Consensus statement on the association between the discharge of patients from hospitals and COVID in care homes
Published 26 May 2022
The authors are Bruce Guthrie, Adelina Comas-Herrera, Dimple Chudasama, Jackie Cassell, Rich Fry, Ian Hall, Éamonn O’Moore and wider Social Care Working Group participants.
Summary
Reason for bringing to the Department of Health and Social Care
This report is a direct commission, see the ‘Motivation’ section below.
Conclusions
Any person infected with COVID-19 going into a care home could introduce infection into the care home. Hospital discharge to care homes connects 2 high contact environments, where contact rates with carers in the course of care are high, and potential consequences of COVID-19 in vulnerable populations severe.
Overall, we interpret the identified studies as showing that at least some care home outbreaks were caused or partly caused or intensified by discharges from hospital.
However, based on the very much larger associations between care home size (a proxy for all footfall) and outbreaks, hospital discharge does not appear to have been the dominant way in which COVID-19 entered care homes.
Hospital discharge of people to care homes without testing early in the pandemic is highly likely to have caused some outbreaks or been one of the often multiple introductions of infection to care homes which experienced an outbreak. However, it is highly unlikely to have been the dominant driver of all care home outbreaks in wave 1.
Proposed next steps
See the ‘Recommendations for further and future action’ section below.
Motivation
The Public Accounts Committee (PAC) recommended in summer 2020 a review be undertaken of which care homes took discharged patients and how many went onto have outbreaks.
The Department of Health and Social Care (DHSC) commissioned a consensus statement on the impact of hospital discharge from the Social Care Working Group (SCWG) in November 2020 to take into account work already undertaken by NHS England (NHSE) and Public Health England (PHE) and any relevant analysis from the devolved administrations.
In July 2021 when it became apparent that NHS England and Improvement (NHSEI) data and analysis would not be available DHSC revised the ask to cover Public Health England (PHE), Public Health Wales (PHW), Public Health Scotland (PHS) and the Department of Health Northern Ireland (DoH NI) studies.
Interpretation based on all available evidence
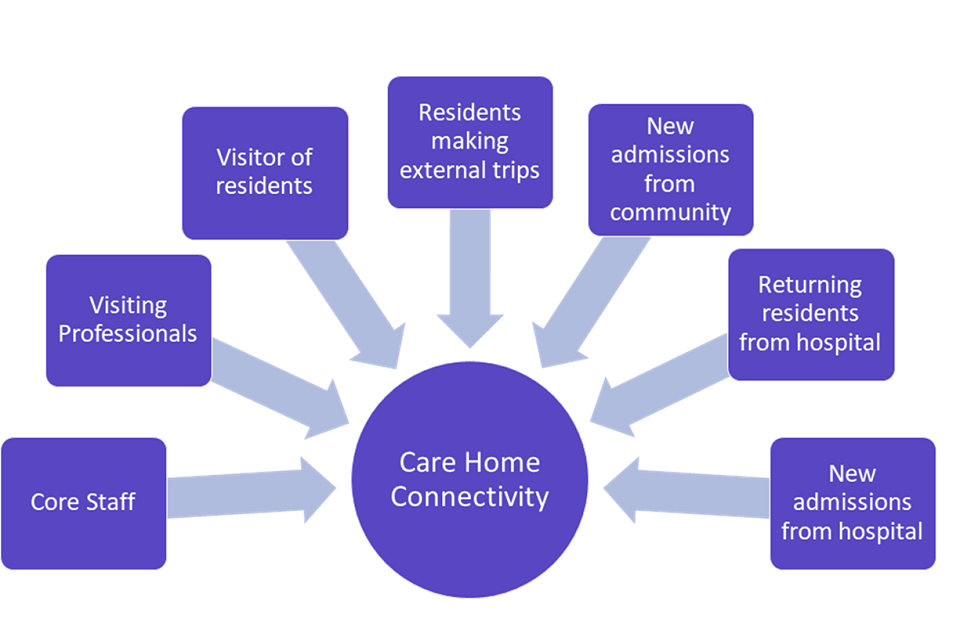
Any person going into a care home could introduce COVID-19 to the care home. The main groups of people crossing the threshold of care homes, shown in figure 1, are listed below in terms of frequency of contact with residents:
- care home staff including non-care staff such as cleaners and cooks (present and in contact with residents)
- professionals visiting like GPs and district nurses, ambulance crews and voluntary helpers (not necessarily on the payroll of care home and potentially working in multiple locations; even when present daily at care homes likely having less contact with residents)
- family and friends visiting (although this was minimal in many care homes for most of the period examined)
- residents themselves visiting settings outside the home (as above this should be minimal after 23 March 2020)
- existing or new residents discharged from hospital to the care home
- new residents admitted direct from the community (occupancy rates reduced during pandemic and so new admissions may not have contributed greatly)
Figure 1: schematic showing the potential routes of ingress of COVID-19 into care home settings
Evaluating all these routes contemporaneous to the period of discharge is not possible due to testing capacity at the time and variation in policy around visiting and staff. Data on the number of visitors could be extracted from log books but this is likely a huge effort to digitise and there is no routine system for systematically collecting electronic visitor data (family or professionals).
