Aurum pre-filled syringes phased introduction of new 10ml CONNECT syringe barrel: Importance of selecting compatible needle-free connectors to minimise the risk of syringe blockage (DSI/2025/02)
Due to a phased introduction of the new 10ml CONNECT barrel design for the Aurum pre-filled syringes, the range of compatible needle free connectors (NFCs) differs between the established syringe barrel and the new syringe barrel. It is vital that healthcare professionals use compatible NFCs with Aurum pre-filled syringes to prevent delays to delivery of emergency medication.
Update: 22 August 2025
We have issued a minor update to this Device Safety Information to remove the reference to the Naloxone pre-filled syringe as this product, which uses a 2 ml barrel, is not affected by the 10ml CONNECT introduction. All other content remains unchanged.
Summary
The use of incompatible needle-free connectors (NFCs) with Aurum pre-filled syringes has led to adverse incidents in emergency situations. Attaching an incompatible NFC can block the syringe and prevent delivery of emergency medicine.
A new 10ml barrel design for the Aurum pre-filled syringes, CONNECT, will be compatible with a wider range of NFCs to minimise the occurrence of these adverse incidents. For the transition period, both the new syringe and the established syringe will be available for affected products.
The CONNECT syringe will be introduced to the market in a phased manner. The 10ml CONNECT barrel for the Adrenaline 1:10,000 pre-filled syringe is scheduled for market introduction in July 2025. This will be followed by the phased rollout of the 10ml CONNECT barrel for the Amiodarone and Calcium Chloride pre-filled syringe products.
All NFCs compatible with the established syringe design remain compatible with the CONNECT syringe design. However, the reverse is not applicable. Therefore, during the transition period, until March 2028, there remains an increased risk of inadvertently using an incorrect NFC.
Healthcare professionals should refer to the list of compatible NFCs under the product information listings below which can be used with both designs of the Aurum pre-filled syringes to eliminate the risk of NFC incompatibility during the transition period. This can also be viewed by scanning or clicking the below QR code:

Advice for healthcare providers:
- refer to the list of compatible needle-free connectors (NFCs) under the product information listings (see summary section above) for the Aurum pre-filled syringes, and procure compatible NFCs for use with the established and/or new CONNECT syringe barrel
- ensure that the specification of NFCs which are integral to vascular access devices are checked for compatibility before procuring or putting into use in areas where Aurum pre-filled syringes may be used
- refer to the compatibility warnings and Luer size limits in the instructions for use of the NFC
- train administration and emergency teams and ensure procedures are in place to determine the compatibility of NFCs with the Aurum pre-filled syringes
- consider displaying a list of compatible NFCs for use with Aurum pre-filled syringes in clinical areas containing the emergency drug boxes and resuscitation training rooms - see additional information section
- if healthcare providers with access to the established syringe barrel only are unable to procure compatible NFCs, it is recommended that they complete a risk assessment as per local clinical protocol, and refer to the product information listings (see summary section above) to identify specified NFCs for use with appropriate adaptors
- when appropriate adaptors and specified NFCs are procured for use with the established syringe barrel, ensure that they are stored with the Aurum pre-filled syringes in the location where they will be used (for example, emergency trolleys or kits)
Advice for healthcare professionals:
- use compatible needle free connectors (NFCs) with the established or new CONNECT syringe barrel for the Aurum pre-filled syringes, refer to the list of NFCs available under the product information listings (see summary section above)
- if no compatible NFC is available for the established syringe barrel, complete a risk assessment as per local clinical protocol prior to using specified adaptors in conjunction with NFCs, as also listed under the product information listings.
- remove adaptors immediately after use to reduce the risk of infection and air embolus
- ensure that the luer lock fitting attached to the line is the correct size and type for the NFC used
Advice for Healthcare Professionals to Provide to Patients:
- there is no related advice for healthcare professionals to provide to patients
Advice for Distributors:
- there is no advice for distributors related to this DSI
Explanation of identified safety issue
Incompatibility between needle-free connectors (NFCs) and Luer tips of the Aurum Range pre-filled syringe barrel can lead to blockage and delayed delivery of medication. The MHRA has received reports where, due to use of incompatible NFCs, fragments from the NFC have broken off and blocked the syringe outflow. In some cases, such damage resulted in a delay in administering emergency medication. The MHRA issued a Device Safety Information (DSI) in August 2023 to raise awareness of this safety issue. Refer to the reference images below for the Aurum Range of pre-filled syringes.
