Start smart then focus: antimicrobial stewardship toolkit for inpatient care settings
Updated 12 September 2023
Applies to England
Start smart then focus: toolkit summary
This document is an update of the ‘start smart then focus’ (SSTF) antimicrobial stewardship toolkit, originally published in 2011 and last updated in 2015.
It takes into account recommendations from the Chief Medical Officer (CMO) (1), the UK 5-year antimicrobial resistance national action plan 2019 to 2024 (2), a 2017 Cochrane Review (3), the 2022 ESPAUR report (4), and the antimicrobial stewardship (AMS) guideline produced by the National Institute for Health and Care Excellence (NICE).
SSTF is evidence-based guidance for secondary care clinicians and leaders, designed to reduce the risk of antimicrobial resistance (AMR) while safeguarding the quality of care for patients with infection. The scope is limited to inpatient care settings (including acute, community and mental health trusts) as an environment with relatively high intensity of antimicrobial use where patients are monitored over time, facilitating review and revision of the initial diagnosis and treatment regimen. The TARGET antibiotics toolkit provides AMS resources for primary care settings.
In England, registered healthcare organisations are required to provide evidence of prudent prescribing and AMS as a standard of compliance; as stated in the Health and Social Care Act 2008: Code of Practice on the prevention and control of infections (6). This should be achieved through an ongoing programme of audit and action which is usually monitored by AMS oversight groups or local medicines optimisation groups. Adherence to the Code of Practice is considered by the Care Quality Commission (CQC) when it inspects organisations.
Within healthcare organisations, such as NHS Trusts, consultants and other senior members of the healthcare team should assume a leadership role for quality improvement of antimicrobial prescribing in their specialist areas, in collaboration with consultant microbiologists and infectious diseases specialists, AMS nurses, AMS pharmacists and pharmacy technicians. AMS initiatives should engage with patient facing members of the multidisciplinary healthcare team to develop a wider understanding of AMS throughout the organisation, implement well-informed and successful strategies, and ensure that patients receive optimal antimicrobial treatment.
The toolkit provides an outline of evidence-based AMS in inpatient care settings and supports adherence to the Code of Practice. It is divided into 3 main parts:
- toolkit summary: overview of the SSTF AMS toolkit available for quick reference
- sections 1 to 3: SSTF principles and ‘components of best practice’; in-depth detail and references for SSTF, and recommendations on good clinical practice and measures for assessing compliance through audits
- section 4: ‘antimicrobial stewardship programmes’; for use by AMS teams and committees or equivalent
Figures 1 and 2 show the AMS – clinical management algorithm and the AMS surgical prophylaxis algorithm.
Start smart: assess, investigate, prescribe and document (AIPD)
Assess
Assess patient for clear evidence of infection (see figure 1) – to establish whether the patient is likely to benefit from antimicrobials as unnecessary use can increase the risk of patient harm.
Perform a comprehensive patient risk assessment to guide selection of proportionate treatment and determine the appropriate care environment. This should include considering disease severity, immunocompromised patients, likelihood of resistant pathogen, prior exposure to antimicrobials, and patients with factors commonly associated with health inequalities where appropriate.
Investigate
Obtain appropriate specimens for culture prior to commencing therapy where possible, including blood cultures before starting antimicrobial treatment if appropriate (but do not delay treatment in cases of severe sepsis) – to guide targeting of treatment in the event of subsequent deterioration and to support de-escalation to narrow-spectrum treatment.
Follow local guidelines for ordering appropriate laboratory investigations (biochemistry, haematology, immunology, organ function) and medical imaging where available.
Implement any required source control interventions as soon as medically or surgically practical – to reduce the risk of treatment failure.
Prescribe
Initiate prompt antimicrobial treatment for patients with severe sepsis or life-threatening infections based on local guidelines – to reduce avoidable morbidity and mortality.
Comply with local antimicrobial prescribing guidance informed by local resistance patterns or national guidance (as appropriate) – to improve clinical outcomes for patients.
Take a detailed drug allergy history, document and consider de-labelling allergies where appropriate – to ensure patients are not denied access to the most effective therapy.
Avoid indiscriminate use of broad-spectrum antimicrobials – to preserve the effectiveness of these agents, reduce collateral damage to the patient’s microbiota and reduce the risk of opportunistic infection (such as C. difficile).
For surgical prophylaxis: Prescribe single dose antimicrobials where single dose antimicrobials have been shown to be effective – to minimise post-operative surgical site infection at an acceptable risk of harm from antimicrobial exposure (see figure 2).
Document
Document evidence of infection, working diagnosis (and disease severity), drug name, dose, formulation, and route on the prescription chart and in the clinical notes – in accordance with good clinical record-keeping.
Consider using the ‘antibiotic review kit (ARK) decision aids’ to categorise prescribing for possible or probable infection – to facilitate subsequent review and refinement of the treatment plan.
Include treatment duration where possible or specify a review date – to avoid unnecessarily prolonged treatment.
Record a clear clinical plan for patient management – to ensure safe handover of care between clinical teams.
If clinically essential to consider medical prophylaxis with antimicrobials, document clearly the indication and plan for review – to inform subsequent clinical decisions.
Then focus – antimicrobial review
Review and revise the clinical diagnosis and the continuing need for antimicrobials at 48 to 72 hours (see note 1) and document a clear plan of action from the antimicrobial review outcomes.
The 5 antimicrobial review outcomes (CARES) are to:
- Cease antimicrobial prescription if there is no evidence of infection – to reduce the risk of harm from antimicrobial treatment in the absence of benefit.
- Amend antimicrobials – ideally to a narrower spectrum agent – or broader if required – to ensure that treatment is effective and proportionate.
- Refer to non-ward based antimicrobial therapy services (such as ‘complex outpatient antimicrobial therapy’ – COPAT, or virtual wards) for appropriate patients if available – to facilitate timely discharge from hospital and reduce risk of acquisition of healthcare-associated infection.
- Extend antimicrobial prescription and document next review date or stop date – to avoid inappropriately prolonged treatment.
- Switch antimicrobials from intravenous to oral according to national intravenous to oral switch (IVOS) criteria – to facilitate timely discharge from hospital and reduce the risk of harm from intravenous administration.
It is essential that the review and subsequent decision is clearly documented in the clinical notes and on the drug chart where possible, for example ‘stop antimicrobial’.
The antimicrobial review may encompass more than one of the outcomes depending on clinical circumstances.
Note 1: 48 to 72 hours is the anticipated typical interval before ‘review and revise’ as results of diagnostic investigations are expected within 72 hours; however, factors such as rapid diagnostics and acute changes in clinical circumstances may facilitate or mandate earlier review and/or intervention.
It is recommended that healthcare organisations should monitor adherence to the SSTF principles regularly in all clinical areas (at least annually) and, in England, evidence adherence to the Code of Practice, developing an action plan for improvement as required.
Figure 1: AMS clinical management algorithm

Figure 1 shows a flowchart of the 9 steps taken during clinical management of an infection. The first box has the title ‘Evidence of infection’ and 6 bullet points. These points are: history, signs and symptoms, physical examination, laboratory results, diagnostic test results and medical imaging.
There is an arrow from the evidence of infection box to the second box which says ‘Start Smart’.
A second arrow links to a third box. The third box has the title ‘Assess’ with 2 bullet points: evidence or suspicion of infection and patient risk (severity, immunocompromise, resistance).
A third arrow links to the fourth box. The fourth box has the title ‘Investigate’ with 4 bullet points: cultures, laboratory investigations (biomarkers, haematology, immunology, organ function), imaging and source control.
A fourth arrow links to the fifth box. The fifth box has the title ‘Prescribe (taking into consideration)’, with 4 bullet points: urgency, guidelines (local), allergy and contra-indications and spectrum (proportionate).
A fifth arrow links to the sixth box. The sixth box has the title ‘Document’ with 4 bullet points: working diagnosis, certainty (possible or probable infection), treatment regime and plan plus review date.
A sixth arrow links to the seventh box which says ‘Then Focus’. A seventh arrow the links to the final box with the title ‘Post-prescription review outcome options’. This box contains 5 bullet points: cease, amend, refer, extend and switch.
Figure 2: AMS surgical prophylaxis algorithm
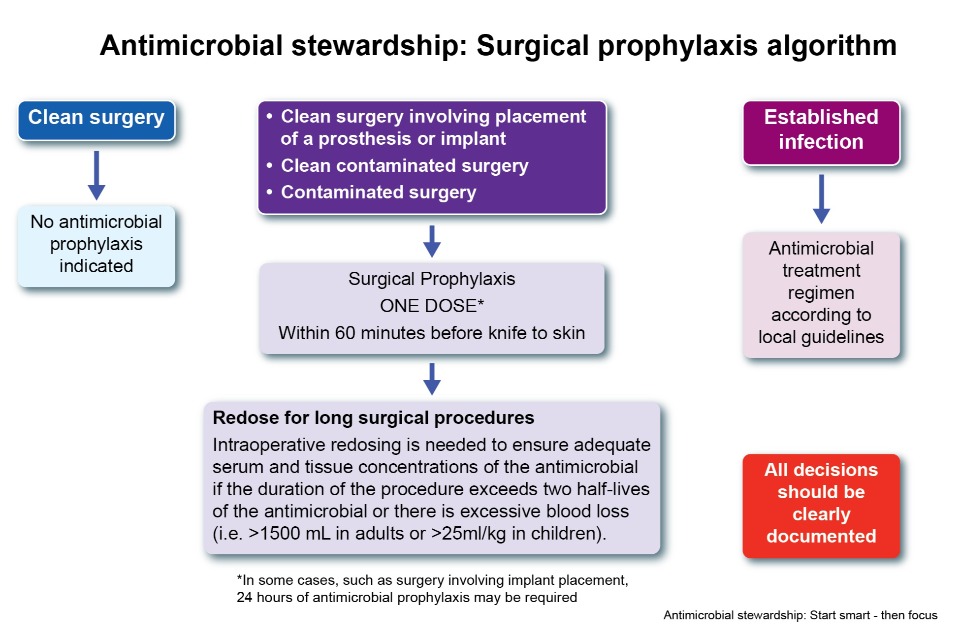
Figure 2 contains information on the surgical prophylaxis algorithm. Boxes are arranged in 3 columns.
The first column contains 2 boxes; the top box says ‘Clean surgery’. An arrow links from the top box to the box underneath. The second box says ‘No antimicrobial prophylaxis indicated’.
