Veterinary Medicines Pharmacovigilance Annual Review 2017: Summary
A summary of results from surveillance work carried out by the VMD's pharmacovigilance team concerning reported adverse events.
Pharmacovigilance
This highlights the key results from the Veterinary Medicines Pharmacovigilance Annual Review 2017.
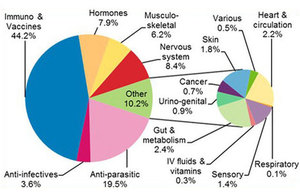
The annual review summarises the 6,721 UK adverse events in animals, humans and the environment after use of veterinary medicines and other products reported to VMD in 2017. This includes a link to a data dashboard, where you can filter the year’s pharmacovigilance data by the species of animal affected and the type of product used. There is also a list of changes made to product literature, as a result of pharmacovigilance information received during this and previous years.