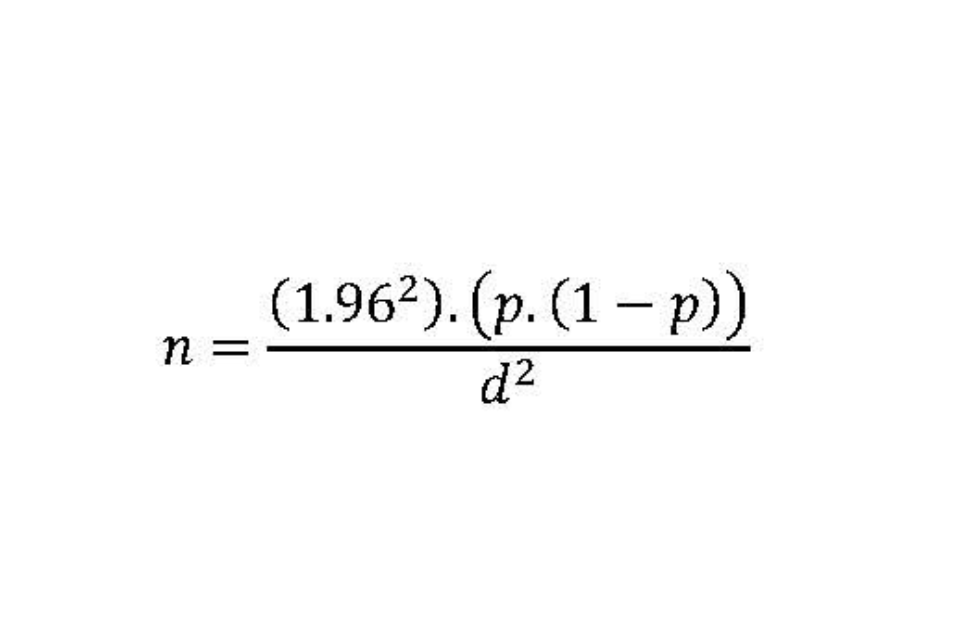
Thus, if we anticipate the prevalence to be 0.5% (0.005) and we wish to measure this precisely; that is, a half width of a 95% CI of 0.005; then a sample size of around 750 subjects would be required.
Cluster sampling of exposed individuals within affected premises requires an inflation to the sample size to allow for the design. The design effect (DE) equals one plus the following calculation: the square of the coefficient of variation (CV) of the number of recruits across the affected premises (cluster size), plus one; multiplied by the average number of recruits per premises; then subtract one; multiplied by the intraclass correlation coefficient (p). This can also be expressed as the following equation (with the average number of recruits per premises expressed as a lower-case ‘n’ with a line above it):
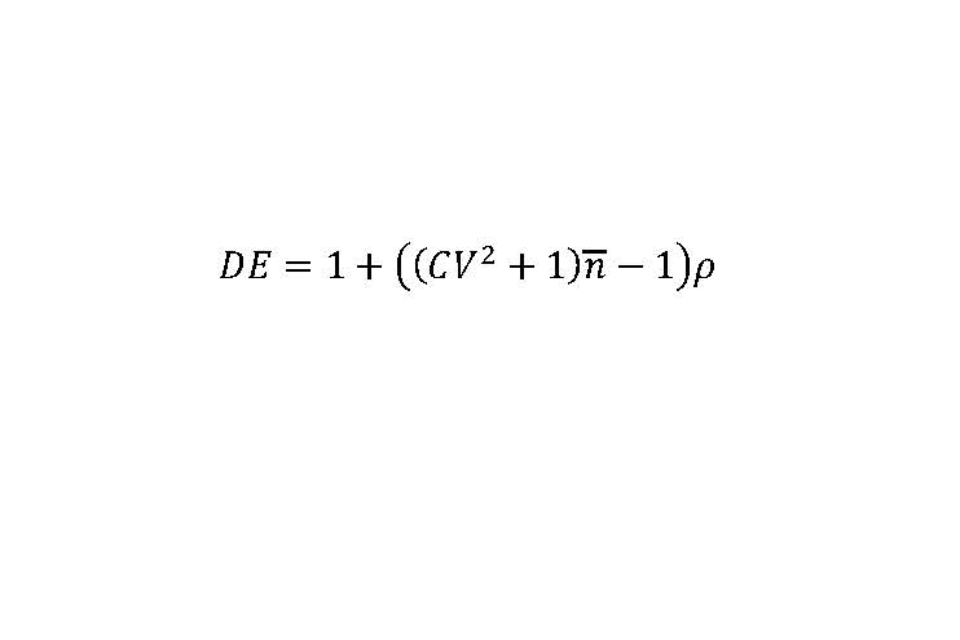
The intraclass correlation coefficient (p) is a measure of homogeneity in prevalence between clusters.
