Private coronavirus (COVID-19) testing validation: government response
Updated 14 February 2022
1. Executive summary
The Department for Health and Social Care held a public consultation, opening from 8 April 2021 and closing on 5 May 2021, on its proposal to introduce a requirement that all COVID-19 tests placed on the UK market undergo a mandatory validation process to assess their performance, above and beyond CE marking. The consultation response summarises the responses to the consultation, and how the department has used them to shape the policy.
The government received broad support for the policy aims outlined in the consultation, in particular the need to implement a minimum performance standard for COVID-19 tests available on the UK market and provide clarity for consumers on the tests they purchase. This set out a clear signal that stakeholders support intervention to address the issues, by specifically pursuing a policy to introduce a requirement for the validation of COVID-19 tests.
The majority of respondents to the question agreed that the validation process should be paid for through fees rather than subsidised by the taxpayer. However, in response to some of the concerns raised about the cost of validation and in particular, the proposed fees, we have decided to set a lower rate for small and medium sized enterprises (SMEs). In addition, in response to consultation feedback, we continue to assess options for lower fees for the verification process.
After considering the responses to the consultation, we have decided to take steps to lay a Statutory Instrument under the Medicines and Medical Devices Act 2021 (MMDA) to create a regulatory requirement for the mandatory approval of antigen and molecular diagnostic tests for COVID-19. However, at this stage, we have taken the decision not to make the laboratory element of validation a mandatory requirement. Initially, we have decided to implement mandatory validation of COVID-19 tests via a desktop review delivered by DHSC. The government intends to introduce mandatory laboratory technical validation at a later date.
Extensive guidance will set out more detail on some of the questions raised throughout the consultation, including on the transitional arrangements for products already on the market and the verification process.
2. Introduction
2.1 About the consultation
The government has proposed establishing a requirement that all antigen and molecular detection COVID-19 tests that exist on the UK market undertake a mandatory validation process, making it a legal requirement to do so.
This public consultation was carried out in order to obtain feedback from businesses involved in the manufacture, distribution and retail of COVID-19 tests on the proposals put forward. In addition, the Medicines and Medical Devices Act (MMDA) requires that, before making regulations under section 15, a public consultation must be carried out.
The consultation response will present a summary of the feedback received from stakeholders and the government’s view in light of this feedback. In this document we have grouped the analysis of consultation and government responses in the same way the sections appeared in the consultation document.
2.2 Respondents and engagement
We had 43 written responses to the public consultation, 27 of which were from organisations and 16 were from individuals. The consultation ran for 4 weeks throughout April, closing on the 5 May 2021. The organisations that responded included large and small manufacturers, chemists, retailers, trade associations, professional bodies, local authorities, universities and individual experts.
During the consultation period, virtual stakeholder engagement meetings were held to gather feedback and comments on the government’s proposals. We held 2 well attended roundtables with industry stakeholders, one of which was a Ministerial roundtable chaired by Lord Bethell shortly after the close of the consultation. The proposal to introduce mandatory validation for COVID-19 tests is a UK wide policy. As such, we have sought to work closely with colleagues in the Devolved Administrations (DAs) and we have engaged on a weekly basis with officials and updates on the policy progress shared for their input. DA contributions have been taken on board and incorporated into the regulations.
2.3 Policy objective
Testing is a vital part of the United Kingdom’s response to COVID-19. The government wants to ensure that test kits sold privately in the UK are of the same high quality as those used by the NHS. The NHS and government procurement of COVID-19 tests has included rigorous clinical evaluation to assess the quality of the test product. The government’s proposals aim to ensure all tests available in the UK meet the same high standards through the introduction of this legislation.
This is to avoid undermining national health efforts in containing the pandemic by poorly performing tests giving inaccurate results. We also want to make it easier for consumers and employers to compare the performance of COVID-19 tests to help them make informed decisions on the tests they purchase.
Entry into the private SARS-CoV-2 (COVID-19) test product market is currently controlled by CE marking, which is a self-declaration process for the majority of the COVID-19 test products available on the UK market. The performance declaration made as part of CE marking is not required to be independently verified ahead of sale and there is no legally binding or consistent process for establishing the performance of COVID-19 tests. Evidence shows that a significant number of COVID-19 tests have failed to replicate their stated performance for their intended use during independent validation. In addition, there is currently no minimum threshold for performance of a COVID-19 test product in terms of its ability to detect positives and negative test results accurately. As such, we propose that government intervention is necessary in order to enforce minimum performance standards for COVID-19 test products to protect the health and interests of the public.
2.4 The government’s proposal
The government is proposing to introduce legislation under the Medicines and Medical Devices Act (MMDA) 2021, to create a regulatory requirement for the mandatory authorisation of diagnostic tests for COVID-19. This will make it mandatory for antigen and molecular detection tests for COVID-19 to undergo an independent validation process to assess whether they meet a minimum performance threshold. This will provide the confidence that tests for sale in the UK meet minimum standards in their sensitivity and specificity. Those tests that do not pass the validation assessment and meet a minimum performance threshold will not be legal for sale in the UK.
We are proposing an approval regime that would require persons seeking to bring a test to market in the UK to register via an online portal. CE marking will be a prerequisite for applying for validation. They would then submit data for a thorough desk-based assessment including evidence of laboratory validation already conducted.
A register of all tests that pass the validation process would be published on GOV.UK, providing consumers with clear comparable data, making it easier to assess the performance of different tests on the market and make informed decisions when purchasing a test.
Retailers, distributors and manufacturers of tests that attempt to sell unauthorised tests would face sanction. This mandatory authorisation requirement will be subject to the enforcement mechanisms provided for in the MMDA. This would include compliance notices, suspension notices, safety notices and information notices which could be served in respect of breaches (or suspected breaches) of the new requirement.
3. Consultation response
3.1 Main findings from the consultation
There was broad support for the policy aims outlined in the consultation. The majority of respondents agreed and recognised that there is a need to set and assure a minimum performance standard for COVID-19 tests and to provide clarity to consumers on their performance. The majority of respondents agreed with the proposed process for validating COVID-19 tests, although there were some concerns and questions regarding post market surveillance and ongoing quality assurance.
A few respondents raised concerns that the government’s proposed approach does not align with plans for future regulation, specifically the planned EU regulations In-vitro Diagnostic Medical Device Regulations (IDVR) in May 2022 and potential future UK regulation. Some raised the concerns that the proposals would mean an inconsistent approach to the existing and future regulatory landscape.
Respondents raised concerns about the cost to applicants, particularly the risk that the cost of validation could be a barrier for small and medium-sized enterprises (SMEs) getting products on to the UK market. Some also highlighted the additional costs to those manufacturers that have previously undergone validation and questioned whether they would be charged a reduced fee.
In addition, we heard some concerns about whether validation could unintentionally present a barrier to innovation in the COVID-19 diagnostics market. For instance, some questioned whether the regulation would discourage companies from seeking to make minor iterative improvements to COVID-19 tests due to the requirement to repeat validation.
There were a number of questions raised about what transitional arrangements would be in place for those tests already on the UK market. In addition, assurances were sought whether there was sufficient capacity built into the system to deliver the proposed validation to ensure products could progress through the assessment in a timely manner. Respondents also sought clarity on how COVID-19 tests that have already been validated would be processed and what a ‘verification’ process would entail.
The consultation analysis below will provide a response to some of the issues raised during the consultation. We will develop extensive guidance which will provide clarity on many of the questions raised, including the transitional arrangements and the proposed verification process for tests where robust performance evidence already exists.