In the middle column the top box contains 3 bullet points: clean surgery involving placement of a prosthesis or implant, clean contaminated surgery, contaminated surgery. An arrow links to a second box underneath the first, containing the text ‘Surgical prophylaxis, one dose within 60 minutes before knife to skin’. An asterisk after the line one dose links to the caveat ‘In some cases, such as surgery involving implant replacement, 24 hours of antimicrobial prophylaxis may be required’. A second arrow links from the second to the bottom box with the title ‘Redose for long surgical procedures’ and the text ‘Intraoperative redosing is needed to ensure adequate serum and tissue connections of the antimicrobial if the duration of the procedure exceeds 2 half-lives of the antimicrobial or there is excessive blood loss (for example >1500mL in adults or >25ml per kg in children)’.
The final column contains 3 boxes. The top box contains the text ‘Established infection’. An arrow links to the box underneath which contains the text ‘Antimicrobial treatment regimen according to local guideline’. A final box in the bottom right of the figure contains the text ‘All decisions should be clearly documented’.
Introduction
Aim
The aim of this multipurpose toolkit is to provide an evidence-based framework for antimicrobial stewardship (AMS) and promote a SSTF approach to antimicrobial prescribing in secondary healthcare settings.
Context
Antimicrobial resistance (AMR) is defined as the ‘loss of effectiveness of any anti-infective medicine, including antiviral, antifungal, antibacterial and antiparasitic medicines’ (5). The prevalence of AMR has risen markedly over the last 40 to 50 years, further compounding the problem is that few truly novel antimicrobials are being developed. This has led to increased pressure on existing antimicrobials and greater challenges in treating patients effectively.
Inappropriate use of antimicrobials also increases the risk to patients of colonisation and infection with resistant organisms and subsequent transmission to other patients. Clinical evidence demonstrates that the inappropriate use of broad-spectrum antimicrobials is associated with the selection of AMR microbes. This includes extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria (7, 8). Methicillin resistant Staphylococcus aureus (MRSA) (9 to 12) and the induction of Clostridioides difficile infection (CDI) (13 to 15) and can cause long-lasting harmful changes to the body’s protective microbial flora (16, 17).
Broad-spectrum antimicrobials (including cephalosporins, fluoroquinolones, co-amoxiclav, piperacillin-tazobactam, carbapenems and clindamycin) have been most strongly associated with CDI, but all antimicrobials should be avoided unless there are clear clinical indications for their use. Antimicrobials should be used for the shortest duration possible that results in a successful clinical outcome. They should also be managed within a multifactorial programme (including infection prevention and control precautions) aimed at reducing healthcare-associated infections (HCAIs) and minimising the risk of AMR (5, 6).
AMS is defined as ‘an organisational or healthcare-system-wide approach to promoting and monitoring judicious use of antimicrobials to preserve their future effectiveness’ (5). It is an important element of key national initiatives to address AMR and remains an integral focus of healthcare policy and clinical practice.
The aims of AMS initiatives are to improve the safety and quality of patient care and to contribute significantly to reductions in the emergence and spread of AMR. A strong and robust AMS programme is a key component of tackling AMR, reducing waste, and improving patient safety and outcomes (18).
The NICE AMS guideline recommends that organisations should establish an AMS programme taking account of the resources needed to support good AMS across all care settings (5). It is not the responsibility of specialists alone to champion AMS efforts within an organisation. The success of AMS programmes is dependent on the support of hospital management and senior clinical staff; the Trust board, leadership team and staff are all responsible for establishing, maintaining and supporting a co-ordinated approach to AMS (6). It is important to note that AMS is also a key responsibility of individual healthcare professionals.
Past reports have highlighted the failure to embed AMS programmes into local practice. A 2009 National Audit Office report suggested that one-third of Trusts in England did not have a robust strategy to review antimicrobial prescriptions automatically within a defined period (19). A national survey of AMS activites detailed in the 2014 ESPAUR report revealed that although a large majority (87.9%) of Trusts reported reviewing the SSTF document formally or informally; only 48% of Trusts reported implementing a SSTF action plan after a review.
In addition, whilst 79% of Acute Trusts collated data on at least one of the recommended audits in SSTF, there was a low uptake of audits that can be correlated to patient outcomes (for example time to first dose in severe sepsis, post prescription review and documentation at 48 hours) (20).
In England, the ‘Code of Practice for the prevention and control of infections’ applies to all healthcare and adult social care organisations under the Health and Social Care Act 2008 and sets out 10 criteria for cleanliness and infection control towards which organisational compliance is assessed (6).
The guidance states:
Procedures should be in place to ensure prudent prescribing and antimicrobial stewardship. There should be an ongoing programme of audit, revision and update with feedback to management, prescribers and administrators. In healthcare settings this is usually monitored by the antimicrobial management team or local primary care medicine optimisation team (6).
The Code of Practice is considered by the Care Quality Commission (CQC) when it inspects organisations. Outcome 8 of the CQC`s Essential Standards for Quality and Safety on cleanliness and infection control also refers to the Code of Practice which further emphasises the importance of adherence for organisations (21). In addition to this, the NHS Standard Contract mandates that healthcare organisations are required to have and comply with a HCAI reduction plan for each contract year that reflects local and national priorities, including AMR, and is agreed with healthcare commissioners.
Why use the toolkit
The SSTF AMS toolkit provides an evidence-based AMS framework for use in secondary healthcare settings; this includes all providers of inpatient care. It is divided into 2 main parts:
Sections 1 to 3
SSTF principles and ‘components of best practice’. These sections encompasses good clinical practice. It is relevant to all prescribers of antimicrobials in inpatient care settings and AMS teams as it includes recommendations on measures for assessing compliance through audits.
Section 4
‘Antimicrobial stewardship programmes’. This section is recommended for use by AMS teams and committees or equivalent, in conjunction with the Code of Practice, ‘Clostridioides difficile: how to deal with the problem’ guidance (22), and NICE guideline (NG15) ‘Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use’ to ensure a strong AMS programme (5). This should be accompanied by a robust programme of auditing activities that promote safe and appropriate use of antimicrobials and a thorough education and training approach for all healthcare professionals (23).
The toolkit can be used as part of quality improvement strategies for patient safety, enhancing stewardship in antimicrobial usage, and most importantly, to ensure optimal patient care and safety by reducing inappropriate prescribing and promoting effective AMS. In England, compliance with this toolkit and auditing can be used as evidence of compliance with the Code of Practice on infection prevention and control and incorporated into HCAI reduction plans.
Section 1: SSTF principles – start smart
Assess
Assess patient for clear evidence of infection – to establish whether the patient is likely to benefit from antimicrobials as unnecessary use can cause patient harm.
Clinicians should ensure that patients clinically require antimicrobials prior to initiation. This is to preserve the future effectiveness of antimicrobials by keeping AMR in check; the widespread and often unnecessary use of antimicrobials produces the strong selective pressure which drives the evolutionary response in microbes.
It is equally important to protect patients from harm caused by unnecessary antimicrobial use. Antimicrobial therapy can disturb gut microbiota; this negatively impacts patients by reducing microbial diversity, altering the function and formation of microbiota, and the selection of antimicrobial resistant strains making patients more susceptible to infection with opportunistic pathogens such as C difficile (24 to 27). Where there is an absence of convincing evidence of bacterial infection and the patient is clinically stable, antibiotics may be withheld, pending the results of investigations.
Perform a comprehensive patient risk assessment to guide selection of proportionate treatment and determine the appropriate care environment. This should include considering disease severity, immunocompromised patients, likelihood of resistant pathogen, prior exposure to antimicrobials, and patients with factors commonly associated with health inequalities where appropriate.
Clinicians should consider patient and infection specific factors when reviewing patients with infections. This should include establishing patient vulnerability (for example immunocompromise and immunosuppressant use; vaccination status), infection severity and risk of mortality, likelihood of infection with a pathogen resistant to usual first-line treatment (for example due to prior antimicrobial exposure, recent hospitalisation, foreign travel) and factors commonly associated with health inequalities where appropriate.
Immunocompromised patients have an increased susceptibility to infections for a wide variety of reasons; some may also be prescribed antimicrobial prophylaxis which is important to consider when prescribing further antimicrobial treatment (28). The risk of AMR is increased for patients who have received multiple courses of antimicrobials, particularly within the previous 6 months, increasing the likelihood of treatment failure for standard first-line treatment. Expert advice should be sought when appropriate to select effective empirical treatment (29).
Investigate
Obtain appropriate specimens for culture prior to commencing therapy where possible, including blood cultures before starting intravenous treatment if appropriate (but do not delay treatment in cases of severe sepsis) – to guide targeting of treatment in the event of subsequent deterioration and to support de-escalation to narrow-spectrum treatment.
Clinicians need to know the source of infection and antimicrobial susceptibility of infectious organisms to identify and prescribe the most appropriate and targeted antimicrobial therapy for patients. Microbiological investigations are critical for patient safety, supporting targeting of treatment to effectively treat resistant pathogens, de-escalation from broad-spectrum to narrow-spectrum therapy to reduce collateral damage to the microbiota, cessation of therapy when cultures indicate infection is unlikely, and for epidemiological surveillance.
Blood cultures should ideally be obtained prior to commencing intravenous treatment, to significantly improve successful pathogen yield. It is recommended at least 2 sets of blood cultures (2 aerobic and 2 anaerobic bottles) should be cultured from adult patients for optimum detection of bloodstream infections; these 2 sets should provide a volume of 8 to 10ml per bottle. They should be sent immediately for analysis as samples need to be incubated within 4 hours.
Empirical treatment should be prescribed promptly however to patients with potentially life-threatening infections, in accordance with NICE guidance, as evidence suggests delay to antimicrobial treatment is associated with a greater risk of hospital mortality (30 to 35).
Follow local guidelines for ordering appropriate laboratory investigations (biochemistry, haematology, immunology, organ function), and medical imaging where available.
Clinicians should review the results of relevant laboratory investigations including inflammatory markers such as serum C-reactive protein and white blood cell count to ascertain the presence of infection and determine its location and severity. This is important to guide appropriate empirical treatment for patients, according to local clinical guidelines. Reviewing results of laboratory investigations in addition to symptoms and signs during clinical assessment reduces diagnostic uncertainty.