The core evidence for this report is described in detail below (the ‘Summary of evidence’ section) where analyses used different methods in different UK countries.
Another source of evidence is the VIVALDI-1 survey[footnote 1] which included the question ‘How many residents have returned to the care home from hospital since March 1 [2020]?’.
This study found an association between each one unit increase in the number of new admissions to the care home and infection in residents (relative to baseline) in the adjusted analysis (Residents: adjusted odds ratio [aOR] 1.01, 95% CI 1.01 to 1.01; Staff: aOR 1.00 (95% CI 1.00 to 1.01). Furthermore, the study found an association with the odds of outbreaks (one or more cases in the care home) but not large outbreaks (more than a third of the total number of residents and staff combined testing positive, or with more than 20 residents and staff combined testing positive) (Outbreaks: aOR 1.08, 95% CI 1.05 to 1.10; Large outbreaks: aOR 1.00, 95% CI 0.99 to 1.02).
The VIVALDI-1 survey therefore presents some evidence of an association between admissions and infections and outbreaks, with the major caveat that this was a survey where it was not possible to look at the temporal relationship between hospital discharges and outcomes.
Viral genomic analysis can help identify whether care home outbreaks are due to a single introduction of infection or whether outbreaks are driven by multiple separate introductions of infection. A large scale study in the East of England found that only 5.8% of care home cases were definitely hospital acquired.[footnote 2] When the genomes of care home residents were compared to a control group of community cases the diversity of the genomic lineages was similar suggesting hospital discharges were not the primary driver. There was epidemiological evidence linking some outbreaks in different care homes to professional visitors.
Similarly an outbreak involving 6 care facilities in a small geographical area of Norfolk and Suffolk, identified a genomically distinct lineage which was not found in community surveillance or in hospital inpatients.[footnote 3] Only 2 out of 89 cases were definitely hospital acquired, suggesting that these care homes cross infected each other, most likely because of shared staff or professional visitors.
An international review of the use of genomics to understand COVID-19 outbreaks in care homes[footnote 4] supports the UK study findings. When mass screening was undertaken during outbreaks, non-resident facing staff usually had identical or near identical genomes to residents. Even when infection control measures were in place, when an outbreak occurred usually all cases were the same lineage with identical or near identical genomes. Due to the slow mutation rate of SARS-CoV-2, the directionality of transmission cannot be ascertained from genomics alone and requires additional epidemiological investigation.
Overall, we interpret the evidence across the UK as showing that at least some care home outbreaks were caused or partly caused or intensified by discharges from hospital (highly likely). However, based on the very much larger associations between care home size (a proxy for all modes of disease ingress) and outbreaks, hospital discharge does not appear to have been the dominant way in which COVID-19 entered care homes (highly likely). This reflects the relative rareness of hospital discharge compared to other kinds of footfall into the care home (staff and other professional visitors like GPs and district nurses).
The PHS analysis likely has the best ascertainment of discharges to care homes in the first period of the pandemic. 843 (77.8%) of Scottish care homes for adults received at least one discharge in March to May 2020. The number of discharges to these 843 care homes varied from 1 to 46, with an average of 6 discharges in the 3 months which can be compared to many hundreds of staff contact days per home over same time frame for instance.
Hospital discharge to care homes almost certainly connects 2 high contact environments, where contact rates with carers in the course of care are high, and potential consequences of COVID-19 in vulnerable populations severe. They will remain a high priority for preventive actions. Hospital discharge is nevertheless highly likely an uncommon event relative to the many other elements of care home connectivity during the pandemic. Care home staff and visiting professionals are likely to dominate routine connectivity.
Recommendations for further and future action
-
The data analysis published to date would be usefully complemented by the NHSEI completing and making available the analysis started in late 2020 replicating the Secure Anonymised Information Linkage Databank (SAIL) analysis on English data. This is because the population of England is larger and so should provide greater power to detect small associations.