4. Questions on policy aims
The consultation included information and questions relating to the overarching policy objective, the problem that government is looking to solve and the proposal to make validation of COVID-19 tests mandatory.
4.1 Summary of responses
Should COVID-19 detection tests be validated beyond the verification and assurance provided for CE marking?
Of those respondents who answered the question, 78% agreed and 22% disagreed. A total of 43 were asked the question and 14% provided no response.
Do you agree or disagree that independently validating COVID-19 detection tests is the right approach to reduce the number of false results from tests and assure consumers that the tests they buy meet minimum quality criteria (such as sensitivity and specificity thresholds)?
Of those respondents who answered the question, 83% agreed and 17% disagreed. A total of 43 were asked the question and 19% provided no response.
Do you agree or disagree that a legally backed and enforceable UK-wide regime is the best approach?
Of those respondents who answered the question, 88% agreed and 12% disagreed. A total of 43 were asked the question and 21% providing no response.
Do you agree or disagree that categorising COVID-19 test performance measures as part of the validation process will ensure more standardised, comprehensive data or test performance measures from private companies?
Of those respondents who answered the question, 88% agreed and 12% disagreed. A total of 43 were asked the question and 23% providing no response.
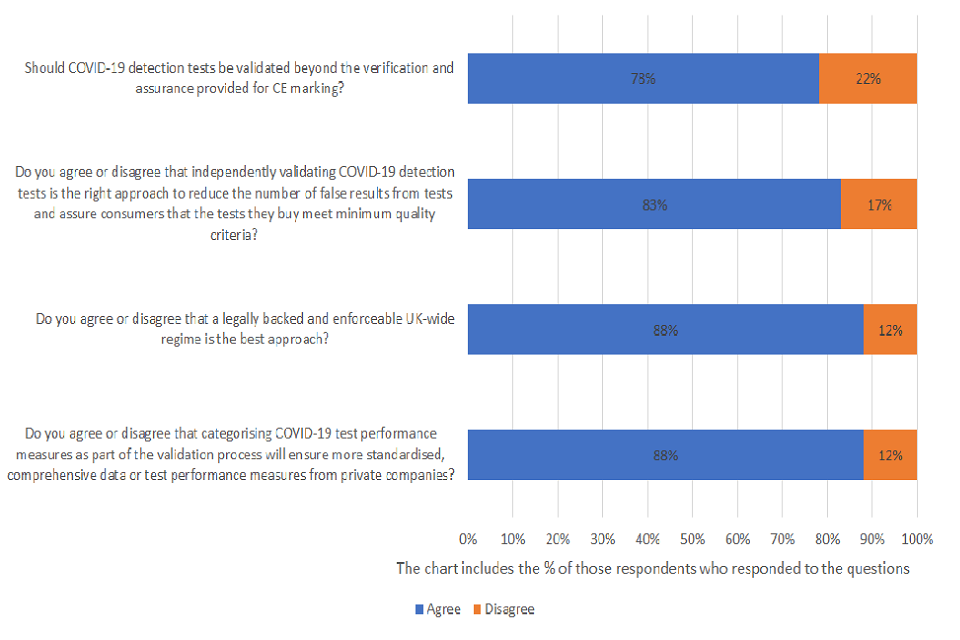
Almost 80% of respondents who answered the question agreed that COVID-19 detection tests should be validated beyond the verification and assurance provided for CE marking. Many respondents further stated that CE marking was insufficient and expressed concerns over whether a satisfactory minimum standard could be achieved through this alone. For example, we received the following response from a national industry association for manufacturers and distributors:
Current performance claims are unreliable and can easily make use of cherry-picked data. There are no set standards for sensitivity and specificity to be evaluated against, and therefore no objective way of comparing 2 tests.
Many felt that independently validating tests was a sensible approach to reduce the number of false test results as it would provide confidence that technologies would be suitable and identified as valid.
Another major theme among respondents was in noting the importance of accessibility and training, both with regards to understanding how the validation process works and the importance of consumers having a better understanding of the performance of COVID-19 tests. Some highlighted that user error risks producing false results. For example when tests developed primarily for use by healthcare professionals are used by untrained users. In addition, to ensure that users can understand and employ tests for intended use, some stressed the importance of consideration for the skills, expertise and experience of any workforce at such private test providers.
One UK-based quality and regulatory affairs consultancy highlighted the importance of improving consumers understanding of COVID-19 testing:
There are special circumstances relating to controlling the pandemic. It is important that consumers should understand the purpose of validation, as well as the purpose and outcome of the tests themselves, and the inherent limitations of any diagnostic testing.
The idea of having a legally backed and enforceable UK-wide regime was well received by most respondents who agreed that it was important that all tests on the market meet a minimum threshold of performance.
A number of respondents commented that they found the current regulatory landscape relating to COVID-19 diagnostic tests confusing and that they would welcome further clarity and consolidation as part of the changes we are looking to implement.
We received the following response from a global healthcare manufacturer:
The current landscape is confusing as a testing company trying to navigate.
Overall, respondents agreed that the validation process will ensure more standardised, comprehensive data and emphasised the importance of a set of standards for all providers to follow.
A common theme expressed by respondents was the need to ensure clarity, accessibility and training, both for manufacturers to comply, but also consumers to ensure test performance can be easily interpreted. This was expressed in the following response from a UK-based manufacturing membership and services organisation:
What is essential is clear labelling and guidance to ensure that purchasers and end users understand any constraints of a product.
4.2 Government’s response
The government’s objective is to ensure that COVID-19 tests available on the UK market are of sufficiently high quality and to ensure that consumers have clarity that tests they use or purchase have appropriate performance characteristics. We were pleased that so many respondents supported the overarching rationale for our proposals.
We have taken on board the feedback on the need for clear guidance and concerns raised throughout the consultation regarding the complexity of the current regulatory landscape. Clear guidance for manufacturers and consumers will accompany any new processes we put in place. The government’s communications plan will also play a key role in delivering on these objectives. We will ensure manufacturers are made aware of any new requirements and raise awareness among consumers and purchasers of tests of the new validation regime for COVID-19 tests.
The government welcomes the overarching agreement and support for the policy objectives, which gives us strong signals of support for market intervention.
4.3 The government’s proposals
The consultation provided detail about the government’s proposal to introduce mandatory validation of COVID-19 tests. This section of the consultation also outlined the intended scope of validation (covering antigen and molecular detection technology), and the intention to implement transitional arrangements for those COVID-19 tests already on the market.
4.4 Summary of responses
Do you agree or disagree that a mandatory validation of tests prior to their entry on to the market is the best approach given the need to quickly establish confidence in them as a step to reopening the economy?
Of those respondents who answered the question, 73% agreed and15% disagreed. 12% didn’t know. A total of 43 were asked the question and 21% provided no response.
