Summary of responses to the Call for Evidence on the Effectiveness of the Medicines and Medical Devices Act 2021 for Veterinary Medicines
Published 1 September 2025
1. Overview
The Medicines and Medical Devices Act 2021 MMD Act provides the primary powers for the Veterinary Medicines Regulations 2013 (as amended) (VMR).
The objectives of the MMD Act, for veterinary medicines, are to:
-
provide a clear legislative framework for veterinary medicines and medicated feed
-
ensure this framework is fit for purpose to protect animal health, public health and the environment by assuring the quality, safety and efficacy of veterinary medicines
-
give the regulator oversight to ensure the requirements are met and action can be taken when they are not
-
give the regulator the power to charge fees
The MMD Act also allows the Veterinary Medicines Directorate (VMD) to amend and supplement the requirements for marketing, manufacturing, prescribing, supplying and using veterinary medicines.
These amendments were previously made under the European Communities Act 1972 which was repealed when the UK withdrew from the EU.
Without the MMD Act, the Government would not have the power to make amendments to the VMR, such as the Veterinary Medicines (Amendments etc.) Regulations 2024, which made significant changes to this legislation.
The MMD Act sets out the requirements when making changes. This includes the need for a report to be published within 5 years of the act being passed. The report must include an assessment of whether:
-
some or all medicines and medical devices legislation should be consolidated or otherwise restructured
-
provisions of medicines and medical devices legislation should be included in regulations or Acts of Parliament
-
powers to make regulations should be modified or repealed
2. The Call for Evidence
The MMD Act call for evidence for veterinary medicines was published on 1 September 2025 and closed on the 26 September 2025.
The purpose of this review was to evaluate whether the MMD Act is now operating as intended, if it continues to effectively protect animal and public health and the environment, and if it avoids imposing unnecessary or excessive regulatory burdens.
The review also looks at the structure of the legislation and whether restructuring or consolidation would make the regulations clearer or easier to implement.
We asked for views from all interested parties, including:
-
veterinary medicine marketing authorisation holders (MAHs)
-
manufacturers and distributors
-
animal health professionals such as vets, vet nurses, vet pharmacists and suitably qualified persons (SQPs)
-
feed business operators
-
farmers and other professional keepers of animals
-
pet owners
The call for evidence was to gain insight into every aspect of the veterinary medicines supply chain and to understand whether the MMD Act allowed the VMD to keep the VMR up-to-date and fit-for-purpose to protect animal health, public health and the environment.
3. Summary of Responses
We received 78 responses to this call for evidence from both individual stakeholders and organisations covering a wide area of the veterinary medicines sector. There was a range of responses covering England, Wales, Scotland and Northern Ireland, as well as responses from stakeholders representing international organisations.
As the focus of this call for evidence was to evaluate the effectiveness of the MMD Act, we will not be responding in this document to comments about the specific provisions and requirements under the VMR, however, we will be taking these concerns on board for future reviews of the VMR.
We are unable to comment on areas outside of the MMD Act and VMR remit, such as controlled drugs and the clinical assessment of animals, or the review of the cost of veterinary medicines being carried out by the Competition and Markets Authority (CMA), or on the cost of veterinary medicines which is not regulated under the VMR.
3.1 Effectiveness of the MMD Act
30.77% answered that the legislation was ineffective and does not adequately protect animal health.
Figure 1: How well do you think the current legislation protects animal health and welfare?
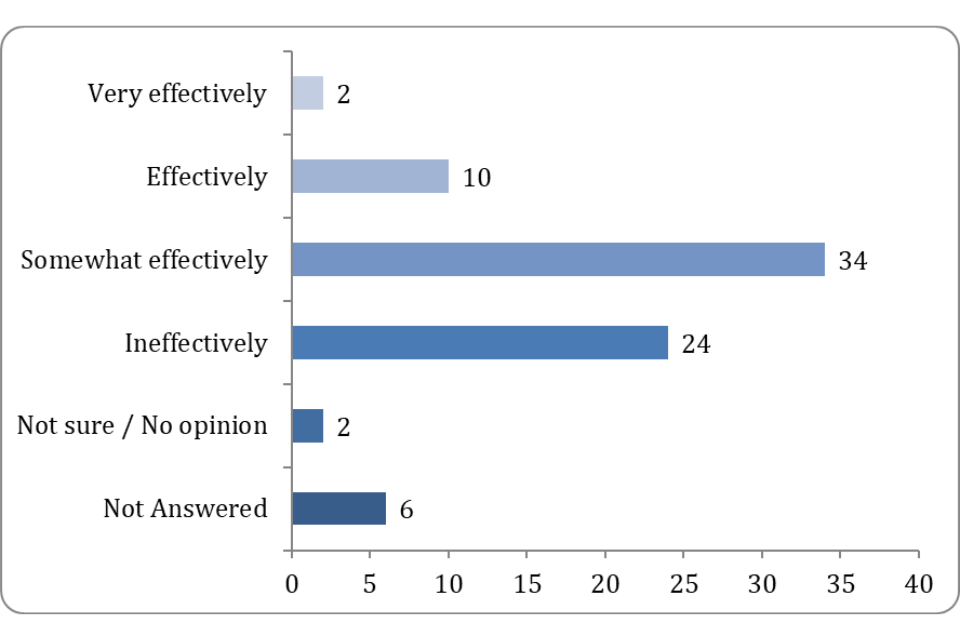
Points raised included the risk of the VMR not being able to keep up with the pace of improvements in technology and medicine, which risks the situation where new types of products are not regulated or where outdated regulation results in difficulties approving novel products.