-
In any future epidemic with high consequence in older and vulnerable people, greater consideration should be given to minimising potential exposure in care homes. This includes consideration of how to safely transfer existing or new residents to care homes from hospitals or from the community, but also active consideration of ways to minimise all possible routes of infection entry to the care home, and to minimise spread within the care home if infection is introduced (including support for infection prevention and control and rapid access to personal protective equipment (PPE) and other control measures).
-
A key limitation of the analyses presented here is the lack of good quality routine data in social care, by comparison with the NHS. This reflects longstanding weaknesses in social care data collection. No UK country can routinely identify who is resident in care homes (including people with short stays for rehabilitation and respite), who is receiving social care at home or who works in or visits a care home or a person’s home. There is an urgent need to collect Social care Episode Statistics (akin to Hospital Episode Statistics which identify who is admitted to hospital). These should identify all permanent and temporary residents of care homes, and all those in receipt of publicly funded care at home. As well as being critical to support understanding and improving care in normal times, this is vital to ensure that data sources are in place to monitor and mitigate the introduction and transmission of infection in future pandemics.
Background and context
COVID-19 had a devastating effect on the population of residents in care homes in the UK: a high risk setting for COVID-19 impacts. This is because this population has a range of hazards (sources of harm) that aggregate to a cumulative risk (the harm itself). These hazards are:
- the relatively large number of non-residents necessarily coming into the care home each day
- close contact interactions within the home
- a relatively large pool of residents and staff allowing large outbreaks to arise
- residents with high vulnerability to severe outcome to COVID-19 infection
Control measures before the availability of vaccine were inadequate for the challenges of a highly infectious virus in such a vulnerable population. Vaccination of staff has reduced the likelihood of ingress, while vaccination among residents and staff has reduced the probability of onward transmission and severity of outcomes, and therefore outbreak size and consequence. This was summarised in more detail in the May 2021 SAGE paper.
This report is intended to focus on the specific evidence for the role of hospital discharges in seeding disease outbreaks in the wave 1 context of the prioritising of building capacity in the NHS. The challenge in this evaluation is the very limited testing of asymptomatic cases in wave 1 of the pandemic (see figure 2). It should also be noted that hospital discharge is not the only mode of ingress into a home, for example staff, visitors, new residents and visiting professionals also all have the potential to seed or reseed outbreaks due to the majority of cases having non-specific symptoms, and in the context of limited community testing in wave 1. At times of high community prevalence multiple introductions may have occurred making outbreak control more complex.
To illustrate the challenge in identification figure 2 shows the time series of weekly deaths registered from 14 March 2020. Since September 2020 testing of residents has been sufficient to allow better ascertainment due to COVID-19 in care homes. Since June non-COVID-19 notifications are mostly constant.
Figure 2: number of weekly deaths of care home residents registered from 14 March 2020 to 2 April 2021, England and Wales[footnote 5]
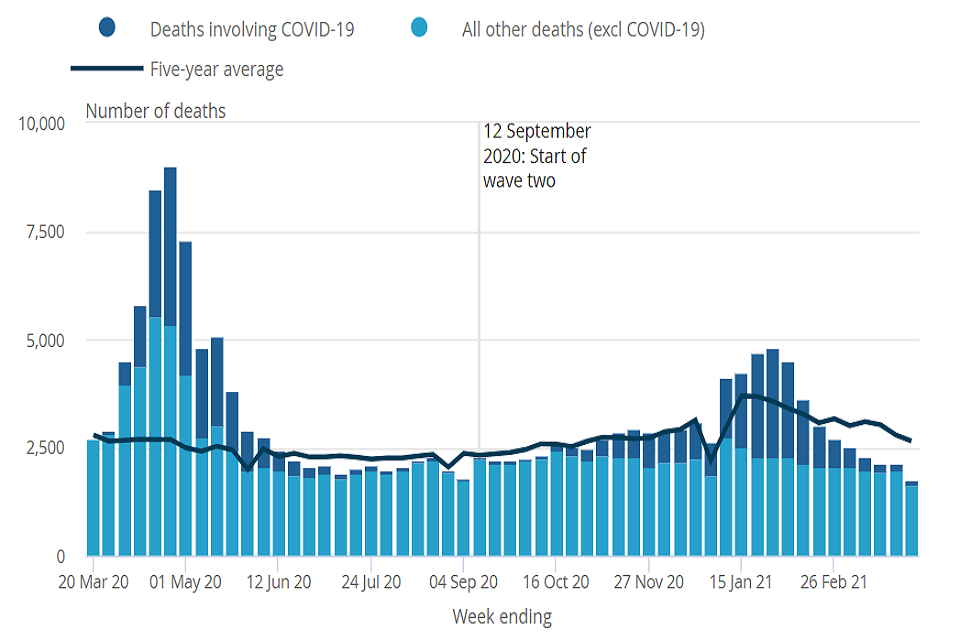
Excess deaths, the additional deaths observed in a given period compared to the number usually expected, better capture direct and indirect mortality impacts in wave 1 (but are unreliable subsequently because bed occupancy (and hence the total care home population) has been lower making historical comparisons problematic). In wave 1 (the first 23 weeks of the pandemic, from 4 March 2020 to 7 August 2020), it has been estimated that there were about 29,542 excess deaths in all care homes in England. However, almost all excess deaths were recorded in the quarter (27.4%) of homes which experienced any COVID-19 related fatalities during the first wave of the pandemic. This suggests higher proportions of COVID-19-related excess deaths than flagged in the official death data. In one analysis, up to 10,000 more care home residents may have died from COVID-19 than officially reported but concentrated in homes with subsequently declared outbreaks.[footnote 6]