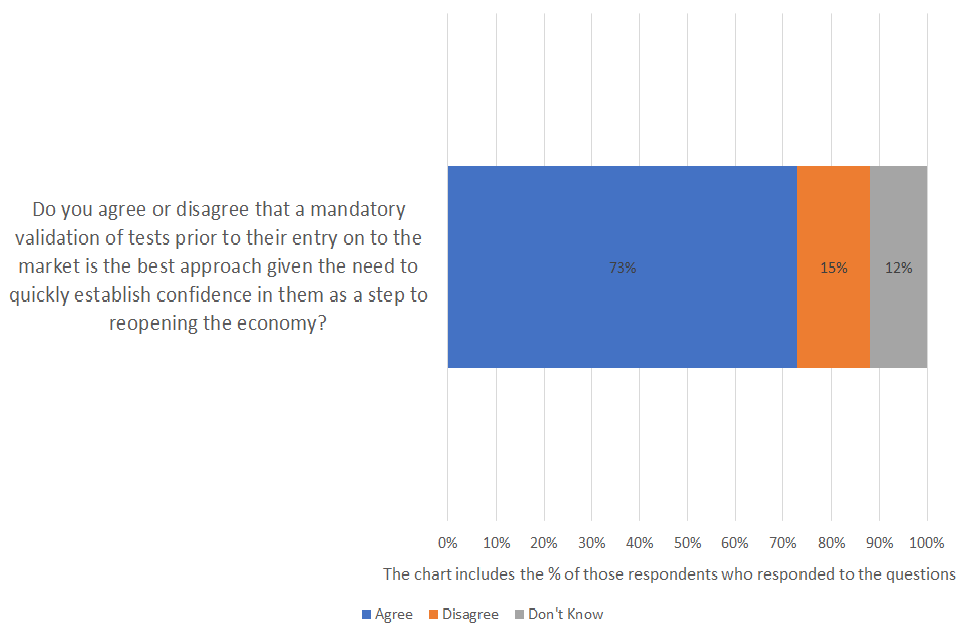
Over 70% of respondents who answered the question agreed that the mandatory validation of tests prior to their entry on the market is the best approach given the urgent need to establish confidence in them. Many commented that the benefits of this approach and agreed that the accuracy of tests and reopening the economy are a priority. A number of comments received from regulatory authorities and professional bodies, were particularly supportive and encouraging of our approach:
a robust testing service is critical on getting the country back to its full economic potential.
(quote from a local trading standards body)
Independent validation will make sure tests from both the private market and public market are of equal quality and that the test product is of sufficient quality and accuracy and can be used to make decisions.
(quote from a UK trade association)
Some respondents suggested that the government should consider including all COVID-19 testing technologies within scope of validation. Others also questioned whether a standardised process for validation risks being too restrictive. Both concerns link to comments made throughout the consultation about whether the proposed validation process could hamper innovation in COVID-19 diagnostics technology.
The following response from a medical device distributor elaborated on this concern around the impact upon innovation:
a new and better technology may not meet the hurdles because they do not conform to a rigid, inflexible validation process designed for a different form of testing.
A few respondents raised concerns that the government’s proposed approach does not align with plans for future regulation, specifically the planned EU regulations In-vitro Diagnostic Medical Device Regulations (IDVR) in May 2022 and potential future UK regulation. Some raised the concerns that the proposals would mean an inconsistent approach to the existing and future regulatory landscape.
Some respondents felt that third party conformity assessments would be a preferred approach to assure ongoing quality assurance, batch to batch controls and post-market surveillance.
The following suggestion was also made by a leading industry body:
COVID-19 tests without existing good quality supportive evidence can be added to the highest risk category for in vitro diagnostic medical devices within MDR 2002. This will ensure tests meet minimum performance specifications as set out by UK Government, with independent assessment by a UK Approved Body who will ensure test performance has been properly validated.
4.5 Government’s response
We are pleased that a majority of respondents who answered the relevant questions agreed that validating tests before permitting them for sale on the UK market is the best approach to establish confidence in tests and reopen the economy.
The government has been clear that the proposal does not prevent the future development of new or emerging technologies. We want to encourage the private sector to bring a number of testing products and services to market to meet the differing needs of businesses and individuals. We are keen to encourage innovation and market growth while ensuring tests meet minimum performance standards on which consumers can depend. That is why we have decided to limit the scope of technologies within scope of the proposed regulation to mature existing technologies that we have an established validation process for. If new products became well established, we will revisit the policy and consider whether they should be considered in scope.
We recognise that some respondents felt that third party conformity was a preferred approach to get assurance on the quality of testing and to ensure robust post market surveillance and quality control. We also acknowledge that the proposed legislative route is a more intensive market intervention than would usually be taken as a first step for regulation. However, due to the urgency presented by the COVID-19 pandemic, we believe that a rapid intervention over and above CE marking is required to address the quality issue in tests urgently and create a strong regulatory framework. It is vital that consumers can buy tests with confidence and without confusion.
We have therefore considered and discounted the option to introduce third party conformity assessment due to the time it would take to implement and potential capacity constraints. However, as part of the planned review process we will consider other options again once the immediate market failure has been remedied.
Strong market intervention can make a strong impact. For example, when HIV test kits were banned in 1992. At that time, there was no effective treatment for HIV and while testing was becoming easier there were serious concerns around people getting tested without any pre-test counselling, under duress or without consent. There were also concerns that test results could be misinterpreted or not confirmed since they would not be supervised by a qualified healthcare professional. Since then, improvements in the treatment and self-test kits for HIV led the government to remove the 1992 regulation. Removing the regulation helped to identify more cases to deliver treatment, while also creating a market for manufacturers. We want to introduce those high standards for COVID-19 tests and build that public confidence in those tests, while continuing to support the private sector to play a critical role in our pandemic recovery.
We have considered and decided against introducing a requirement relating to how and where COVID-19 tests may be sold. We did not receive any feedback from stakeholders on proposals to introduce such requirements. However, we have decided that placing a legal requirement on manufacturers and retailers of tests is sufficient to deliver on our policy aims to implement a minimum standard for COVID-19 tests and to make it clearer for consumers to make informed choices when purchasing tests.
5. Private testing market
The consultation asked respondents to consider the private testing market supply and the potential impacts on the favourability of the UK to carry out research, development and manufacture of COVID-19 tests.
5.1 Summary of responses
Do you agree or disagree a mandatory validation scheme will not significantly reduce the supply of high quality COVID-19 detection tests?
Of those respondents who answered the question, 71% agreed and 29% disagreed. A total of 43 were asked the question and 21% provided no response.
Do you agree or disagree a government-run validation process will increase the likelihood of the UK being seen as a favourable place in which to carry out research relating to COVID-19 tests, develop COVID-19 tests and manufacture or supply COVID-19 tests?
Of those respondents who answered the question, 56% agreed and 44% disagreed. A total of 43 were asked the question and 26% provided no response.
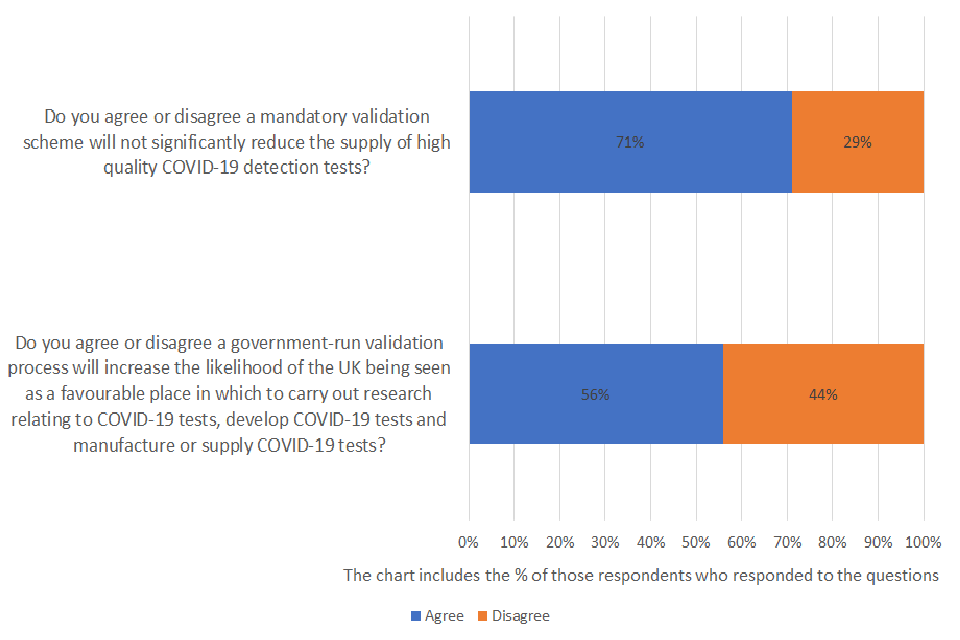
The majority of respondents agreed that a mandatory validation scheme will not significantly reduce the supply of high quality COVID-19 detection tests. Many of them acknowledged the benefits of the approach and that it will have a potential positive impact on quality control within the UK market:
a government run scheme could provide a level playing field and thus a good benchmark for testing.