Evidence from a large cohort study demonstrated that a complex clinical decision rule involving 28 clinical features to support diagnosis of infection was not able to provide exact discrimination of infection; therefore, additional investigations continue to be necessary for confirmation (36 to 40).
Implement any required source control interventions as soon as medically/surgically practical – to reduce the risk of treatment failure.
Clinicians should ensure that source control (for example incision and drainage of pus, removal of infected prosthetic material such as urinary and intravascular catheters) is implemented promptly where appropriate. Source control of suitable infections is associated with reduced treatment failure and improved survival. It should be achieved as soon as possible following initial resuscitation.
Published clinical experience suggests that without adequate source control, many severe infections will not stabilise or improve despite treatments such as rapid resuscitation and antimicrobial therapy. This includes intra-abdominal infections, soft tissue infections, and infections associated with intravenous lines and indwelling urinary catheters where source control is generally feasible and impactful (41 to 44).
Prescribe
Initiate prompt antimicrobial treatment for patients with severe sepsis or life-threatening infections based on local guidelines – to reduce avoidable morbidity and mortality.
Clinicians should use local guidelines to initiate prompt effective antimicrobial treatment after diagnosis in patients with life-threatening infections such as severe sepsis. The urgency of initiating antimicrobial treatment of adult and paediatric patients with suspected sepsis should be guided by National Early Warning Scores in secondary care settings (NEWS2 for adults, PEWS for children), combined with clinical and laboratory assessments of severity, urgency and probability of infection (41, 45 to 47).
Comply with local antimicrobial prescribing guidance informed by local resistance patterns or national guidance (as appropriate) – to improve clinical outcomes for patients.
Clinicians should be aware of and take into consideration local and national antimicrobial prescribing guidelines before making clinical and prescribing decisions. Antimicrobial prescribing guidelines are designed to establish acceptable standards of care, emphasise quality improvement, and improve patient outcomes. Antimicrobial prescribing guidelines are developed by infection specialists with knowledge of pathogen epidemiology, local resistance patterns, drug penetration to the site of infection and clinical trial evidence of effectiveness. Guideline-adherent empirical therapy is associated with a relative risk reduction for mortality of 35% (48 to 55).
Take a detailed drug allergy history, document and consider de-labelling allergies where appropriate – to ensure patients are not denied access to the most effective therapy.
Clinicians should ensure that a thorough and accurate drug allergy history is taken in the context of prescribing antimicrobial therapy to allow patients to receive the most appropriate treatment. This is especially important in the context of penicillin allergies. Evidence demonstrates that greater than 90% of patients labelled with a penicillin allergy are not truly allergic to penicillins and associated β-lactams; this results in patients being denied or unnecessarily avoiding potentially lifesaving first-line treatments. Inappropriate penicillin allergy labels may negatively impact antimicrobial stewardship by leading to use of potentially less effective and broader spectrum antimicrobials increasing the risk of antimicrobial resistance and impacting patient care. Penicillin allergy de-labelling initiatives are a becoming a key component of antimicrobial stewardship programmes (56 to 60).
Avoid indiscriminate use of broad-spectrum antimicrobials – to preserve the effectiveness of these agents, reduce collateral damage to the patient’s microbiota and reduce the risk of opportunistic infection (such as C. difficile).
Clinicians should avoid the inappropriate use of broad-spectrum antimicrobials where possible (61). This is necessary to preserve their future effectiveness; excessive use of broad-spectrum antimicrobials is a strong factor in the emergence of antimicrobial resistance (62). Using indiscriminate broad-spectrum antimicrobials can also increase the risk of collateral damage to the patient’s gut microbiota and increase the risk of subsequent opportunistic infection (for example C. difficile) and inflammatory bowel disease (24 to 26,63).
For surgical prophylaxis: Prescribe single dose antimicrobials where single dose antimicrobials have been shown to be effective – to minimise post-operative surgical site infection at an acceptable risk of harm from antimicrobial exposure.
Clinicians should prescribe single dose prophylactic antimicrobials where they have been shown to be effective in reducing surgical site infections. To maximise the benefits and reduce adverse effects, appropriate antimicrobials must be selected and administered within 60 minutes prior to surgical incision or tourniquet inflation. This enables peak blood levels to be present at the start of the surgical procedure (64 to 67).
Intraoperative redosing is needed to ensure adequate serum and tissue concentrations of the antimicrobial if the duration of the procedure exceeds 2 half-lives of the antimicrobial or there is excessive blood loss (for example >1500 mL in adults, >25 ml/kg in children) (68). A treatment course of antimicrobials may also need to be given (in addition to appropriate prophylaxis) in cases of dirty surgery or infected wounds. The appropriate use and choice of antimicrobials should be prescribed in line with local guidelines. Complex cases may require discussion with infection specialists (69).
Document
Document evidence of infection, working diagnosis (and disease severity), drug name, dose, formulation, and route on the prescription chart and in the clinical notes – in accordance with good clinical record-keeping.
Clinicians should ensure that antimicrobial prescriptions are documented sufficiently, accompanied by an indication and a clear duration/review date that supports colleagues to change or stop therapy when appropriate; using the antibiotic review kit (ARK) decision aids can help standardise the process depending on clinical circumstances (70). Evidence shows that prescribers are often reluctant to modify antimicrobial prescribing decisions made by others; even when a patient’s clinical status has changed, and treatment should be stopped or adjusted (71).
Consider using the ARK decision aids to categorise prescribing for possible or probable infection – to facilitate subsequent review and refinement of the treatment plan (70).
Clinicians should consider using interventions such as the ARK decision aids to help facilitate antimicrobial prescribing decisions and reduce antimicrobial use in hospital. This includes documenting whether infection is ‘possible’ or ‘probable’ and implementing a review-and-revise approach at 48 to 72 hours. Lack of information about an antimicrobial prescription is a key barrier to its discontinuation (47). Antimicrobial prescribing decisions are influenced by sociocultural and behavioural factors which include the fear of adversely affecting health outcomes for patients, intolerance of uncertainty, and professional hierarchy (72). ARK decision aids can help overcome these barriers.
Include treatment duration where possible or specify a review date – to avoid unnecessarily prolonged treatment.
Clinicians should adopt the recommended ‘review and revise’ approach when prescribing antimicrobials; this is a key to reduce unnecessary continuation of antimicrobials, reduce selection pressure on bacteria to develop resistance, and to protect patients from adverse effects and antimicrobial resistant pathogens. It is not always necessary to ‘complete the course’ of antimicrobial therapy; duration of treatment in hospital should be guided by patient risk factors and response to treatment (73 to 78).
Record a clear clinical plan for patient management – to ensure safe handover of care between clinical teams.
Clinicians should take care to produce and document accurate clinical plans when handing patient care over to different healthcare professionals or teams as they improve patient safety through strengthened communication and ensure appropriate continuity of care. This includes when patients are transferred between organisations and/or are discharged from secondary care. Plans, where possible, should include contact details of prescribers for potential queries. Inadequate handovers increase the risk of patient harm and can result in increased costs of delivering healthcare and longer hospital stays, delays in diagnosis, duplication of diagnostic tests and potential for clinical errors (79).
Provide exact indication on the drug chart for medical prophylaxis.
Clinicians should clarify exact indication when prescribing medical prophylaxis as this may provide greater insight and context regarding the patient’s clinical state and impact the antimicrobial treatment they require.
Section 2: SSTF principles – then focus
Review and revise the clinical diagnosis and the continuing need for antimicrobials by 48 to 72 hours and document a clear plan of action – the antimicrobial review outcome.
Antimicrobials are often initiated before a patient’s full clinical picture is known; this is usually to provide treatment in potentially life-threatening infections as per clinical guidelines. Following treatment commencement, additional information from diagnostic tests usually begin to emerge. It is vital to consider de-escalation of antimicrobials at this point, where appropriate. This is a critical component within antimicrobial stewardship programmes and involves discontinuing or switching to narrower spectrum antimicrobials if possible (80).
Prescribers should consider the information obtained within this time period and adopt a ‘review and revise’ approach to re-evaluate why therapy was initiated and review whether adjustments are needed. The timeline for review depends on various factors such as timing of when necessary diagnostic results are received, the patient’s clinical status, and recommendations from clinical guidelines.
Evidence suggests few bloodstream infections are detected after 48 hours of culture incubation; therefore, prescribers may usually de-escalate from empirical antimicrobial cover at this time with minimal risk. Some evidence suggests it may be safe to discontinue antimicrobials within 24 hours of prescribing for patients with no signs of infection due to growing field of rapid diagnostics, however more research is needed to fully support this de-escalation strategy. (81 to 84).
Antimicrobial review outcomes
The 5 antimicrobial review outcomes: ‘CARES’ – to cease, amend, refer, extend or switch.
Cease
Cease antimicrobial prescription if there is no evidence of infection – to reduce the risk of harm from antimicrobial treatment in the absence of benefit.
Clinicians should cease antimicrobials prescriptions if there is no evidence of infection. Studies which reviewed stopping antimicrobials early when signs of infection are lacking demonstrated better survival rates. Taking unnecessary antimicrobials can cause harm to patients and increase the likelihood of antimicrobial resistant infections in the future. Therefore, de-escalation of antimicrobials and discontinuation should be considered in appropriate circumstances (80).
Evidence suggests that conservative strategies focused on limiting selective pressure such as reducing unnecessary usage of antimicrobials and preventing situations which select for and promote resistance, are needed to prevent further AMR developing (24 to 27, 55).
Amend
Amend antimicrobial prescription – ideally to a narrower spectrum agent, or broader if required – to ensure that treatment is effective and proportionate. Prescribers should seek expert advice when necessary.
Clinicians should amend antimicrobials where appropriate. Evidence suggests that adopting a ‘review and revise’ approach towards managing antimicrobial therapy results in a reduced relative risk of death by 56%. In these circumstances, targeting of antimicrobials to narrower spectrum agents is recommended as part of AMS efforts (80).
Studies have demonstrated that broad spectrum antimicrobials are not linked to improved clinical or patient-centred outcomes compared with narrow spectrum antimicrobials and are associated with higher rates of adverse effects. The recommendation is therefore to use narrow spectrum antimicrobials where possible 55, 85 to 91).
Refer
Refer to non-ward based antimicrobial therapy services, such as complex outpatient antimicrobial therapy (COPAT) or virtual wards for appropriate patients if available – to facilitate timely discharge from hospital and reduce risk of acquisition of healthcare-associated infection.