There were also comments raised that veterinary medical devices are not currently regulated, a possible concern given the growing use of artificial intelligence (AI). This could mean devices are being used without any evidence to support their effectiveness, quality or safety.
3.2 Blockers and Burdens
73.08% answered to say they had encountered issues, blockers or areas of ambiguity when using the regulations.
Figure 2: Have you encountered any issues, blockers, or areas of ambiguity when using the regulations?
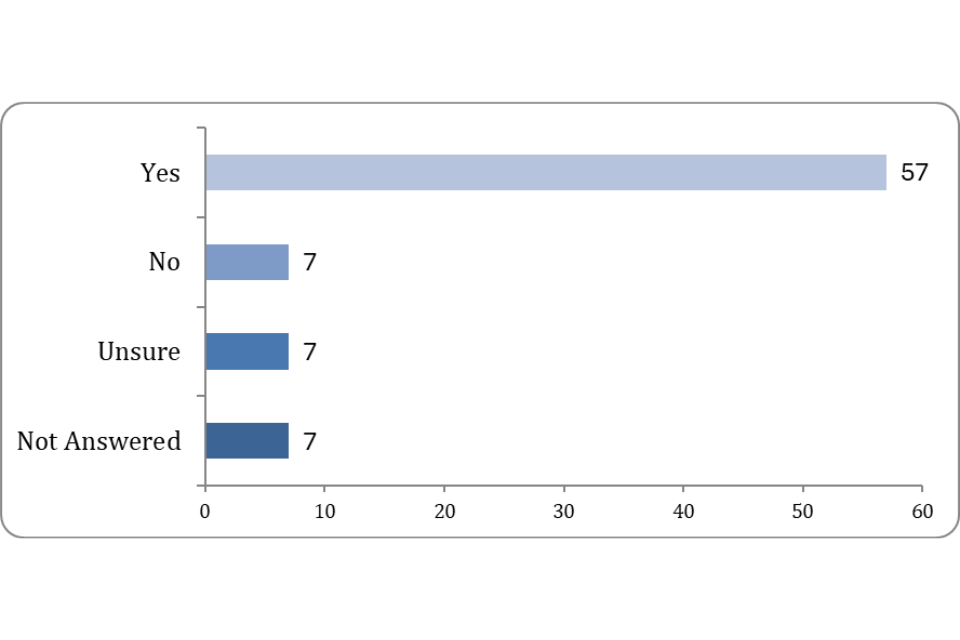
50% answered to say they had experienced areas of the regulations that impose unnecessary or excessive burdens.
Figure 3: Are there any particular areas of the regulation which you consider impose unnecessary or excessive regulatory burdens?
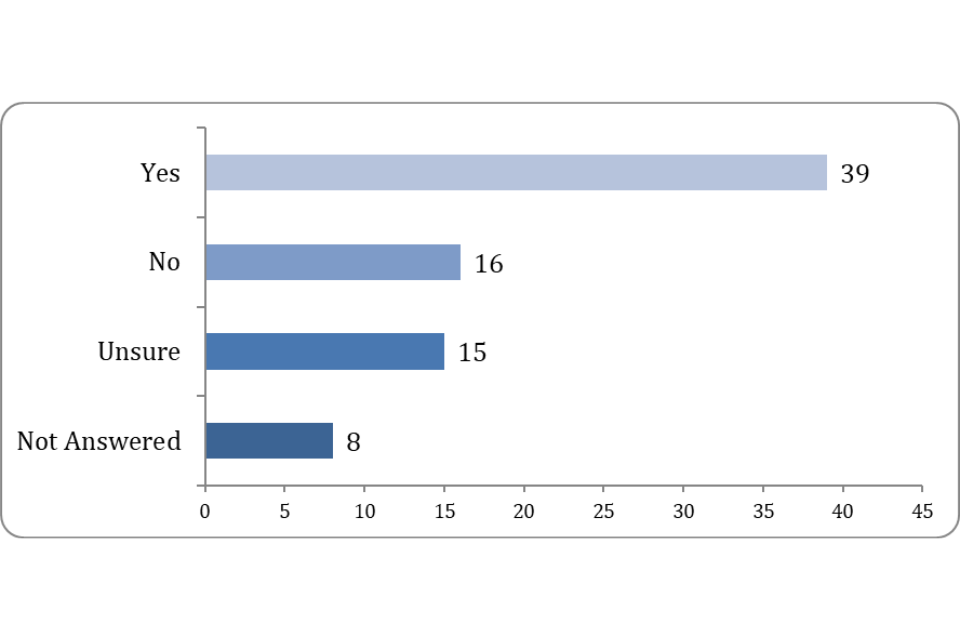
These responses show that further updates to the VMR or the supporting guidance could be required to ensure that provisions are clear. This could reduce familiarisation costs for stakeholders and increase compliance with the requirements.