(quote from a UK distributor of COVID-19 tests)
if tests get a reputation for being unreliable, people will stop purchasing them so in the long run, it would be counterproductive for testing manufacturers not to have their tests validated.
(quote from a UK based Industry Association)
Just over half of the respondents who answered this question agreed that a government-run validation process will increase the likelihood of the UK being seen as a favourable place in which to carry out research relating to COVID-19 tests. Many emphasised the importance of ensuring transparency throughout the validation process. Some highlighted that one of the additional benefits of a high-quality UK validation process is that this could provide a commercial advantage:
UK Approval may ultimately be seen as useful badge of merit that is accepted in export markets and gives some competitive advantage which would be a useful spin off benefit.
(quote from large technical testing and analysis company)
However, some respondents had concerns that tighter regulation and costs could negatively impact supply. A number of respondents raised concerns over whether other international markets may become more favourable for manufacturers due to the additional administrative and financial costs of mandatory validation.
Some respondents had concerns about the potential that validation could increase costs for the consumer. The following response was from a medical device distributor:
The cost and time lag for entry into the UK market may discourage manufacturers from addressing the UK as a priority target market.
The UK is recognised as a centre excellence, and our approvals process is regarded as second to none because it is predictable and fast. We should not risk losing this advantage by making the process more cumbersome.
(quote from a manufacturing membership and professional services organisation)
5.2 Government’s response
We acknowledge concerns that were raised during the consultation regarding the risks to the supply and favourability of COVID-19 tests. However, we have found limited evidence that the type of validation we are proposing, nor the fees or time required, will act as a deterrent to most manufacturers of quality tests due to their experience of dealing with such processes. The upfront fees are in line with those used for other similarly processes such as conformity assessment both domestically and internationally. We have also further addressed concerns regarding barriers to SMEs by proposing a discounted fees regime. This will further ensure that we remove any potential barriers to innovation or market entry for SMEs that could impact supply.
We believe the creation of a highly respected validation process could in fact create a market efficiency. This is because high quality tests are likely to seek out the UK market, whereas poor quality tests could self-allocate themselves out. In addition, as outlined in the consultation, we believe introducing validation will drive up standards for performance of COVID-19 tests, as manufacturers strive to raise their standards to a sufficiently high level to pass validation. In either scenario, the government believes that the supply of high-quality tests is unlikely to be impacted.
We are also taking steps to ensure that the supply of high-quality tests is not constrained through the provisions for transitional arrangements for those products already on the UK market.
We are pleased that some respondents recognised the benefits in introducing COVID-19 tests with regards to the UK’s favourability. We agree with comments made that a successful highly respected validation regime could in fact benefit the UK’s favourability, while also making the market for COVID-19 tests more equitable.
6. Delivery body
This section of the consultation asked respondents to consider the appropriateness of the Department for Health and Social Care as the proposed delivery body for validation of COVID-19 tests.
7. Summary of responses
Do you agree or disagree that the Department of Health and Social Care is the appropriate delivery body to provide UK-wide validation?
Of those respondents who answered the question, 67% agreed and 33% disagreed. A total of 43 were asked the question and 23% provided no response.
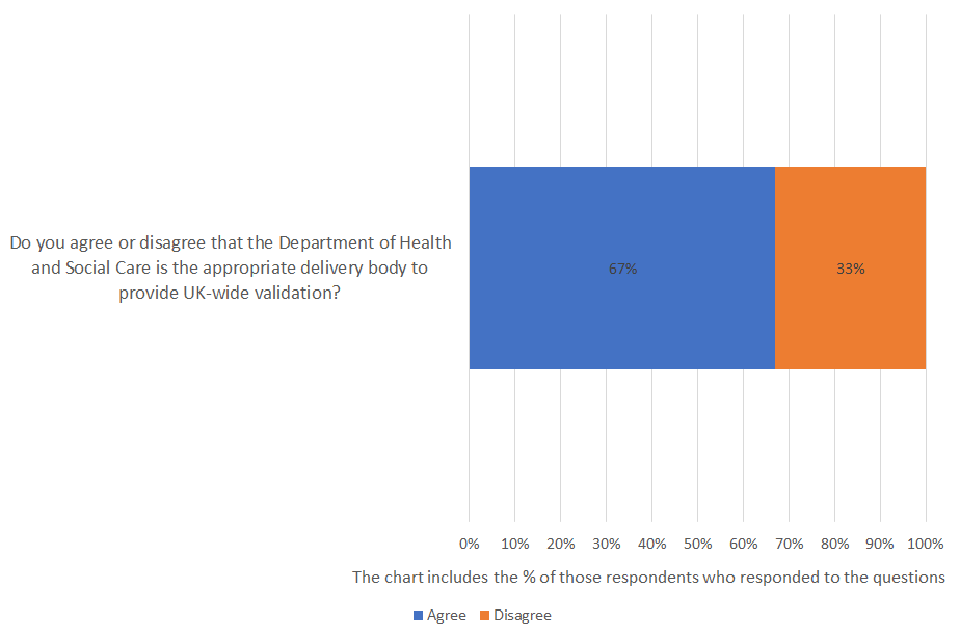
Two-thirds of all respondents who answered the question agreed that the Department of Health and Social Care is the appropriate delivery body to provide UK-wide validation. Respondents emphasised the importance of the Department’s continued transparency throughout the validation process to maintain both producer and consumer confidence.
Respondents highlighted that the critical success of validation is dependent upon sufficient capacity and expertise. Some expressed concerns surrounding the deliverability of validation and whether sufficient capacity would be built into the system to manage the demands within an appropriate timescale. Responses that were centred on this theme, included those from a multinational healthcare company who offered the following evaluation:
Critical success to this process will be to have sufficient capacity and expertise… we would urge that the appropriate skills and resources are assigned accordingly to ensure right tests are available in a timely manner in the UK.
Some had questions about whether there would be an appeals process to appeal against decisions that were perceived as unfair. Some respondents suggested establishing an independent body to oversee the validation regime as a safeguard and measure to tackle any problems with the system. Many noted the importance that the MHRA continue to play a key role in any process that is put in place.
8. Government’s response
The government agrees with some of the comments raised regarding the importance of appropriate training and ensuring that the validation process is led by individuals with the relevant expertise. That is why the validation process will be delivered by expert scientists with experience in validating COVID-19 test products. The UK Health Security Agency will be the delivery body that provides and manages the validation process.