COVID-19 is often asymptomatic or has non-specific symptoms in staff (as in the general population) and in care home residents (where symptoms may be atypical or masked by other conditions). The first systematic examination of 4 large outbreaks in London care homes in early April 2020 did PCR testing of all staff and residents after an outbreak started, identifying that half of residents and a third of staff with a positive test did not report any symptoms before testing or in the subsequent 14 days.[footnote 7] This is broadly similar to the wider population.
There was limited testing of the entire population early in the pandemic, and any testing done was typically in symptomatic people. Before April 2020 this would have only been symptomatic cases, while in the summer of 2020 in the care home sector there was a move to routine whole home testing once a case was identified. There is therefore incomplete identification of index (first) cases and underestimation of outbreak size in wave 1. This makes it difficult to be certain when and how COVID entered care homes in wave 1.
Subsequent viral genomic analyses of the same 4 London outbreaks with additional data from 2 other care homes, indicated multiple introductions in each home.[footnote 8] This is inconsistent with a single seeding event, as might arise from a hospital discharge (although it does not exclude hospital discharge as a source of an introduction).[footnote 9] In contrast, a systematic review of viral genomic studies internationally concluded that most outbreaks were from a single introduction of the virus, although the review also found evidence of transmission between care homes, possibly because of staff working in more than one home or because staff from different homes had community connections.[footnote 10]
Building on the evidence above and in order to consider the question of the impact of discharges the SCWG has conducted a rapid review of the evidence, focussed on the UK, which has revealed 2 types of analysis using routine data. A more structured search of scientific evidence[footnote 11] on COVID-19 and long-term care up to the 18 October 2020, focusing on papers linking hospital discharges to COVID-19 outbreaks in long-term care facilities identified a retrospective genomic analysis of COVID-19 cases from the East of England that covered 700 care home residents[footnote 12] (discussed in interpretation section below), as well as the studies by UK Health Security Agency (UKHSA), PHS and PHW included in this report. It did not identify other international studies.
Firstly, studies looking at epidemiological associations of outbreaks with observed discharges of people with a positive test, and secondly studies looking at all discharges. This review began in autumn 2020 and has been updated as studies have been peer-reviewed. This consensus statement is intended to review the methods, results and limitations of the science and does not consider policy implications though recommendations on data collection in future and further analysis are made given the scientific basis found. Unsurprisingly given the nature of the pandemic response and expertise in the UK, authors of studies include participants of SCWG. We now discuss those studies that have been conducted, all of which are UK based, and then discuss limitations.
Summary of evidence
Care home outbreaks epidemiologically associated with hospital discharges of people with a previous positive test
These analyses identify people discharged from hospital to a care home who have had a previous positive test for COVID-19, and then examine whether any care home outbreak is ‘epidemiologically associated’ with such a discharge. ‘Epidemiologically-associated’ means that the timing of the positive test in the person discharged, and the timing of the outbreak in the care home are consistent with what we know about how SARS-CoV-2 is transmitted. This does not mean that the discharge actually caused an observed outbreak, but it helps identify outbreaks where the discharge could have contributed to the outbreak.