Clinicians should refer appropriate patients to non-ward based antimicrobial therapy services if they are available. Availability and type of services may vary depending on organisation. Examples include outpatient parenteral antimicrobial therapy (OPAT), COPAT, virtual wards, as well as other services designed for delivering monitored antimicrobial therapy outside of the hospital setting.
OPAT services have been demonstrated to be safe and clinically effective, with low rates of complications/readmissions and high levels of patient satisfaction. OPAT services are evolving to become COPAT services; this refers to both outpatient intravenous therapy in addition to the use of oral regimens allowing for early intravenous-to-oral switch or as an alternative to intravenous antimicrobial therapy (92 to 96).
With large-scale expansion of models of care such as virtual wards in the NHS; it is important to consider how patients requiring longer courses of antimicrobials can be treated appropriately in these circumstances. All non-ward based antimicrobial therapy services should follow ‘UK Good Practice Recommendations for Outpatient Parenteral Antimicrobial Therapy’ guidance and use it as quality indicators for service evaluation and quality improvement (92).
Extend
Extend antimicrobial prescription and document next review date or stop date for IV and oral antimicrobials – to avoid inappropriately prolonged treatment.
Clinicians should extend antimicrobial prescriptions, in appropriate clinical circumstances, for as long as is necessary to treat the patient’s infection. It is vital to specify a review or stop date when documenting this decision. Patients are put at unnecessary risk from antimicrobial resistance when treatment is given for longer than required with no added benefit.
Studies have demonstrated that for patients who receive shorter courses of antimicrobials, recurrent infections with multidrug resistant pathogens develop less frequently than in those who receive longer antimicrobial courses. Whilst there are clinical situations where prolonged therapy is beneficial and necessary, prolonged duration of antimicrobial therapy is generally associated with increased resistance, side effects, high costs and adverse drug reactions (76, 97, 98).
Switch
Switch antimicrobial formulation from IV to oral according to national IVOS criteria – to facilitate timely discharge from hospital and reduce the risk of harm from intravenous administration (99).
Clinicians should switch antimicrobial formulation where possible; switching antimicrobials from intravenous to oral (IVOS) as soon as clinically appropriate has many beneficial outcomes. Numerous studies have demonstrated the equal efficacy of an early IVOS compared to a full course of intravenous therapy.
Early IVOS has many indirect advantages, such as reduced incidences of catheter-related infections, decreased length of hospital stays and significant decreases in costs. Evidence suggests it improves patient experience, frees up nursing time, reduces the incidence of bloodstream infections, reduces adverse events, reduces healthcare associated infections, reduces carbon footprint, reduces dosing errors, and reduces broad-spectrum antimicrobial exposure (100 to 108).
It is essential that the review and subsequent decision be clearly documented in the clinical notes. The decision should also be documented clearly on the antimicrobial prescription.
Section 3: components of best practice for antimicrobial prescribing
The following information summarises key criteria of best practice with regards to safe and effective antimicrobial prescribing tethered to robust antimicrobial stewardship. There are also suggestions on how their implementation may be evaluated through potential audits.
Treatment: components of best practice for antimicrobial prescribing
Treatment of infection emergencies
A delay in starting adequate antimicrobial therapy in severe infection is associated with increased morbidity and mortality.
Audit the treatment of severe sepsis and septic shock against clinical standards. This should include an audit of the time from the onset of severe sepsis to the administration of the first dose of antimicrobial therapy.
Communication of the decision to prescribe antimicrobials
Communication between healthcare teams is vital to ensure safe and effective patient care. The requirement to document prescribing decisions discourages antimicrobial prescribing where evidence of infection is lacking. The ARK decision aids can be utilised to help facilitate this process (70).
Audit the documentation of the decision to start antimicrobial therapy along with the indication or provisional (working) diagnosis and level of certainty (for example possible or probable) in the clinical notes and on the drug chart. This should include the clear identification of the prescriber and their contact details.
Allergy status
Determining true allergy status is crucial in delivering safe and effective care. Evidence shows that > 90% of patients labelled with a penicillin allergy are not allergic to penicillins and associated β-lactams; this often results in them receiving second line antimicrobials associated with worse outcomes and higher economic costs (58).
Audit the documentation of penicillin allergy status and documentation of the source of this information
Diagnostic tests including microscopy, culture and sensitivity
The availability of microscopy, culture and sensitivity information will facilitate the prompt de-escalation of broad-spectrum agents or the tailoring of therapy in cases of treatment failure.
Audit the appropriateness of specimens (for specific infections) obtained for microscopy, culture and sensitivity. This should conform to local guidelines.
Audit action taken following diagnostic test results.
Antimicrobial consumption
The unnecessary continuation of antimicrobials is associated with HCAIs and contribute to the development of AMR.
Audit the consumption of antimicrobial agents and provide feedback to prescribers by specialty.
Choice of antimicrobial agent(s)
Inappropriate antimicrobial therapy is associated with HCAIs, the development of AMR and the associated risks of unnecessary drug exposure.
Audit the choice of antimicrobial therapy. This should be according to local guidelines where available. This audit may also opt to include the dose and route of administration of the antimicrobials prescribed.
Review date for prescribed antimicrobials
An expected duration or review date should be documented on antimicrobial prescriptions. This practice will discourage open-ended prescriptions.
Audit the review of antimicrobials at 48 to 72 hours after initiation. This should capture the documentation of the decision to continue current therapy and subsequent specified review or stop date.
Duration of IV antimicrobial therapy
Treatment with IV antimicrobials should not continue beyond 48 to 72 hours unless recommended by local guideline or consultant microbiologist or infectious diseases specialist.
Unnecessary continuation of IV treatment increases the risk of line infection, increases demands on nursing staff and can extend hospital stay.
Where IV antimicrobials are continued at 48 to 72 hours after initiation, audit the documentation for continuing treatment.
Audit the relative consumption of IV and oral antimicrobials.
IV-to-oral antimicrobial switch
Treatment with IV antimicrobials should be switched to oral therapy promptly once national IVOS switch criteria are met (or antimicrobials discontinued if appropriate) (99).
Unnecessary continuation of IV treatment increases the risk of line infection and patient adverse effects, increases demands on nursing staff and can extend hospital stay.
Audit compliance with local IV to oral switch policy across all medical and surgical specialties.
or
Benchmark use of the IV route of administration by comparing with peer organisations the number of IV antimicrobial treatment days prescribed per admission and proportion of antimicrobial treatment days prescribed intravenously.
Total duration of antimicrobial therapy
Treatment with antimicrobials should not continue beyond 5 to 7 days (IV plus oral) unless stipulated in guidance or recommended by a local or national guideline or consultant microbiologist or infectious diseases specialist
Evidence consistently shows that short course durations of antimicrobials are as effective as longer courses for uncomplicated infections and are associated with less adverse effects for patients and reduced selection pressure for resistance (29, 78, 109).
Audit compliance of antimicrobial prescribing for each infective episode with local guidelines for total duration.
Peri-operative prophylaxis: components of best practice for antimicrobial prescribing (peri-operative prophylaxis)
Need for antimicrobial prophylaxis
For certain clean procedures, evidence suggests a lack of benefit of antimicrobials.
The clinical indication should comply with NICE guideline NG125.
Audit the indication for antimicrobial prophylaxis. Practice should conform to local guidelines.
Choice of antimicrobial agent(s) for peri-operative prophylaxis
Antimicrobial prophylaxis should ensure adequate coverage of expected pathogens according to surgical site. Whenever possible the prophylaxis should avoid cephalosporins, clindamycin and fluoroquinolones.
Where necessary, appropriate alternatives should be prescribed for patients with penicillin or beta-lactam allergy, or those colonised with resistant organisms, for example MRSA.
The choice of antimicrobial agent or agents should be prescribed according to local guidelines.
Audit the choice of antimicrobial therapy. This should be according to local guidelines where available.
Timing of antimicrobial prophylaxis
Antimicrobial prophylaxis should be administered within 60-minutes prior to incision (or tourniquet) or according to local guidelines (69). The lowest surgical site infection rates associated with optimal timing of pre-incision administration of antimicrobials.
Audit the time between the administration of antimicrobial prophylaxis and skin incision.
Repeat doses of antimicrobial prophylaxis
Single dose is indicated for the majority of procedures and should be implemented unless there is clear evidence that multiple or post-operative dosing improves outcomes.
Reason for antimicrobial administration beyond one dose should be documented and/or comply with criteria below agreed criteria.
Audit cases of multiple or post-operative antimicrobial prophylaxis.
MRSA positive patients
Decolonisation therapy is recommended prior to surgery and antimicrobial prophylaxis should include cover for MRSA.
Audit MRSA decolonization practice (normally collected by IPC teams).
Section 4: antimicrobial stewardship programmes
Current guidance and published evidence recommend AMS programmes should include the following:
- AMS teams
- evidence-based antimicrobial prescribing guidelines
- assessment of AMS activities
- education, training, and responsibilities of ward-based teams and clinicians (across multiple professions) directly responsible for patients care
AMS programmes are associated with a reduction in inappropriate antimicrobial prescribing, a lower incidence of HCAIs, cost effective prescribing, and a reduction in the emergence of AMR (3, 55). A vital priority for antimicrobial stewardship programmes is to ensure patient safety and improve the quality of patient care.
Elements of AMS such as using empirical therapy according to guidelines, de-escalation of therapy, early switch from intravenous to oral therapy, therapeutic drug monitoring, restricting use of certain antimicrobials (in particular, antibiotics in the ‘Watch and Reserve’ categories), and bedside consultations have been identified as leading to significant benefits for patient clinical outcomes and in the reduction of adverse events. Treatment according to guidelines and de-escalation of therapy can have significant effects on decreasing mortality (55, 120).
Antimicrobial stewardship teams
It is recommended that UK secondary care hospitals have the following teams set up to develop and implement an AMS programme for all adults and children admitted to hospital (5, 110).
- an AMS committee
- a ward-focused AMS team
These teams may have different names in practice depending on the preference of different organisations.
Membership of the AMS committee will vary depending on organisations but should be multidisciplinary and representative in accordance with NICE guidance. Recommendations for inclusion are a consultant microbiologist or infectious diseases specialist, an AMS pharmacist, an acute care physician, a surgeon, a senior member of the pharmacy management team, an anaesthetist, a paediatrician, an AMS or senior nurse, primary care representation (to ensure a whole healthcare economy approach), and a Director of Infection Prevention and Control (DIPC). The aim is to ensure a multidisciplinary approach and improve engagement across the organisation (121).