We do not believe there is a need to establish an independent body for oversight of the validation process. We are confident that a robust complaints and reconsideration process will be sufficient to spot and address any defects or reports of problems within the system. It will remain open to applicants to seek a remedy in the courts. In addition, we do not believe the volumes coming through the system would justify establishing a new entity which would duplicate the work of the validation process.
We have consulted closely with the MHRA throughout the consultation process and they will continue to be responsible for enforcement. We agree with respondents who highlighted the need for a procedure, which allows some review of the validation process to take place. This is why a robust complaints procedure and a process to conduct reviews is built into the policy. Further details on compliance and enforcement are included below.
9. Validation process
This section of the consultation set out a proposed process for validating COVID-19 tests and asked respondents to consider these proposals. It also asked respondents to consider the proposals implications on the safety of COVID-19 tests.
9.1 Summary of responses
Do you agree or disagree that the proposed mandatory validation process set out in the consultation document will increase the safety of COVID-19 tests and reduce the risks presented by poor quality tests?
Of those respondents who answered the question, 79% agreed and 21% disagreed. A total of 43 were asked the question and 23% provided no response.
Do you agree or disagree that a desktop review followed by a laboratory testing is the most effective way to validate tests?
Of those respondents who answered the question, 75% agreed and 25% disagreed. A total of 43 were asked the question and 26% provided no response.
How much time do you think is reasonable to complete an application to validation? Please explain.
Do you have any further comments you would like to make about your expectations around the validation process or user experience?
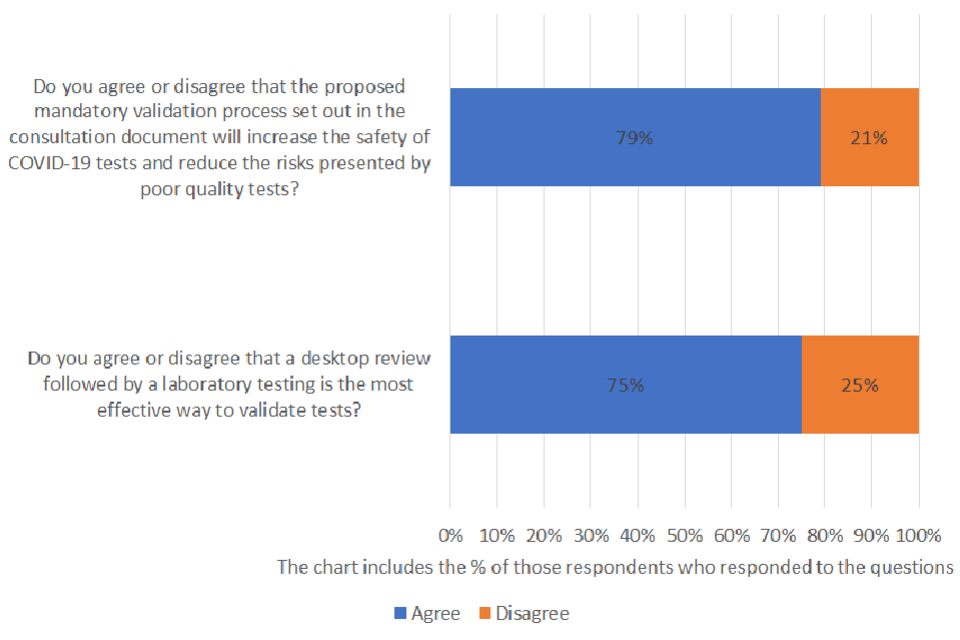
The majority of those who responded to the question agreed that the proposed mandatory validation process set out in the consultation document will increase the safety of COVID-19 tests and reduce the risks presented by poor quality tests. Most respondents also agreed that a desktop review followed by a laboratory testing is the most effective way to validate tests.
Respondents emphasised the importance of transparency and fairness across all aspects of the process for both manufacturers and consumers. Others also emphasised the importance of specialised training and expertise of staff throughout the validation stages, to ensure results are correctly interpreted.
The validation procedure would need to be open to all suppliers and not have impossible hurdles to jump for the smaller companies, which could exclude very effective and viable tests.
(quote from an anonymous individual in response to the consultation)
Access to samples was raised as a key issue throughout the consultation. Ensuring there would be enough access to samples to deliver independent validation was raised on a number of occasions. Respondents also sought assurance regarding the types of samples that DHSC intend to use for validation, to ensure that the process is fair.
A national industry association for in vitro diagnostic devices commented:
The IFU and recommended sample type would need to be followed. Any contrived samples would need to meet the matrix required for the product being tested as stated in the manufacturer’s IFU.
Some respondents offered alternative suggestions to the proposed approach. Some felt that the stated aims of the proposed mandatory validation process could all be achieved within the existing regulatory framework. As outlined in the previous section, others proposed third party review of performance data and quality management systems by UK Conformity Assessment bodies to maintain public confidence. A number of respondents highlighted the importance of having a robust post market surveillance program to enable sufficient oversight of product performance.
One large manufacturer of COVID-19 tests made the following comment:
any solution should utilise existing infrastructure and regulatory environments to minimise any potential for confusion or inefficiency in any process.
A leading industry association wished to particularly capture the issue of batch variation:
The proposal does not address batch to batch variation or improvements in safety and performance. Nor does it include standards for design and manufacturing quality, or include any requirements for post market surveillance.
75% of respondents who answered the question agreed that a desktop review followed by a laboratory testing is the most effective way to validate tests. However, a number of respondents sought clarification on the process for re-validation of tests or services that have been modified or enhanced and the ‘verification’ process for tests that have previously undergone validation.
A global safety science company elaborated further, stating that:
“Desktop review also enables an assessment of prior data to enable better targeted validation in laboratory studies.”
A number of respondents commented that they felt the existing regulatory framework was confusing and hard to navigate. Many noted that they would welcome consolidation and clarity as part of the proposed validation process. Some respondents felt that the proposed bespoke validation process is too complex and incongruous with stated ‘light touch’ rationale governing the proposal. In light of this, respondents also underlined the importance of continued collaboration with the United Kingdom Accreditation Service (UKAS) with regards to test validation and laboratory accreditation:
to ensure that there is no confusion nor that the processes do not inadvertently undermine one another.
Most respondents believed that one month or less would constitute a reasonable timeframe to complete an application to validation. Respondents consistently highlighted a concern that if the mandatory validation process exceeded a reasonable timeframe, this could impact availability of acceptable tests on the UK market and the subsequent risk to public health.
A leading UK healthcare partner provided the following assessment:
This timeframe would enable tests to move on to the market quickly and support patient access.
9.2 Government’s response
We welcome all the feedback from stakeholders on the proposals for the validation process set out in the consultation document. We have taken the decision to proceed to legislate to implement a validation process which includes only a desktop review stage at this time. At a later stage, it is the government’s intention to implement laboratory validation as outlined in the proposals in the consultation. The reason for this change to the policy is that laboratory validation is not deliverable in time to address the immediate market failure swiftly.
However, we believe the risks that poor performing tests pose to public health and the government’s COVID-19 response still warrants urgent intervention to obtain that assurance over and confidence in the performance of COVID-19 tests. Therefore while we believe that the full 2 stage validation including laboratory assessment as outlined in the consultation would provide the greatest assurance, the desktop review will provide us with adequate assurances in the short term to deliver upon our aims to address the immediate market failure, while we prepare to implement laboratory validation in the future.
We are committed to ensuring the validation process is clear and transparent. In order to facilitate this we will develop extensive guidance for manufacturers and consumers to support the validation process. We heard the feedback which highlighted that people find the existing regulatory framework confusing. We intend for the new system to make it easier for manufacturers and end users to navigate.