Only 3 analyses were found which look at this type of association; given the need for rapid data access, they are by national public health agencies.
UK Health Security Agency (formerly Public Health England)[footnote 13]
UKHSA analysis examined the period 30 January to 12 October 2020. Care home residents were identified by address matching COVID-19 positive case level data, followed by linking to secondary care hospital admission data to obtain information on hospital discharges. Healthcare acquired COVID-19 was then determined in these cases, by utilising the European Centre for Disease Prevention and Control (ECDC) international definitions.[footnote 14] By examining the timing of the discharge from hospital of these people and the timing of any outbreaks of COVID-19 in the care home they were discharged to, the analysis can identify ‘epidemiologically associated’ outbreaks.
The analysis identified 43,398 laboratory confirmed cases of COVID-19 in care home residents across 4,382 care homes (with some care homes experiencing multiple outbreaks), of whom 13, 971 died, over the study period. 11,777 residents with COVID-19 had recently had a hospital admission, of whom 1,744 had a definite, probably or possible hospital-acquired infection (defined based on time in hospital). Of the outbreaks in care homes, 97 were epidemiologically associated with hospital discharge, representing 1.6% of the care home outbreaks identified in this analysis. These 97 outbreaks involved 804 (1.2%) residents, of whom 286 died (died within 60 days of first specimen date, or COVID-19 mentioned on death certificate).
Public Health Scotland (PHS)[footnote 15]
PHS analysis first identified people discharged from hospital to a care home, using data from multiple sources, and with manual validation in clinical records where data were ambiguous. Importantly, the analysis identifies discharges for short or temporary stays in care homes, which represented 730 (14.1%) out of 5,198 discharges to care homes between 1 March and 31 May 2020. Outbreaks were defined as one or more residents having laboratory confirmed COVID-19. Some 2,344 discharges were to care homes which did not experience an outbreak, 1,019 were to care homes with an existing outbreak, and 1,822 were to care homes at some point before an outbreak started.
349 people had laboratory confirmed COVID-19 at some point before their hospital discharge, of whom 243 had a subsequent negative test before discharge. Similar to the PHE analysis but with slightly different definitions, the timing of care home outbreaks in relation to the discharge to 79 care homes of the 106 people with a positive test but no negative test before discharge were examined.
Of 348 outbreaks in Scottish care homes in March to May 2020, 5 (1.4%) were defined as having a ‘possible epidemiological association’ with a hospital discharge of a person whose last test was positive, and 5 (1.4%) were defined as having an ‘uncertain association’. For 3 of these 10 outbreaks, the discharged person’s test sample had been sequenced. For 2 of these, other sequencing data were available for other care home residents, allowing a limited examination of whether the discharged person was the likely source of the outbreak.
In one care home with 12 laboratory confirmed cases in residents, 8 had been sequenced finding 2 UK lineages consisting of 4 phylotypes, consistent with multiple introductions of infection.
In one care home with 7 laboratory confirmed cases in residents, 3 had been sequenced finding 2 UK lineages and 2 phylotypes. Although very limited data, this is some evidence that the outbreak was not due to a single introduction.
Northern Ireland report for Minister of Health[footnote 16]
In Northern Ireland a report to the Minister for Health identified the weekly pattern of care home outbreaks during the first wave of COVID-19 appears to be more closely correlated with COVID-19 hospital admissions rates during the same week (a reasonable surrogate for general community transmission and infection) than with the associated rates of unscheduled discharges to care homes.
Patient-level analysis of those testing positive during weeks when the number of people discharged to care homes was more than usual (weeks 11 and 13), found that only about 1% (5 out of 465) of those people tested positive for COVID-19 in the fortnight after discharge to a care home based on testing of symptomatic residents. It did not support a hypothesis that this group of people was a substantial cause of COVID-19 outbreaks in care homes.
Uniquely the NI report includes a survey of 130 consultants responsible for discharge decisions suggesting no change in discharge decision making in respondents (31 out of 130 response rate).
Summary
Both the UKHSA and PHS analyses identify a small proportion of outbreaks which could have been seeded by a hospital discharge of someone who had tested positive for COVID-19. The PHS genomic analysis is limited by the data available, but the 2 epidemiologically associated outbreaks examined are consistent with multiple introductions of COVID-19 to the care home rather than the discharge being the sole cause of introduction. Analyses are constrained by the available data, since most people discharged were not tested in March and April.