The AMS committee should report AMS activities and antimicrobial prescribing trends to the organisation’s Trust Board directly or via DIPC or infection control committee, and/or the drugs and therapeutic committee (or equivalent). Key roles of the AMS committee are to:
- ensure that evidence-based local antimicrobial guidelines (or endorsed national guidelines for use locally) are in place and reviewed regularly or when new evidence is published
- ensure regular auditing of the guidelines, AMS practice and quality assurance measures
- report a regular formal review of the organisation’s retrospective antimicrobial consumption data (especially highlighting the use of broad-spectrum antibiotics such as cephalosporins, co-amoxiclav, piperacillin-tazobactam, fluoroquinolones and carbapenems). UKHSA and ESPAUR measure antimicrobial consumption as defined daily doses (DDD) per 1000 admissions (see note 2)
- identify actions to address non-compliance with local guidelines, general AMS issues and other prescribing issues
Note 2: The presentation of DDD per admissions rather than bed-days reflects hospital activity for admissions rather than those who are in hospital only. This measurement would allow comparison with national data and consistent benchmarking between organisations.
In some organisations, such as non-acute community and mental health Trusts, where the setup of an AMS committee may not be feasible, oversight may instead be provided by the drug and therapeutics committee or equivalent.
In addition to the AMS committee, the formation of a ward-focused AMS team is recommended. This should include the AMS pharmacist and consultant microbiologist or infectious diseases specialist that report to the AMS committee or equivalent. The ward-focused team would be expected to review patients receiving antimicrobials at ward level as part of multi-disciplinary AMS ward rounds to ensure they are receiving the most appropriate care.
Improving antimicrobial prescribing and stewardship is dependent on strong clinical leadership. Within local healthcare organisations, such as NHS Trusts, medical and surgical teams, in particular consultants and other senior members of the healthcare team should take a leadership role for quality improvement of antimicrobial prescribing in their specialist areas to have a greater impact on practice and influence the habits of their junior colleagues (122, 123). This should be done in collaboration with consultant microbiologists or infectious diseases specialists, AMS nurses, and AMS pharmacists.
AMS initiatives should engage with patient facing members of the multidisciplinary healthcare team to develop a wider understanding of AMS throughout the organisation, implement well-informed and successful strategies, and ensure that patients receive optimal antimicrobial treatment.
Evidence-based antimicrobial prescribing guidelines
It is recommended that each organisation draw up a local AMS policy and utilise national and/or local antimicrobial prescribing guidelines.
Local guidelines should be evidence-based taking into consideration national guidance (for example from the British National Formulary, NICE or UKHSA). They should be relevant to the local healthcare setting and consider local AMR patterns. They should cover diagnosis and treatment of common infections and prophylaxis of infection. Prescribers should adhere to these guidelines and compliance should be monitored and supported by senior clinicians and pharmacists. Responsibility for guideline implementation should reside with the AMS committee, drugs and therapeutics committee or equivalent and the DIPC.
The local AMS policy should contain:
- a policy statement that outlines the need for clear clinical case definitions and associated evidence of infection to minimise unnecessary prescribing of antimicrobials (124, 125)
- an emphasis of the urgent need to start treatment with effective antimicrobial agents for severe sepsis or life-threatening infections (41, 45, 126, 127)
- a reminder for prescribers to use antimicrobial agent(s) with an adequate spectrum to cover only the expected pathogens for less severe infections; to highlight that broad-spectrum antibiotics are sometimes not as potent in vitro as their narrower-spectrum counterparts against certain pathogens (128)
- a reminder for prescribers to consider the risk of resistant pathogens such as MRSA or ESBL-producing organisms and offer alternative treatment regimens accordingly or encourage prescribers to seek expert advice (129 to 131
- a description of the importance of confirming the allergy status of recommended antimicrobial agents in patients as there may be a need to offer alternative treatment choices for those who are allergic (132); in line with NICE guidance on drug allergy, patients with a history of such allergies should be assessed, and the allergy label removed where it is not correct, in order to improve patient outcomes(133)
- an outline for clinical teams to take appropriate specimens for culture and sensitivity testing prior to commencing antimicrobial treatment; however, they should not delay starting treatment in patients with severe sepsis or life-threatening infections (41)
- a recommendation for intravenous (IV) administration only to patients who are severely ill, unable to tolerate oral treatment, or where oral therapy would not provide adequate coverage or tissue penetration
- an outline for prescribers to review microbiology results daily and to de-escalate to pathogen-directed narrow-spectrum treatment promptly where appropriate (134, 135)
- a recommendation for prescribers to document the next review date or stop date and switch to the oral route of administration promptly in accordance with local IV-to-oral switch guidance (136)
Antimicrobial prescribing guidelines should be guided by evidence and local susceptibility data (for example by area team where available). Guidelines should include the following:
- clinical diagnosis, to include case definition, evidence of infection, severity assessment and relevant microbiology investigations
- recommendations for non-antimicrobial treatment (for example fluid resuscitation, source control or surgery)
- empirical antimicrobial treatment recommendations: initial antimicrobial therapy (according to infection severity and patient risk) prior to availability of microbiology results or if a microbiological diagnosis is not going to be possible (see note 3)
- directed antimicrobial treatment when microbiology results are known and advice to contact clinical microbiologists or infectious diseases specialists if required (see note 3)
- oral switch guidance to highlight which oral agents to switch to and when (100)
- duration of therapy for IV and oral agents (97)
- specific guidance for exceptions and special cases if appropriate
- provide advice regarding monitoring and follow-up and contingency advice for treatment failure (5, 136, 137)
- guidance for prophylaxis for surgery or procedures; hese should also include the aim of prophylaxis (for example reduce surgical site infection), where prophylaxis is required and where it is not, distinction between risk groups (for example patients colonised with multi-drug resistant organisms such as MRSA, ESBL and CRE), alternatives where penicillin or other allergy exists and recommendation of single dose surgical prophylaxis regimens as appropriate and redosing frequency when more than one dose is required (68, 69)
Note 3: Empirical and directed treatment recommendations should specify the choice of drug(s), route of administration and dose. In addition, a reminder for prescribers to adjust dosing for specific patient factors, for example renal or hepatic impairment (137).
Assessment of antimicrobial stewardship activities
Organisations should demonstrate that they assess AMS activities against the SSTF AMS toolkit, and develop and adhere to an action plan to provide assurance to their Trust board of safe, effective, and appropriate antimicrobial prescribing.
It is recommended that as a minimum, healthcare organisations should develop an action plan and monitor adherence to SSTF principles regularly in all clinical areas (at least annually).
Organisations should monitor:
- evidence of documenting indication and duration or review date on the drug chart or clinical notes
- evidence of AMS review of antimicrobials at 48 to 72 hours after initiation and documentation of the antimicrobial review outcome on the drug chart or clinical notes
- the time between the onset of sepsis related hypotension and administration of appropriate antimicrobials – this may be part of ‘surviving sepsis’ related audits within the Trust (unless escalation to acute care organisation required)
- adherence with local guidance on the choice of antimicrobial therapy (or documented reason for non-compliance)
- AMR and consumption trends
The use of a self-assessment tool such as the AMS peer review tool may be a useful additional resource to enable such an appraisal (112). There are also a number of published quality indicators and checklists that may be useful (75, 113 to 116).
Procedures should be in place to ensure prudent antimicrobial prescribing and AMS. This requires an ongoing programme of audit, revision and update and should be monitored by the organisation’s AMS committee. It is recommended that a multi-disciplinary quality improvement or audit programme for AMS should be developed and sustained in every acute Trust.
Regular (at least annual) feedback of adherence to audits recommended within the SSTF toolkit should be provided to the Trust board (as part of the annual infection control committee report), prescribers, lead clinicians, microbiologists or infectious diseases specialists, nurses, pharmacists and the DIPC. The AMS committee and the DIPC should review antimicrobial consumption trends regularly (at least annually). Organisations should utilise technology such as organisational and national clinical surveillance tools where appropriate; this is important as many AMS activities can be labour intensive.
For organisations who conduct 6-monthly or annual point prevalence studies (PPS), data obtained may be used to monitor compliance with the organisation’s AMS programme and the Code of Practice on infection prevention and control. Organisations should consider formal investigating, via an existing clinical governance framework, cases of repeated non-compliance (without clinical justification), inappropriate prescribing particularly when these result in adverse patient outcomes (for example development of an HCAI, prolonged length of stay, and so on), and unexpected trends in prescribing. These should be documented and reported, for example in minutes of the AMS committee meetings.
The medical director or DIPC should challenge clinicians whose prescribing practice is found to be repeatedly inappropriate. It is also important for organisations to monitor patient outcomes to ensure that qualitative or quantitative alterations (changing, reducing, restricting) to antimicrobial prescribing do not have unintended detrimental effects for example increased time to clinical cure, increased mortality or increased readmission rate (138).
Education, training, and responsibilities of ward-based teams
Providers should ensure that all health and care workers involved in prescribing, dispensing and administration of antimicrobials receive induction and appropriate training in prudent antimicrobial use and the principles of antimicrobial stewardship (6)
All healthcare professionals involved in the management and treatment of patients with infections throughout their hospital admission have a duty to ensure good antimicrobial stewardship. They should have a clear understanding of their responsibilities and implement these in practice.
The following demonstrates some of the key responsibilities of doctors and non-medical prescribers, nursing staff, and pharmacy teams within antimicrobial stewardship.
Overview of prescribing, nursing, and pharmacy responsibilities
Doctors and non-medical prescribers
Doctors and non-medical prescribers should use the antimicrobial prescribing and stewardship competency framework to help develop their practice in relation to prescribing antimicrobials (139). Physician associates also have a role within AMS and can also provide support where appropriate.