We know how important it is to ensure that the validation process is efficient for manufacturers and that there are no unnecessary barriers to strong performing tests getting on to the UK market. This has been carefully considered in the design of the policy. Within the implementation design, we have been cognisant of the need to ensure the right level of capacity required to process applications in a timely manner.
We recognise that access to samples is critical to the success of the laboratory element of validation. To ensure that there will be a reliable supply of samples biobanking of clinical samples has been initiated. This will provide access to a reliable source of the various different swab types that would be required for laboratory assessments of test products. While laboratory validation will not be a mandatory requirement initially this work and further work to develop reliable and appropriate contrived samples will ensure there are sufficient stocks of each swab type when required.
We recognise that some tests have already passed rigorous validation such as those approved for UK public procurement. The government does not want to create any unnecessary barriers to entry to the COVID-19 testing market, nor does it want to require onerous or repetitive work for validation. As such, we will ensure the validation process can use existing evidence where our scientists are satisfied it is of sufficiently high quality to avoid any duplication and we are exploring implementing a lighter touch expedited verification process to sit as an alternative to the future laboratory validation. We believe it is important that there is consistency between the future validation and verification process to give DHSC and the public that assurance and to ensure the process is fair and equitable. Further details on the verification process and the criteria for appropriate documentation and data that can be used for verification will be outlined in published guidance.
As outlined in the previous section on the government’s proposals, we considered and discounted the option of third-party conformity assessments at this time.
10. Fees
This section asked respondents to consider proposals to charge a fee for the validation process and asked for views on the level at which they should be set.
10.1 Summary of responses
Do you agree or disagree that the costs of the validation process and associated running costs should be covered through fees rather than subsidised by the taxpayer?
Of those respondents who answered the question, 68% agreed and 32% disagreed. A total of 43 were asked the question and 23% provided no response.
Do you think that the proposed fee between £55,000 and £75,000 per test for the total cost of the validation service is reasonable?
Of those respondents who answered the question, 40% agreed and 60% disagreed. A total of 43 were asked the question and 30% provided no response.
Can you name other national or international diagnostic testing validation services that require payment, and what the cost is of those services?
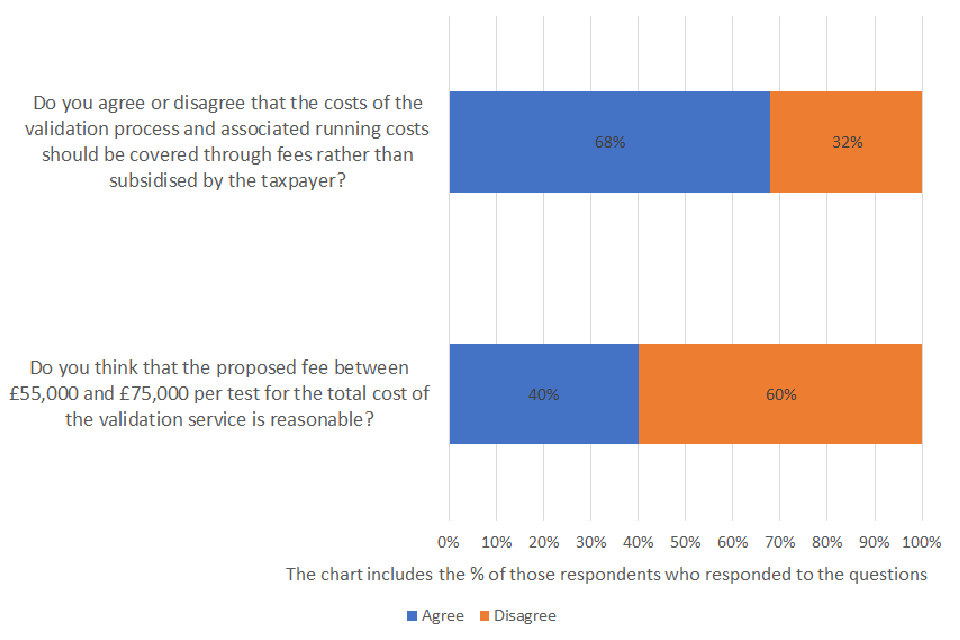
The majority of respondents who answered the relevant question agreed that the costs of the validation process and associated running costs should be covered through fees rather than subsidised by the taxpayer.
However, 60% of respondents to the question disagreed that the proposed fee between £55,000 and £75,000 per test for the total cost of the validation service is reasonable. Despite this, we did not receive any alternative proposals during the consultation that would make the validation regime cost neutral to the taxpayer at lower fee rates. Nonetheless, a prevailing theme among respondents was that the proposed costings are too high and the associated implication could inhibit market innovation. Respondents contended that such fees may deter manufacturers from submitting tests for validation and have a subsequent negative impact on the United Kingdom’s test supply. Some manufacturers also questioned whether this could translate into increasing costs for consumers and an impact on the affordability of tests:
Such a high cost would be crippling.
(quote from developer and manufacturer of in vitro test kits based in Northern Ireland)
Many respondents expressed concern that the proposed level of fees risked incurring a disproportionately negative impact on Small to Medium Enterprises (SMEs) and could pose a barrier to entry to the UK market. Many asked us to consider introducing a fee structure that considers size and turnover to address this issue:
SMEs may not be able to risk their financial futures on this cost level.
(quote from a large trade association)
One organisation delivering professional scientific and technical activities highlighted that differences in the impact of fees depending on the size of the organisation:
A £75,000 is a large amount of money for an SME but a drop in the ocean for a multi-national organisation.
Some highlighted the risk that a standard fee for all applicants could unfairly benefit those larger companies, including one large manufacturer:
This process will benefit larger players by removing potential competition from small SME’s focussed on product innovation.
Many were also keen to highlight the substantial fees paid by companies under the existing regulatory framework of CE marking:
Fees should be minimised for such an approach reflecting the significant investment already made by companies.
10.2 Government’s response
We agree with the majority of respondents who felt that the cost of validation should be covered through fees rather than the taxpayer. Our ambition is to achieve as close to full cost recovery as possible for the validation process. The intention is this will be cost neutral by recovering the costs from applicants so as not to burden the taxpayer, while also keeping fees as low as possible.
We have not found sufficient evidence that the type of validation we are imposing, nor the fees or time the process will require, will act as a deterrent to most manufacturers of quality tests, given their experience with dealing with such processes. The upfront fees are in line with those used for other similarly processes such as conformity assessment both domestically and internationally. In addition, it is not expected that these costs will be passed onto consumers through higher prices, this is based on the high sales volumes expected and sufficient competition between test products ensuring prices will not rise.
However, we recognise that some stakeholders were of the opinion that the proposed costs may present a to Small and Medium-sized Enterprises (SMEs). We acknowledge that this issue is particularly important due to the make-up and structure of the UK’s diagnostics market, in which the majority of businesses are SMEs. Analysis of the ONS’ UK Business Workbook suggests that: 93% of businesses involved in the manufacture of basic pharmaceutical products are micro or small (76% and 17% respectively). For this reason, we have set a lower rate for smaller entities employing less than 250 people.
Similarly, there were a number of questions raised about whether businesses that have previously undergone a validation process as part of the NHS procurement route will be charged a reduced fee. We are considering options that will enable us to charge a lower fee for businesses that are deemed to have previously met validation requirements through NHS procurement.
The government anticipates that private sector provided testing will form a crucial part of day-to-day testing as we move into the long-term management of COVID-19 and reopen the economy. We therefore expect that this will require considerable expansion of domestic production and potentially an increase in imports to ensure there is sufficient supply to meet demand.