Comments were raised that the requirement to amend and supplement the VMR (rather than revoke and replace) caused confusion and a lack of clarity for key stakeholder groups.
The responses also show that regular updates of the VMR are crucial to ensure that they remain fit-for-purpose and provide enough regulatory oversight without unnecessary restrictions.
Points were raised on the restrictive nature of the VMR requirements for authorised veterinary medicines and the regulatory burden placed on MAHs, particularly in relation to labelling and pharmacovigilance obligations. Stakeholders were also concerned about the impact of legislation on the supply of medicines in Northern Ireland.
There were also comments on the impact that burdens could have on reducing animal testing methods.
Other points raised were on the prescription and supply of veterinary medicines, particularly on animal charities’ access to veterinary medicines for wild animals, or the capacity for rescue charities to manage the numbers of animals they have. Stakeholders also raised the issue of advertising for veterinary medicines and the possible access to unregulated information online.
Answers also highlighted blockers for novel areas such as cannabidiols (CBD), bacteriophages and products used under the cascade such as stem cells.
The VMD will review the concerns raised as part of future reviews of the VMR to ensure that stakeholders are able to better understand the VMR and continue to provide for the effective and safe treatment of animals.
Only 7.69% of respondents agreed that there was an appropriate balance of flexibility to respond to new technologies or emerging animal health issues, and robust regulatory oversight, with none strongly agreeing to this. Compared to 51.29% who either disagreed (34.63%) or strongly disagreed (16.67%) that there was appropriate balance.
Figure 4: Do the regulations provide the appropriate balance of flexibility to respond to new technologies or emerging animal health issues, and robust regulatory oversight?
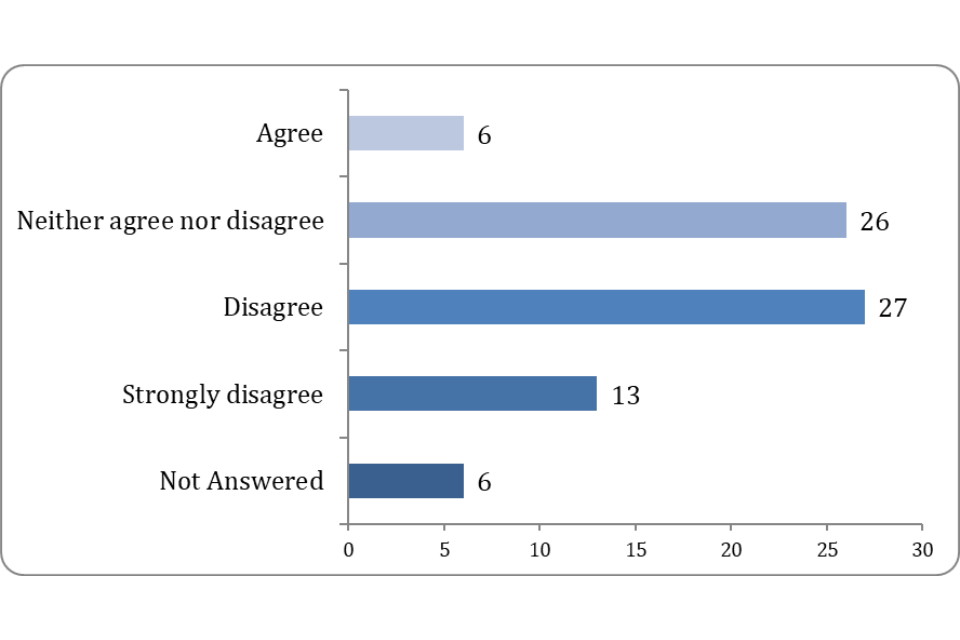
The concerns raised above would suggest that stakeholders believe a greater degree of flexibility is required to address the concerns raised with the regulations.
3.3 Legislation, regulation and guidance
19.23% of respondents believed that there was an appropriate balance of legislation and guidance, with 21.79% believing there was too much legislation and 19.23% believing there was too much guidance.
Figure 5: Do you think the current balance between what is set out in legislation versus what is provided in supporting guidance is appropriate?

84.62% of respondents believed that changes were needed to the legislation, although only 34.62% believed that the legislation would benefit from major changes or restructuring. 7.69% of respondents believed that the potential downsides of restructuring the legislation would outweigh the benefits, compared with 43.59% who believed the benefits of a restructuring would outweigh the downsides.
Specific points included that the legislation meant that the VMR was slow to react to changes.
The VMR serve as a comprehensive and accessible ‘one-stop shop’ for all technical and operational requirements, and stakeholders value this clarity. The views of stakeholders were that moving technical provisions into primary legislation would risk creating unnecessary complexity.
This suggests that stakeholders would be in support of minor changes to the MMD Act, however a major restructure could be unnecessary.