Examples of prescribing responsibilities include:
- demonstrating principles of diagnostic stewardship by recognising signs and symptoms of infection, taking appropriate specimens for culture, and interpreting test results – this includes prioritising the right test, for the right patient, to ensure the right outcome and ensuring person centred care (140)
- adhering to SSTF approach when treating patients in need of antimicrobial treatment and contributing to prescribing audits where possible
- Lliaising and communicating with all members of multidisciplinary healthcare team, including pharmacy and nursing staff, regarding prescribing decisions and monitoring requirements to ensure continuity of care and that patients receive antimicrobial treatment safely and effectively
Nurses
Nursing teams have a significant role to play in limiting the threat posed by AMR (141, 142). A targeted education strategy ensures that nurses can manage the use of antimicrobials effectively; this includes questioning and highlighting the duration of therapies and prescription of medications where these do not meet with established organisational guidelines, and ensuring patients receive appropriate information regarding their diagnosis and treatment (143 to 145).
Examples of nursing responsibilities include:
- supporting the diagnosis of infection and appropriate use of antimicrobials by recognising signs and symptoms of infection, escalating concerns to prescribers about unwell patients, taking appropriate specimens for culture, interpreting test results where appropriate, and informing prescribers if an antimicrobial prescription does not specify an indication, duration, or review date
- ensuring that first doses of antimicrobials are administered promptly; especially in high-risk patients with potential sepsis or septic shock
- prompting the prescriber to consider IV to oral switching where appropriate, in accordance with national IVOS criteria, and alerting the prescriber promptly if a patient is unable to receive their antimicrobial treatment by the prescribed route
- querying prescriptions continuing beyond specified review dates with the prescriber, and seeking immediate advice from prescriber or pharmacy team regarding missed dose (146)
Pharmacists
Pharmacist interventions, such as routine reviews of antimicrobial prescriptions, improve the appropriate use of antimicrobials, reduce waste, and contribute to patient safety. As well as optimising antimicrobial use, pharmacists should educate colleagues, patients, and carers about the appropriate use of antimicrobials (147). Pharmacy technicians also have a role within AMS and can provide further support.
Examples of pharmacy responsibilities include:
- clinically checking antimicrobial prescriptions to ensure they comply with guidelines, alerting prescribers when prescriptions have not been reviewed, monitoring patients for response to treatment, and liaising with prescribers when microbiology results are available to suggest changes to regimens if appropriate
- facilitating IV-to-oral switch, therapeutic drug monitoring, advising on dosing according to renal or hepatic function, providing advice on alternative treatment options in patients with allergies, reviewing course lengths, and ensuring restriction polices are adhered to
- ensuring continuity of treatment, if appropriate, by informing prescribers to review antimicrobial prescriptions which run out during evenings and weekends (less staffed hours)
- supplying antimicrobials safely and ensure timely delivery of treatment
- contributing to audits and provide feedback to support AMS
Appendix 1: Resources
Antibiotic awareness resources: 2019 resources
Antimicrobial prescribing and stewardship competency framework
Dental antimicrobial stewardship toolkit
FutureNHS Platform – antimicrobial resistance programme
National antimicrobial intravenous-to-oral switch (IVOS) criteria for early switch
Start smart then focus: resource materials – audit tools, review stickers and drug charts
TARGET antimicrobial prescribing toolkit for primary care
Appendix 2: SSTF update contributors
SSTF update group members
-
Tanya Miah: Update Project Lead – Chief Pharmaceutical Officer’s Clinical Fellow 2022 to 2023, UKHSA
-
Professor Diane Ashiru-Oredope: SSTF Project Lead – Lead Pharmacist for HCAI, AMR, AMU, Fungal and Sepsis Division, UKHSA
-
Dr Kieran Hand: National Pharmacy and Prescribing Clinical Lead for Antimicrobial Resistance, NHSE
-
Susan Bowler: AMS Nurse, Nottingham University Hospitals NHS Trust
-
Dr Aaron Brady: Lead Antimicrobial Pharmacist, Belfast Trust
-
Dr Nicholas Brown: Consultant Medical Microbiologist, Cambridge University Hospitals NHS Trust; Director of Public and Professional Engagement, BSAC
-
Kevin Cahill: Chief Pharmaceutical Officer’s Clinical Fellow, NHSE
-
Annette Clarkson: Lead Antimicrobial Pharmacist, Nottingham University Hospitals NHS Trust
-
Lauren Cook: Clinical Nurse Specialist, County Durham and Darlington NHS Foundation Trust
-
Dr Neil Cunningham: National Medical Director’s Clinical Fellow, UKHSA
-
Gill Damant: Regional Antimicrobial Stewardship lead - North West, NHSE
-
Dr Nidhi Desai: Paediatric Teaching Fellow, Newcastle upon Tyne Hospitals NHS Trust
-
Dr Claire Mary Donnelly: Infectious Diseases Consultant, Southern Trust
-
Carole Fry: Infection Prevention and Control Lead, UKHSA
-
Frances Garraghan: Lead Antimicrobial Stewardship Pharmacist, Manchester University NHS Foundation Trust
-
Philip Howard: Regional Antimicrobial Stewardship lead – North East, NHSE
-
Dr Christopher Jones: Clinical Research Fellow and Specialist Registrar – Microbiology and Infectious Diseases, UKHSA
-
Sabina Khanom: AMR Research Translation Lead, NHSE
-
Jo McEwen: Advanced Nurse Practitioner Antimicrobial Stewardship, NHS Tayside
-
Dr Cara McKeating: Consultant Microbiologist, Southern Trust
-
Ceri Phillips: Consultant Pharmacist – Antimicrobials, Aneurin Bevan University Health Board
-
Dr Joe Price: Clinical Research Fellow and Specialist Registrar – Microbiology and Infectious Diseases, UKHSA
-
Sian Price: Welsh Antimicrobial Pharmacy Group Chair
-
Dr Nicholas Reid: All Wales Consultant Antimicrobial Pharmacist – Public Health Wales
-
Claudia Salvagno: Senior Health Protection Practitioner – IPC, UKHSA
-
Dr Anda Samson: Infectious Diseases Consultant, UKHSA
-
Jonathan Underhill: Consultant Clinical Advisor, NICE
-
Vivian De Vittoris: Chief Pharmaceutical Officer’s Clinical Fellow 2022 to 2023, NHSE
-
Laura Whitney: Regional Antimicrobial Stewardship lead – London, NHSE
This update was carried out in collaboration with Dr Kieran Hand (National Pharmacy and Prescribing Clinical Lead for AMR at NHS England).
We acknowledge multidisciplinary colleagues across the UK who provided feedback, including those listed and those whose names are not listed below:
- ESPAUR Oversight Group
- All Wales Antimicrobial Pharmacist Group
- Rebecca Stretch
- Ching-Yan Hui
- Robin Allan
- Christianne Micallef
- Netta Tyler
- Dr Sumita Pai
- Kevin Frost
- Rachel Medcalf
- Bronagh Smyth
- Cerys Lockett
- Naomi Fleming
- Zena Uppal
- Ryan Hamilton
- Conor Jamieson
- Susan Bowler
- Aneeka Chavda
- Mark Gilchrist
Appendix 3: Update process
The SSTF antimicrobial stewardship toolkit for inpatient care settings was initially developed in 2011 by PHE (now known as UKHSA).
A stepwise and systematic approach was undertaken involving 26 multidisciplinary subgroup members (SSTF update group – appendix 2) through meetings and online surveys and 25 multidisciplinary clinicians across 5 professions who provided feedback on the final draft. Data collection to inform the update of the toolkit consisted of focused literature searches, group discussions and questionnaires.
The additional 25 multidisciplinary professionals who provided feedback; consisted of colleagues invited through cascade emails to UK AMS networks and the ESPAUR oversight group, and an expression of interest process, to ensure its broad dissemination and effective implementation. Feedback was incorporated within the final version of the toolkit
References
1. Davies, S.C., Fowler, T., Watson, J., Livermore, D.M. and Walker, D. Annual Report of the Chief Medical Officer: infection and the rise of antimicrobial resistance. The Lancet 381, 1606 to 1609. 2013
2. UK 5-year antimicrobial resistance national action plan 2019 to 2024
3. Davey, P., and others. Interventions to improve antibiotic prescribing practices for hospital inpatients (updated protocol). Cochrane Database of Systematic Reviews. 2017
4. UKHSA. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report. 2022
5. NICE. Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use – NICE guideline NG15. 2015
7. Livermore, D.M. Has the era of untreatable infections arrived? Journal of Antimicrobial Chemotherapy 64, i29 to i36. 2009
8. Hawkey, P.M. and Jones, A.M. The changing epidemiology of resistance. Journal of Antimicrobial Chemotherapy 64, i3 to i10. 2009
9. Lucet, J.-C. Prevalence and risk factors for carriage of methicillin-resistant staphylococcus aureus at admission to the intensive care unit: results of a multicenter study. Archives of Internal Medicine 163, 181. 2003
10. Tacconelli, E., De Angelis, G., Cataldo, M.A., Pozzi, E. and Cauda, R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 61, 26 to 38. 2007
11. Dancer, S.J. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. Journal of Antimicrobial Chemotherapy 61, 246 to 253. 2007
12. Liebowitz, L.D. and Blunt, M.C. Modification in prescribing practices for third-generation cephalosporins and ciprofloxacin is associated with a reduction in meticillin-resistant Staphylococcus aureus bacteraemia rate. Journal of Hospital Infection 69, 328 to 336. 2008
13. Wistrom, J. Frequency of antibiotic-associated diarrhoea in 2,462 antibiotic-treated hospitalized patients: a prospective study. Journal of Antimicrobial Chemotherapy 47, 43 to 50. 2001
14. Freeman, J. and others. The changing epidemiology of Clostridium difficile infections. Clinical Microbiology Reviews 23, 529 to 549. 2010
15. Nelson, R.L., and others. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database of Systematic Reviews, John Wiley and Sons Ltd. 2011
16. Blaser, M. Stop the killing of beneficial bacteria. Nature 476, 393 to 394. 2011
17. Hviid, A., Svanstrom, H. and Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 60, 49 to 54. 2010
18. MacDougall, C. and Polk, R.E. Antimicrobial Stewardship Programs in Health Care Systems. Clinical Microbiology Reviews 18, 638 to 656. 2005
19. National Audit Office. Reducing Healthcare Associated Infections in Hospitals in England. 2009
20. PHE. ESPAUR: report 2014
21. CQC. Essential standards of quality and safety. 2010
22. PHE and DHSC. Clostridioides difficile infection: how to deal with the problem
23. Satterfield, J., Miesner, A.R. and Percival, K.M. The role of education in antimicrobial stewardship. Journal of Hospital Infection 105, 130 to 141. 2020
24. Michael, C.A., Dominey-Howes, D. and Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Frontiers in Public Health 2. 2014
25. Patangia, D.V., Anthony Ryan, C., Dempsey, E., Paul Ross, R. and Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 11. 2022
26. Pérez-Cobas, A.E., and others. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62, 1591 to 1601. 2012
27. Ng, K.M., and others. Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context, and Environmental Reservoirs. Cell Host and Microbe 26, 650 to 665. e654. 2019
28. Dropulic, L.K. and Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiology Spectrum 4. 2016
29. Hanretty, A.M. and Gallagher, J.C. Shortened Courses of Antibiotics for Bacterial Infections: A Systematic Review of Randomized Controlled Trials. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 38, 674 to 687. 2018
30. Lamy, B., Dargère, S., Arendrup, M.C., Parienti, J.-J. and Tattevin, P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Frontiers in Microbiology 7, 697. 2016
31. Scheer, C.S., and others. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clinical Microbiology and Infection 25, 326 to 331. 2019
32. Harris, A.M., and others. Influence of Antibiotics on the Detection of Bacteria by Culture-Based and Culture-Independent Diagnostic Tests in Patients Hospitalized With Community-Acquired Pneumonia. Open forum infectious diseases 4, ofx014. 2017
33. Garcia, R.A., and others. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line–associated bloodstream infections. American Journal of Infection Control 43, 1222 to 1237. 2015
34. Morrell, M., Fraser, V.J. and Kollef, M.H. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial agents and chemotherapy 49, 3,640 to 3,645. 2005