11. Compliance and enforcement
This section had a number of questions relating to the proposed enforcement powers set out in the Medicines and Medical Devices Act and questions around the relevant enforcement authorities.
11.1 Summary of responses
Do you agree or disagree that the range of enforcement powers set out above is appropriate and proportionate as a first line response for breaches of regulation that may arise?
Of those respondents who answered the question, 85% agreed and 15% disagreed. A total of 43 were asked the question and 23% provided no response.
Do you suggest any other additional powers are required to investigate suspected offences?
Do you agree or disagree that MHRA and local authority Trading Standards are the correct enforcement authorities?
Of those respondents who answered the question, 88% agreed and 12% disagreed. A total of 43 were asked the question and 21% provided no response.
Do you agree or disagree that the failure to comply with the enforcement notices should be a criminal offence?
Of those respondents who answered the question, 94% agreed and 6% disagreed. A total of 43 were asked the question and 25% provided no response.
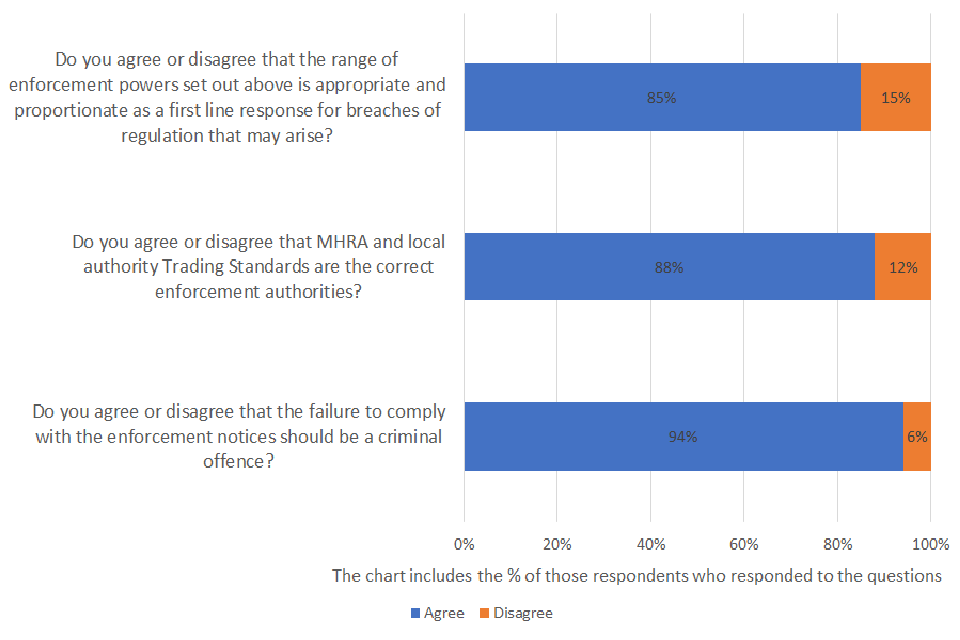
The overwhelming majority of respondents agreed that the range of enforcement powers set out is appropriate and proportionate in relation to any breaches of the proposed regulation. Adherence to regulatory requirements was particularly noted for patient safety and wider public health. However, other respondents highlighted that a suitable transitionary period must be considered and the necessity of enforcement powers should be clearly communicated from the outset.
Some respondents sought clarification within the legislation of the role of ‘Medicines and Medical Devices Regulations’ for the purposes of enforcement.
Over 80% of respondents who answered the question agreed that Medicines and Healthcare products Regulatory Agency (MHRA) and local authority Trading Standards are the correct enforcement authorities.
Over 90% of respondents who answered the question agreed that failure to comply with the enforcement notices should be a criminal offence. Below was a common sentiment shared among respondents was that a more lenient approach may:
dilute the strength of the enforcement notice.
11.2 Government’s response
The government believes it is important that the appropriate powers are in place to ensure that any breach of regulations can be dealt with through appropriate penalties. Under the proposed legislative vehicle, the Medicines and Medical Devices Act (MMDA), compliance notices, suspension notices, safety notices and information notices can be served in respect of breaches (or suspected breaches) of the requirement and failure to comply with any of those notices would be an offence.
The criminal sanctions in the MMDA which relate to a breach of an enforcement notice came into force on 26 May 2021 (see S.I. 2021/610 (C.22)). The specific criminal sanction created by Schedule 3 to the MMDA is not yet in force, but existing criminal sanctions for contravening a prohibition in safety regulations will continue to apply until that Schedule is brought into force.
We will ensure that the guidance and information about any new legal requirements around validation of COVID-19 tests is communicated to manufacturers and the public in advance of legislation coming into force. Clear and accessible guidance will ensure that manufactures have all the information they need to comply with any new regulations.
Transitional arrangements for products already on the market will provide time for products to meet the new legal requirements, without the need to remove products from the market. Further information on transitional arrangements will be included in the published guidance.
12. Section 15 Medicines and Medical Devices Act 2021
The Secretary of State’s overarching objective in making any regulations under section 15 of the Medicines and Medical Devices Act 2021 to give effect to the policy outlined in this consultation document would be to safeguard public health.
We sought feedback from stakeholders on their views regarding the safety, availability of COVID-19 tests and medical devices, as well as the favourability of the UK market.
In considering this policy and the regulations which may be needed to give effect to it, the Secretary of State has had regard to:
- the safety of medical devices within the scope of this policy
- the availability of medical devices within the scope of this policy
- whether the United Kingdom is likely to be seen as a favourable place in which to: research the medical devices within the scope of this policy, develop medical devices within the scope of this policy or manufacture or supply medical devices that come within the scope of this policy
13. Summary of responses
The safety of COVID-19 medical devices themselves was not highlighted as a concern by respondents to the consultation. However many respondents commented on the indirect risk that poor performing COVID-19 tests pose to public health. Reducing the risk of false positives and false negative results was seen very favourably for public health and the economy.
For example, the following excerpt was taken from a response by a UK-based quality and regulatory affairs consultancy:
Any impact of this proposal on the safety of the COVID-19 tests will be positive, since it will ensure that only tests meeting a minimum standard of accuracy are available on the UK market.
Over 70% of respondents who answered the question agreed that the proposal would not restrict the supply of high quality COVID-19 tests. In addition, implementing the proposed validation process may encourage manufacturers to raise their standards to a higher level of performance. The following quotes taken from the response to the consultation demonstrate these sentiments:
If tests get a reputation for being unreliable, people will stop purchasing them so in the long run, it would be counterproductive for testing manufacturers not to have their tests validated. The assurance around quality and accuracy is more important.
(quote from a large UK based Industry Association)
It won’t reduce the supply, it will however reduce the tests that are acceptable in terms of the results that they are producing. It will enhance the quality of the tests available.
(quote from an anonymous individual in response to the consultation)
A mandatory validation scheme will provide the required assurance that the tests provided within healthcare settings such as community pharmacy continue to meet the threshold standards on both sensitivity and specificity.
(quote from a UK-based industry association)
Of those respondents who felt that there would be a detrimental impact upon the supply of COVID-19 tests, a few highlighted concerns that the UK could be seen as a less favourable market due to the additional financial and administrative demands outlined above.
When asked about whether the proposals would impact the UK’s favourability to research, develop or manufacture COVID-19 tests, there was a mixed response. Similar to the concerns raised regarding the supply of tests, respondents raised the issue of additional demands on manufacturers and concerns about the potential to impact innovation.