We anticipate considerable variation in the number of recruits between affected premises, from around 5 on small to 50 on large premises. It has been assumed that the mean number of exposed individuals per affected premise that are recruited is 27.5. If this range represents 6 standard deviations, then an estimate of the CV would be 0.27.
There is no estimate available for the ICC, however, there is a well-recognised relationship between the anticipated prevalence and the ICC (Gulliford and colleagues, 2005). We have therefore assumed that an ICC of 0.01 is reasonable for design purposes as the anticipated prevalence is low which provides an estimate of the DE of 1.29. Thus, the total sample size required is 968, which for practical purposes is rounded to 1,000.
Due to the prospective nature of this study, there is no sampling frame of affected premises. Participants will be able to participate more than once, if recruited again at an alternative site or incident. It would be anticipated that this would require recruiting from around 40 affected premises; however, due to the variation in the size of clusters the study we may have to recruit between 20 and 100 infected premises, based on the number of staff deployed hitherto in previous incidents.
3.2 Analysis of participants data
All data from evaluable participants (those with complete or partial data) will be included in the analysis.
3.3 Surveillance study interim assessment points
As this study will be undertaken over a relatively short time, it should not be necessary to do a formal analysis of the data on an interim basis, but this could be done if required. However, if the sampling determined that some of the participants were infected with avian influenza, immediate actions would be required to control the risk. Therefore, the results of the virological work will be made available through the course of the investigation to the investigators rather than at the end. Should a participant be identified as infected with avian influenza, the investigators will be notified as would the relevant public health protection team and other relevant partners.
3.4 Criteria for stopping the study
This surveillance study may be stopped if after sampling 500 participants, no evidence of infection with avian influenza is discovered in any participant. This would allow proportions of 1% or higher to be ruled out (the upper limit for 95% CI for 0 out of 500 is 0.7%). The decision to stop early would be the decision of the study steering group. Additionally, the study would be terminated if recruitment was not progressing satisfactorily. Depending on the nature of any adverse events, these may also contribute to decisions on terminating the study if deemed sufficiently serious.
3.5 End of investigation
The end of the investigation will be the point which the required number of participants have been recruited (or recruitment ceased for other reasons including inability to recruit sufficient participants), samples analysed, and all the study data has been entered into the database, any issues resolved, and analysis undertaken.
Appendix: Sampling kit warnings in the package insert
Do not use if package arrives damaged or is missing components.
Check the use-by date on the tube and swab. Do not use the test device after its use-by date.
This device is for single use only.
Store out of the reach of children.
These kits are designed for human use only.
Clean spills from the plastic tube using paper towels and household cleaner.
Get medical advice if the solution is accidentally swallowed, or comes into contact with the skin, mouth or eyes.
If you have a nose piercing swab the other nostril. If pierced on both sides, remove the piercing on one side before swabbing that nostril.
If you have had a nosebleed within the last 24 hours, swab the other nostril or wait 24 hours.
Do not use excessive force or pressure or bend the swab when collecting swab samples, as this may break the swab.
Try not to touch your tongue, teeth, cheeks, gums, or any other surfaces with the swab’s fabric tip, as this may spoil your sample. If this does happen, complete and return the test anyway.