35. England, N. Improving the blood culture pathway – executive summary. 2022.
36. Craig, J.C., and others. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15,781 febrile illnesses. BMJ 340, c1594. 2010
37. Simon, L., Gauvin, F., Amre, D.K., Saint-Louis, P. and Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-analysis. Clinical Infectious Diseases 39, 206 to 217. 2004
38. Falk, G. and Fahey, T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Family Practice 26, 10 to 21. 2009
39. van der Meer, V., Neven, A.K., van den Broek, P.J. and Assendelft, W.J.J. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 331, 26. 2005
40. Camille, E., Sandra, I., Fernandez-Carballo, B.L. and Sabine, D. The good and the bad: using C reactive protein to distinguish bacterial from non-bacterial infection among febrile patients in low-resource settings. BMJ Global Health 5, e002396. 2020
41. Evans, L., and others. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Critical Care Medicine 49. 2021
42. Lagunes, L., Encina, B. and Ramirez-Estrada, S. Current understanding in source control management in septic shock patients: a review. Annals of Translational Medicine 4, 330. 2016
43. Sartelli, M., and others. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World Journal of Emergency Surgery 12, 29. 2017
44. Sartelli, M., and others. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World Journal of Emergency Surgery 13, 58. 2018
45. Kumar, A., and others. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 34, 1,589 to 1,596. 2006
46. Academy of Medical Royal Colleges. [Statement on the initial treatment of sepsis V2.0] (https://www.aomrc.org.uk/reports-guidance/statement-on-the-initial-antimicrobial-treatment-of-sepsis-v2-0/). 2022
47. Llewelyn, M.J., and others. Antibiotic review kit for hospitals (ARK-Hospital): a stepped-wedge cluster-randomised controlled trial. The Lancet Infectious Diseases 23, 207 to 221. 2023
48. Asadi, L., and others. Impact of guideline-concordant antibiotics and macrolide/β-lactam combinations in 3203 patients hospitalized with pneumonia: prospective cohort study. Clinical Microbiology and Infection 19, 257 to 264. 2013
49. Asadi, L., and others. Guideline adherence and macrolides reduced mortality in outpatients with pneumonia. Respiratory Medicine 106, 451 to 458. 2012
50. Garber, A.M. Evidence-Based Guidelines As a Foundation For Performance Incentives. Health Affairs 24, 174 to 179. 2005
51. Ament, S.M.C., and others. Sustainability of professionals’ adherence to clinical practice guidelines in medical care: a systematic review. BMJ open 5, e008073. 2015
52. Fleuren, M.A.H., van Dommelen, P. and Dunnink, T. A systematic approach to implementing and evaluating clinical guidelines: The results of 15 years of Preventive Child Health Care guidelines in the Netherlands. Social Science and Medicine 136 to 137, 35 to 43. 2015
53. Grimshaw, J., and others. Toward evidence-based quality improvement. Journal of General Internal Medicine 21, S14 to S20. 2006
54. Tickell, K.D., and others. A mixed method multi-country assessment of barriers to implementing pediatric inpatient care guidelines. PloS one 14, e0212395. 2019
55. Schuts, E.C., and others. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. The Lancet Infectious Diseases 16, 847 to 856. 2016
56.Wanat, M., and others. Mixed-methods evaluation of a behavioural intervention package to identify and amend incorrect penicillin allergy records in UK general practice. BMJ Open 12, e057471. 2022
57. Savic, L., and others. BSACI guideline for the set‐up of penicillin allergy de‐labelling services by non‐allergists working in a hospital setting. Clinical and Experimental Allergy 52, 1,135 to 1,141. 2022
58. Lee, R.U. Penicillin Allergy Delabeling Can Decrease Antibiotic Resistance, Reduce Costs, and Optimize Patient Outcomes. Federal practitioner: for the health care professionals of the VA, DoD, and PHS 37, 460 to 465. 2020
59. Stone Jr, C.A., Trubiano, J., Coleman, D.T., Rukasin, C.R.F. and Phillips, E.J. The challenge of de-labeling penicillin allergy. Allergy 75, 273 to 288. 2020
60. Staicu, M.L., and others. Penicillin Allergy Delabeling: A Multidisciplinary Opportunity. The Journal of Allergy and Clinical Immunology: In Practice 8, 2858 to 2868. e2816. 2020
61. Llor, C. and Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic Advances in Drug Safety 5, 229 to 241. 2014
62. Cižman, M. and Plankar Srovin, T. Antibiotic consumption and resistance of gram-negative pathogens (collateral damage). GMS Infectious Diseases 6, Doc05. 2018
63. Adam, S.F., and others. Antibiotic use as a risk factor for inflammatory bowel disease across the ages: a population-based cohort study. Gut 72, 663 (2023)
64. Prokuski, L. Prophylactic Antibiotics in Orthopaedic Surgery. Journal of the American Academy of Orthopaedic Surgeons 16, 283 to 293 (2008)
65. Boxma, H., Broekhuizen, T., Patka, P. and Oosting, H. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. The Lancet 347, 1133 to 1137. 1996
66. Tourmousoglou, C.E., Yiannakopoulou, E.C., Kalapothaki, V., Bramis, J. and Papadopoulos, J.S. Adherence to guidelines for antibiotic prophylaxis in general surgery: a critical appraisal. Journal of Antimicrobial Chemotherapy 61, 214 to 218. 2007
67. A World Alliance for Safer Health Care – Patient Safety. WHO Surgical Safety Checklist
68. Bratzler, D.W., and others. Clinical practice guidelines for antimicrobial prophylaxis in surgery. American Journal of Health-System Pharmacy 70, 195 to 283. 2013
69. Surgical site infections: prevention and treatment – NICE guideline NG125. 2019
70. Antibiotic Review Kit (ARK)
71. Charani, E., and others. Understanding the determinants of antimicrobial prescribing within hospitals: the role of ‘prescribing etiquette’. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57, 188 to 196. 2013
72. Roope, L.S.J., and others. Why do hospital prescribers continue antibiotics when it is safe to stop? Results of a choice experiment survey. BMC medicine 18, 196. 2020
73. Rice, L.B. The Maxwell Finland Lecture: For the Duration – Rational Antibiotic Administration in an Era of Antimicrobial Resistance and Clostridium difficile. Clinical Infectious Diseases 46, 491 to 496. 2008
74. Warreman, E.B., and others. Determinants of in-hospital antibiotic prescription behaviour: a systematic review and formation of a comprehensive framework. Clinical Microbiology and Infection 25, 538 to 545. 2019
75. Dyar, O.J., and others. ESCMID generic competencies in antimicrobial prescribing and stewardship: towards a European consensus. Clinical Microbiology and Infection 25, 13 to 19. 2019
76. Llewelyn, M.J., and others. The antibiotic course has had its day. BMJ, j3418. 2017
77. Curran, J., and others. Estimating daily antibiotic harms: an umbrella review with individual study meta-analysis. Clinical Microbiology and Infection 28, 479 to 490. 2022
78. Palin, V., Welfare, W., Ashcroft, D.M. and van Staa, T.P. Shorter and Longer Courses of Antibiotics for Common Infections and the Association With Reductions of Infection-Related Complications Including Hospital Admissions. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 73, 1805 to 1812. 2021
79. Ahmed, J., Mehmood, S., Rehman, S., Ilyas, C. and Khan, L.U.R. Impact of a structured template and staff training on compliance and quality of clinical handover. International Journal of Surgery 10, 571 to 574. 2012
80. Masterton, R.G. Antibiotic de-escalation. Critical Care Clinics 27, 149 to 162. 2011
81. Pardo, J., and others. Time to Positivity of Blood Cultures Supports Antibiotic De-escalation at 48 Hours. Annals of Pharmacotherapy 48, 33 to 40. 2013
82. Mizusawa, M. Updates on Rapid Diagnostic Tests in Infectious Diseases. Missouri Medicine 117, 328 to 337. 2020
83. Loo, L.W., and others. Discontinuation of antibiotic therapy within 24 hours of treatment initiation for patients with no clinical evidence of bacterial infection: a 5-year safety and outcome study from Singapore General Hospital Antimicrobial Stewardship Program. International Journal of Antimicrobial Agents 53, 606 to 611. 2019
84. Daniel Markley, J., Bernard, S., Bearman, G. and Stevens, M.P. De-escalating Antibiotic Use in the Inpatient Setting: Strategies, Controversies, and Challenges. Current Infectious Disease Reports 19. 2017
85. Shapiro, D.J., Hicks, L.A., Pavia, A.T. and Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. Journal of Antimicrobial Chemotherapy 69, 234 to 240. 2013
86. Sanchez, G.V., Roberts, R.M., Albert, A.P., Johnson, D.D. and Hicks, L.A. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerging infectious diseases 20, 2041 to 2047. 2014
87. Gerber, J.S., and others. Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. Journal of the American Medical Association 318, 2,325 to 2,336. 2017
88. Chow, A.W., and others. IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clinical Infectious Diseases 54, e72 to e112. 2012
89. Lieberthal AS, Carroll AE, Chonmaitree T, and others. Clinical Practice Guideline: The Diagnosis and Management of Acute Otitis Media. Pediatrics 2013; 131 (3): e964 to e999. Pediatrics 133, 346 to 347. 2014
90. Gupta, K., and others. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical Infectious Diseases 52, e103 to e120. 2011
91. Mandell, L.A., and others. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 44, Supplement 2, S27 to S72. 2007