In contrast, others felt that independent validation could give the UK a competitive advantage and/or could make the UK market more favourable. Some respondents highlighted the fact that the proposed validation would make the UK market for COVID-19 tests more equitable, by ensuring that all manufacturers of COVID-19 tests are all held to the same standards for performance.
In relation to COVID-19 tests specifically, while a number of respondents felt that it would not impact the UK’s favourability, we noted some concerns raised by stakeholders that the additional costs to validate tests might deter manufacturers from selling in the UK market in favour of markets with less regulation. This is outlined in more detail on stakeholder feedback in relation to the UK’s favourability above under the private testing market.
Some respondents commented on the potential impact the proposed policy might have on the UK being seen as a favourable place in which to carry out research, develop, manufacture or supply medical devices more generally. A leading industry association elaborated further, stating that:
The proposal is not a suitable template for future regulation and feels punitive and reactive. It may be viewed as a disincentive for innovation, particularly by smaller companies.
14. Government’s response
Below sets out the government’s response in relation to the safety of COVID-19 tests:
The safety of COVID-19 testing kits do not represent a risk to health, however they do present a risk to the general public health as inaccurate test results could lead to further spreading of the virus. The validation process proposed will ensure that tests are of sufficient quality to protect the public health and instil confidence in testing.
Further qualitative benefits centre on overcoming information asymmetry and instilling public confidence in privately available tests and subsequent behaviours associated with this. If government is successfully to transition the provision of widespread/asymptomatic COVID-19 testing from the public to the private domain while retaining this testing as a policy lever in its strategy to manage the prevalence of COVID-19 it is necessary that the public have (well-founded) confidence in private testing.
Below sets out the government’s response in relation to the availability of COVID-19 tests:
The supply of high quality tests is unlikely to be impacted. We believe that the impact upon supply will only affect the supply of poor performing COVID-19 tests. Our intention is to ensure that there continues to be sufficient supply of COVID-19 tests on the UK market, but only those that meet a minimum performance standard. Therefore, we have assessed that the benefits in restricting the supply of poor performing COVID-19 tests due to the risks that they present to public health outweigh concerns about limiting supply of tests overall.
The exclusion from the market of lower performing devices by definition improves average performance.
Specifically, this will:
-
reduce the number of false positive results / increase the number of true negative results for individuals not carrying COVID-19 at the point of testing
- by removing constraints on social/economic engagement (such as removing the need to self-isolate), reducing false positives will increase the productivity and wellbeing of test participants
- it will also reduce cost pressures on the test and trace system, and the need for contacts to self-isolate (therefore also improving their productivity and wellbeing)
- reduce the number of false negative results / increase the number of true positive results for individuals who are carrying COVID-19 at the point of testing
- by correctly identifying more individuals who are carrying COVID-19, this will reduce the spread of the virus through self isolation and contact-tracing of carriers, which by reducing onward infections improves wellbeing, long-term health, mortality and social and economic participation of prevented onward infections
- this will also marginally reduce the likelihood of future national level interventions like lockdown and marginally slow the emergence of new strains of the virus
- reduce the number of false negative results / increase the number of true positive results for individuals who are carrying COVID-19 at the point of testing
Below sets out the government’s response in relation to the favourability of COVID-19 tests and medical devices.
The government has considered the potential impact of the proposed policy on the continued favourability of the UK as a place to research, develop, manufacture and supply medical devices. While we acknowledge the concern raised about the impact on our proposals for future medical devices innovation, this specific policy is intended only for mature COVID test technologies. The government remains very much committed to supporting innovation in our thriving life sciences sector.
We consider the planned policy to be a proportionate response to address a situation which risks undermining national health efforts in containing the pandemic by poorly performing tests giving inaccurate results. Additionally, we would strongly encourage stakeholders to engage in the upcoming consultation on Medical Devices Regulations, launching later this year, which will consider medical device regulation more widely.
We believe that robust and clear regulation will give business clear and consist rules they can rely on and this will make the UK an attractive market. In addition, we agree that regulating the COVID-19 testing market will ensure a more equitable market.
15. Next steps
The consultation has provided us with valuable feedback and suggestions that have enabled us to refine our proposals in the development of the government’s proposed validation scheme for COVID-19 tests. The overarching support for the policy, and in particular, the policy aims themselves have given a clear signal that stakeholders support intervention to address the issues, by specifically pursuing policy to introduce a requirement for the validation of COVID-19 tests.
We will now take steps to make a Statutory Instrument under the Medicines and Medical Devices Act 2021 (MMDA) to create a regulatory requirement for the mandatory approval of diagnostic tests for COVID-19. The MMDA provides powers to amend and supplement the regulatory frameworks for human medicines (including clinical trials of human medicines), veterinary medicines and medical devices and thereby enable the effective regulation of these fields going forward following the UK’s exit from the EU.
Testing is a vital part of the United Kingdom’s response to COVID-19. The government wants to ensure that test kits sold privately in the UK are of the same high quality as those used by the NHS. This is to avoid undermining national health efforts in containing the pandemic by poorly performing tests giving false results.
A key part of the government’s approach to managing COVID-19 in the long term is to facilitate a thriving private sector market for COVID-19 detection tests, and to supplement and support testing led by NHS Test and Trace. The government wants to encourage the private sector to bring a number of testing products and services to market to meet the differing needs of businesses and individuals. The government is keen to encourage innovation and market growth while ensuring tests meet minimum performance standards on which consumers can depend upon.
Given the urgency presented by the COVID-19 pandemic, a rapid intervention is required to address the quality issue in tests urgently and create a strong regulatory framework so that consumers can buy tests with confidence and without confusion.
To this end the UK government is establishing, through this instrument, a requirement that all COVID-19 tests placed on the UK market undergo a mandatory approval process, to validate them as meeting high quality standards. Following a transition period, tests that fail this process would be barred from sale.
Retailers, distributors and manufacturers of tests that attempt to sell unauthorised tests would face sanction.
As outlined above, we have taken the decision to proceed to legislate to implement a validation process which includes only a desktop review stage at this time. At a later stage, it is the government’s intention to implement mandatory laboratory validation as outlined in the proposals in the consultation. To do so, it will be necessary to undergo a further public consultation to ensure industry and the wider public have the opportunity to input feedback. We believe desktop review will provide us with adequate assurances in the short term to deliver upon our aims to address the immediate market failure, while we prepare to implement laboratory validation in due course.
The proposed approach to monitoring this legislation is to monitor this regulatory regime on an ongoing basis to ensure it remains fit for purpose. The quickly changing nature of both the underlying public health policy issue presented by the COVID-19 pandemic and the market for private testing necessitates this approach. We will engage closely with stakeholders to ensure regulation remains agile and doesn’t present unforeseen and unnecessary burdens on business.
As conditions change, we will look again at some of the options, including some raised in the consultation, which were not suitable in the immediate term to see if they are now more appropriate to address the market situation. We will also consider developments internationally particularly if there are benefits aligning with other regulatory jurisdictions in the longer term. We will also want to capture learning more generally to help in our preparation for future pandemics.
A statutory review clause is included in the instrument. This will require the Secretary of State to publish a report by 31 December 2022 assessing:
- the effectiveness of the validation regime to ensure minimum quality levels for COVID-19 test kits
- the impact on prices for consumers and consumer confidence, impacts on safety and supply of COVID-19 tests
- the impact on the UK as being a good place to research and manufacturer COVID-19 tests
We wish to thank everyone who submitted a response to the consultation and those who engaged with us throughout the process.
