New case study illustrates how MHRA and OxSonics help cancer drugs to go the extra mile
How MHRA and OxSonics help cancer drugs go the extra mile.
OxSonics CEO Dr Colin Story
The Medicines and Healthcare products Regulatory Agency (MHRA) has helped OxSonics take a big step towards developing a revolutionary generation of ultrasound devices which aim to enhance delivery of anti-cancer drugs deep into solid tumours.
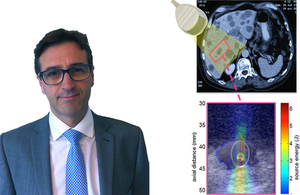
OxSonics is a relatively new company which believes its cutting edge technology has the potential to deliver anti-cancer drugs to those cells that lie farthest from blood vessels.
A case study published today illustrates how the Innovation office at MHRA provided informal access to world-leading expertise. It is hoped that following clinical trials the approach will result in better health outcomes for people currently facing terminal illness.
MHRA director of licensing, Dr Siu Ping Lam said:
Our experts have the knowledge and experience to helpfully support SMEs to develop their innovative products. As well as being a regulator, we are passionate about helping companies develop products that could benefit patients and improve public health. Through the MHRA innovation office and scientific advice service, we are able to offer information and guidance to help companies, large and small, navigate scientific and regulatory requirements effectively.
The new innovation office at MHRA helped by:
- co-ordinating and facilitating an initial meeting to clarify the classification of the particles and the ultrasound hardware – this in turn ensured that OxSonics could work to the correct regulatory standards
- providing advice on the scientific and regulatory aspects of the toxicology studies in order to help OxSonics take their development forward
- offering the opportunity to hold a scientific advice meeting to review the planned pre-clinical toxicology protocols that would need to be in place in order to gain approval to start clinical investigations
Dr Colin Story, CEO, OxSonics said:
MHRA’s innovation office is an excellent resource for companies working to develop innovative technologies. The support offered by MHRA through its innovation office has been invaluable to OxSonics. The innovation office has helped us with product classification and defining pre-clinical studies and MHRA will continue to support us by providing informal access to world-leading expertise.
According to Dr Story, there are no similar devices either on the market or in development.
The case study was developed as part of our commitment to the Medicines Manufacturing Industry Partnership (MMIP). MMIP was launched by the Association of the British Pharmaceutical Industry (ABPI) and the BioIndustry Association (BIA) earlier this year. It brings the UK’s medicines manufacturing industry together to create an attractive and innovation rich environment to drive UK competitiveness, and build international recognition in medicines manufacturing.
Background
- The innovation office, launched in March 2013, is one of the ways MHRA supports the Prime Minister’s Life Sciences strategy. This strategy calls for the encouragement of life science industries as a future growth area for the country. It is designed to help companies overcome barriers and it creates incentives for the promotion of healthcare innovation.
- See MHRA’s innovation office for enquiries
- MHRA is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. Underpinning all our work lies robust and fact-based judgments to ensure that the benefits justify any risks. MHRA is a centre of the Medicines and Healthcare Products Regulatory Agency which also includes the National Institute for Biological Standards and Control (NIBSC) and the Clinical Practice Research Datalink (CPRD). MHRA is an executive agency of the Department of Health. www.mhra.gov.uk
- About OxSonics Limited: OxSonics was established in July 2013 to develop and commercialise ground-breaking advancements in the field of therapeutic ultrasound invented at the University of Oxford’s Institute of Biomedical Engineering. OxSonics “SonoTran” drug delivery platform has the capability to overcome one of the greatest challenges facing solid tumour cancer therapy by delivering drugs throughout tumours, including to the areas that lie furthest from blood vessels. SonoTran can be applied to any cancer drug. A major benefit of OxSonics’ technology is the ability to provide real-time on-screen feedback to the clinician as to where and when drug delivery is taking place. OxSonics is based in Oxford, UK. For more information visit the OxSonics website.
Media enquiries
News centre
MHRA10 South Colonnade
London
E14 4PU
Email newscentre@mhra.gov.uk
Telephone (including out of hours): 020 3080 7651