92. Chapman, A.L.N., and others. Updated good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults and children in the UK. JAC-Antimicrobial Resistance 1. 2019
93. Steffens, E., and others. Outpatient parenteral antimicrobial therapy and antibiotic stewardship: opponents or teammates? Infection 47, 169 to 181. 2018
94. Mahoney, M.V., and others. Recent Updates in Antimicrobial Stewardship in Outpatient Parenteral Antimicrobial Therapy. Current infectious disease reports 23, 24. 2021
95. Chapman, A.L.N., and others. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. Journal of Antimicrobial Chemotherapy 64, 1,316 to 1,324. 2009
96. Chapman, A.L.N., and others. Updated good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults and children in the UK. JAC-Antimicrobial Resistance 1, dlz026. 2019
97. Chastre, J., and others. Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults. JAMA 290, 2588 to 2588. 2003
98. Zilahi, G., McMahon, M.A., Povoa, P. and Martin-Loeches, I. Duration of antibiotic therapy in the intensive care unit. Journal of thoracic disease 8, 3,774 to 3,780. 2016
99. UKHSA. National antimicrobial intravenous-to-oral switch (IVOS) criteria for early switch
100. Mertz, D., and others. Outcomes of early switching from intravenous to oral antibiotics on medical wards. The Journal of antimicrobial chemotherapy 64, 188 to 199. 2009
101. Akhloufi, H., and others. Development of operationalized intravenous to oral antibiotic switch criteria. Journal of Antimicrobial Chemotherapy 72, 543 to 546. 2016
102. Mandell, L.A., and others. Sequential antibiotic therapy: Effective cost management and patient care. The Canadian journal of infectious diseases 6, 306 to 315. 1995
103. Rojo, D., Pinedo, A., Clavijo, E., Garcı́a-Rodriguez, A. and Garcı́a, V. Analysis of risk factors associated with nosocomial bacteraemias. Journal of Hospital Infection 42, 135 to 141. 1999
104. Carratalà, J., and others. Effect of a 3-Step Critical Pathway to Reduce Duration of Intravenous Antibiotic Therapy and Length of Stay in Community-Acquired Pneumonia. Archives of Internal Medicine 172. 2012
105. Haran, J.P., and others. Factors influencing the development of antibiotic associated diarrhea in ED patients discharged home: risk of administering IV antibiotics. The American journal of emergency medicine 32, 1,195 to 1,199. 2014
106. McDowell, S.E., Mt-Isa, S., Ashby, D. and Ferner, R.E. Where errors occur in the preparation and administration of intravenous medicines: a systematic review and Bayesian analysis. Quality and Safety in Health Care 19, 341 to 345. 2010
107. MacGregor, R.R. and Graziani, A.L. Oral Administration of Antibiotics: A Rational Alternative to the Parenteral Route. Clinical Infectious Diseases 24, 457 to 467. 1997
108. Bamford, K.B., and others. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury 42, S24 to S27. 2011
109. Lee, R.A., Stripling, J.T., Spellberg, B. and Centor, R.M. Short-course antibiotics for common infections: what do we know and where do we go from here? Clinical Microbiology and Infection 29, 150 to 159. 2023
110. The Health and Social Care Act 2008. Code of practice for the NHS on the prevention and control of health care associated infections and related guidance. Department of Health and Social Care. 2010
111. Dellit, T.H. Summary of the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Infectious Diseases in Clinical Practice 15, 263 to 264. 2007
112. Oloyede, O., Cramp, E. and Ashiru-Oredope, D. Antimicrobial Stewardship: Development and Pilot of an Organisational Peer-to-Peer Review Tool to Improve Service Provision in Line with National Guidance. Antibiotics (Basel, Switzerland) 10, 44. 2021
113. Thern, J., and others. Selection of hospital antimicrobial prescribing quality indicators: a consensus among German antibiotic stewardship (ABS) networkers. Infection 42, 351 to 362. 2013
114. CDC. Core Elements of Hospital Antibiotic Stewardship Programs
115. Dumartin, C., and others. Antibiotic usage in south-western French hospitals: trends and association with antibiotic stewardship measures. Journal of Antimicrobial Chemotherapy 66, 1,631 to 1,637. 2011
116. Bruce, J., and others. Antibiotic stewardship and consumption: findings from a pan-European hospital study. Journal of Antimicrobial Chemotherapy 64, 853 to 860. 2009
117. Antimicrobial stewardship in Australian health care: 2018. JAC-antimicrobial resistance 1, dlz010. 2019
118. Barlam, T.F., and others. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clinical Infectious Diseases 62, e51-e77. 2016
119. Buyle, F.M., and others. Development and validation of potential structure indicators for evaluating antimicrobial stewardship programmes in European hospitals. European Journal of Clinical Microbiology and Infectious Diseases 32, 1,161 to 1,170. 2013
120. Dik, J.-W.H., and others. Measuring the impact of antimicrobial stewardship programs. Expert Review of Anti-infective Therapy 14, 569 to 575. 2016
121. Cortoos, P.J., De Witte, K., Peetermans, W.E., Simoens, S. and Laekeman, G. Opposing expectations and suboptimal use of a local antibiotic hospital guideline: a qualitative study. Journal of Antimicrobial Chemotherapy 62, 189 to 195. 2008
122. Sahota, R.S., and others. Increasing the documentation of 48-hour antimicrobial reviews. BMJ Open Quality 9, e000805. 2020
123. Papoutsi, C., and others. Social and professional influences on antimicrobial prescribing for doctors-in-training: a realist review. Journal of Antimicrobial Chemotherapy 72, 2418 to 2430. 2017
124. Costelloe, C., Metcalfe, C., Lovering, A., Mant, D. and Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340, c2096. 2010
125. Saeed, K., Dryden, M., Bourne, S., Paget, C. and Proud, A. Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. Journal of Hospital Infection 78, 289 to 292. 2011
126. Kollef, M.H., Sherman, G., Ward, S. and Fraser, V.J. Inadequate Antimicrobial Treatment of Infections. Chest 115, 462 to 474. 1999
127. Tang, C.M. and Macfarlane, J.T. Early management of younger adults dying of community acquired pneumonia. Respiratory Medicine 87, 289 to 294. 1993
128. Melander, R.J., Zurawski, D.V. and Melander, C. Narrow-Spectrum Antibacterial Agents. MedChemComm 9, 12 to 21. 2018
129. Safdar, N. and Maki, D.G. The Commonality of Risk Factors for Nosocomial Colonization and Infection with Antimicrobial-Resistant Staphylococcus aureus, Enterococcus, Gram-Negative Bacilli, Clostridium difficile, and Candida. Annals of Internal Medicine 136, 834 to 834. 2002
130. Owens Robert C, Jr., Donskey, Curtis J., Gaynes, Robert P., Loo, Vivian G. and Muto, Carlene A. Antimicrobial‐Associated Risk Factors for Clostridium difficile infection. Clinical Infectious Diseases 46, S19 to S31. 2008
131. Vernaz, N., and others. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. Journal of Antimicrobial Chemotherapy 62, 601 to 607. 2008
132. Charneski, L., Deshpande, G. and Smith, S.W. Impact of an Antimicrobial Allergy Label in the Medical Record on Clinical Outcomes in Hospitalized Patients. Pharmacotherapy 31, 742 to 747. 2011
133. Drug allergy: diagnosis and management – NICE clinical guideline CG183
134. Alvarez-Lerma, F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Medicine 22, 387 to 394. 1996
135. Alvarez-Lerma, F., and others. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Critical care (London, England) 10, R78. 2006
136. Singh, N., Rogers, P., Atwood, C.W., Wagener, M.M. and Yu, V.L. Short-course Empiric Antibiotic Therapy for Patients with Pulmonary Infiltrates in the Intensive Care Unit. American Journal of Respiratory and Critical Care Medicine 162, 505 to 511. 2000
137. Thomas, J.K., and others. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrobial agents and chemotherapy 42, 521 to 527. 1998
138. Wachter, R.M., Flanders, S.A., Fee, C. and Pronovost, P.J. Public Reporting of Antibiotic Timing in Patients with Pneumonia: Lessons from a Flawed Performance Measure. Annals of Internal Medicine 149, 29. 2008
139. UKHSA. Antimicrobial prescribing and stewardship competencies
140. Fabre, V., and others. Principles of diagnostic stewardship: A practical guide from the Society for Healthcare Epidemiology of America Diagnostic Stewardship Task Force. Infection Control and Hospital Epidemiology 44, 178 to 185. 2023
141. Edwards, R., Drumright, L., Kiernan, M. and Holmes, A. Covering more Territory to Fight Resistance: Considering Nurses’ Role in Antimicrobial Stewardship. Journal of Infection Prevention 12, 6 to 10. 2011
142. Edwards, R., Loveday, H., Drumright, L.N. and Holmes, A. Should nurses be more involved in antimicrobial management? Journal of Infection Prevention 12, 4 to 5. 2011
143. Pulcini, C. and Gyssens, I.C. How to educate prescribers in antimicrobial stewardship practices. Virulence 4, 192 to 202. 2013
144. van Huizen, P., Kuhn, L., Russo, P.L. and Connell, C.J. The nurses’ role in antimicrobial stewardship: A scoping review. International Journal of Nursing Studies 113, 103772. 2021
145. Monsees, E., Goldman, J. and Popejoy, L. Staff nurses as antimicrobial stewards: An integrative literature review. American Journal of Infection Control 45, 917 to 922. 2017
146. Olans, R.N., Olans, R.D. and DeMaria, A. The Critical Role of the Staff Nurse in Antimicrobial Stewardship – Unrecognized, but Already There: Table 1. Clinical Infectious Diseases 62, 84 to 89. 2015
147. Garau, J. and Bassetti, M. Role of pharmacists in antimicrobial stewardship programmes. International Journal of Clinical Pharmacy 40, 948 to 952. 2018