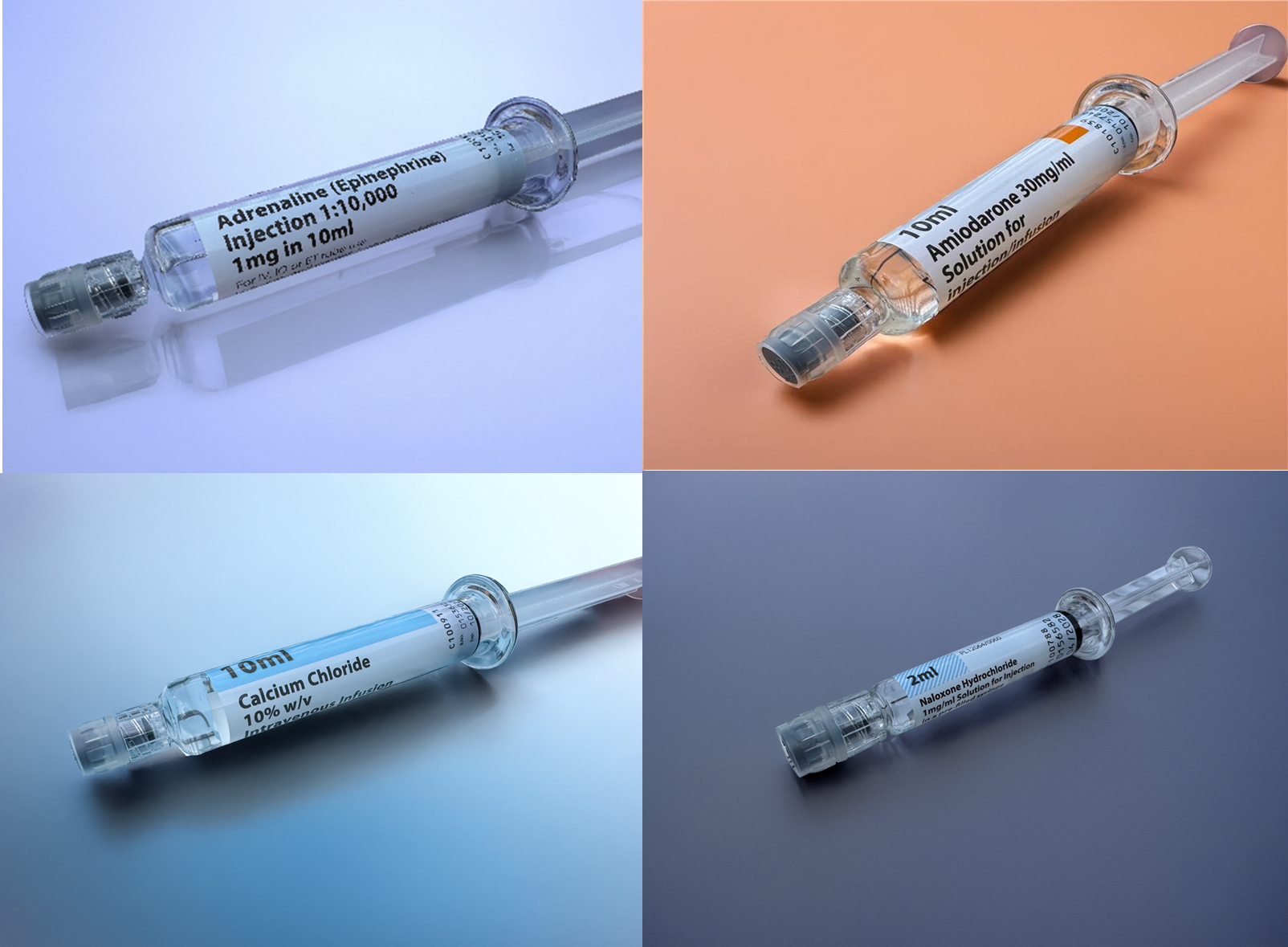
A new 10 ml CONNECT syringe barrel has been introduced by Ethypharm for the Aurum Range of pre-filled syringes. The phased rollout will begin in July 2025 with the new 10ml CONNECT barrel for the Adrenaline 1:10,000 pre-filled syringe, followed by a phased rollout of the 10ml CONNECT barrel for Amiodarone and Calcium Chloride pre-filled syringes.
The CONNECT barrel has a wider internal channel and fixed Luer lock adapter. This change increases the number of NFCs that are compatible with the CONNECT Aurum Range of pre-filled syringes compared to the established barrel design, addressing the current safety concern associated with incompatible NFCs.
Due to the long shelf life of the Aurum Range pre-filled syringes, there will be an extended period where both, the established syringe barrels and the new CONNECT syringe barrels will be available for all products. All NFCs that are compatible with the established barrel will continue to be compatible with the CONNECT barrel. However, the reverse is not applicable. Therefore, there remains a risk of inadvertently using an incorrect NFC. The current DSI highlights and makes recommendations to mitigate this risk.
To aid in-product identification between both barrels, the new CONNECT barrel products have new product codes, and the following changes have been made to the packaging:
- Text: Updated layout with the word “CONNECT.”
- Icon: “Twist to open” icon on CONNECT packaging.
- Colour: Green with white stripes box closure label on CONNECT packaging (previously white).

Healthcare professionals are requested to ensure that only compatible NFCs are procured and used with the Aurum pre-filled syringes.
Reporting advice
Healthcare professionals should report incidents:
- in England and Wales to the Yellow Card website or via the Yellow Card app
- in Scotland to Incident Reporting & Investigation Centre (IRIC) and their local incident recording system
- in Northern Ireland to the Northern Ireland Adverse Incident Centre and their local incident recording system
Download Document
Additional information:
To obtain hard copies of the list showing compatible NFCs for use with Aurum pre-filled syringes from the manufacturer phone 01277 266 600 (select option 2) or email: medinfo@ethypharm.com.
You can sign up to receive email updates on alerts and device safety information from the MHRA.
You can sign up to receive our monthly roundup of safety communications.
For any enquiries, please contact info@mhra.gov.uk
Stakeholder engagement:
- NHS England National Patient Safety Team
- NHS Wales
- NHS National Services Scotland, Incident Reporting and Investigation Centre (IRIC)
- Northern Ireland Adverse Incident Centre (NIAIC)
- National Infusion and Vascular Access Society (NIVAS)
- Royal College of Emergency Medicine