Analysis using all discharges from hospital
These analyses identify people discharged from hospitals to care homes irrespective of whether they were tested for COVID-19 or were known to be positive. They therefore provide alternative evidence than studies of ‘epidemiologically associated’ outbreaks, but the findings are critically dependent on the quality of the data in terms of timeliness and completeness of who are resident in care homes.
Public Health Wales (PHW) survival model
The study population was adults living in residential or nursing care home in Wales, identified by manual review of partially address matched data from COVID-laboratory testing and hospital discharge datasets. 3,115 discharges to 544 (of a total 1068) care homes were identified. Some 330 (30.9%) care homes experienced an outbreak (defined as one or more residents testing positive for COVID) between 22 February and 30 June 2020.[footnote 17]
The analysis used a Cox proportional hazards regression model with the first positive test in the care home as the outcome, with exposure relating to hospital discharge fitted as a time-varying covariate. Model output in relation to hospital discharge therefore compares the hazard of an outbreak starting in the period 7 to 21 days after a hospital discharge versus the hazard in other periods of time (that is, without the exposure). Sensitivity analysis examined whether varying the exposure time period after discharge influenced findings (concluding that it did not).
In univariate analysis, compared to periods without a discharge, there was a statistically significant increased risk of an outbreak in the period 7 to 21 days after a hospital discharge to a care home (unadjusted hazard ratio (HR) 2.47, 95% CI 1.96 to 3.11). After adjustment for other care home characteristics (bed capacity as the measure of care home size, provision of dementia care, service subtype in terms of with versus without nursing, provision of learning disability care, health board), this association was smaller and no longer statistically significant (HR 1.15, 95% CI 0.89 to 1.47). Care home size was very strongly associated with the risk of an outbreak (for care homes with 50 or more beds versus those with less than 10, adjusted HR 17.35, 95% CI 9.65 to 31.19).
Public Health Scotland (PHS) survival model
The study population was the same as for the analysis of ‘potentially epidemiologically associated’ outbreaks described and referenced above.[footnote 18] The analysis replicated the PHW Cox regression analysis described above in terms of methods but with some variation in how care home characteristics were defined due to different data availability. It also additionally examined hospital discharge in terms of whether the person discharged had not been tested, had been tested and shown to be negative before discharge, or whose last test before discharge was positive.
Replication of the PHW analysis
In univariate analysis, compared to periods without a discharge, there was a statistically significant increased risk of an outbreak in the period 7 to 21 days after a hospital discharge (unadjusted hazard ratio (HR) 2.85, 95% CI 2.28 to 3.56). After adjustment for other care home characteristics, this association was smaller and no longer statistically significant (HR 1.18, 95% CI 0.92 to 1.52). Care home size was very strongly associated with the risk of an outbreak (for example, for care homes with 90 and more beds versus those with less than 20, adjusted HR 17.4, 95% CI 7.51 to 40.33), noting that the categorisations of care home size varies between PHW and PHS analysis due to different pattern of building sizes.
Additional analysis stratifying by testing status before discharge
In univariate analysis, compared to periods without a discharge, there were statistically significantly increased risk of an outbreak in the period 7 to 21 days after a discharge. Compared to periods without a hospital discharge, the univariate HR for those discharged with a negative test was 2.13 (95% CI 1.43 to 3.16), compared to univariate HR for those discharged untested 3.06 (95% CI 2.41 to 3.89), and univariate HR for those discharged positive 4.65 (95% CI 1.71 to 12.66). After adjustment for other care home characteristics, associations were smaller and no longer statistically significant. Compared to periods without a hospital discharge, the adjusted HR for those discharged with a negative test was 0.95 (95% CI 0.63 to 1.43), for those discharged untested adjusted HR 1.27 (95% CI 0.97 to 1.66), and for those discharged positive adjusted HR 1.17 (95% CI 0.41 to 3.31). Care home size was very strongly associated with the risk of an outbreak (for example, for care homes with 90 and more beds versus those with less than 20, adjusted HR 17.6, 95% CI 7.58 to 40.86).
Swansea University Medical School multilevel logistic regression
The study population was residents of adult care homes identified by matching GP registered address to a care home index address via a unique address identifier, and included data for 15,772 residents of 923 care homes. Hospital discharge and laboratory testing data for these residents was linked for analysis.[footnote 19]
Analysis used a multilevel logistic regression model where the outcome was an increase in the number of cases over a 2-week period, and the exposure was if any resident had been discharged from hospital in the previous 14 days. Sensitivity analysis varied periods of observation.
In univariate analysis, hospital discharge was associated with an increased risk of a subsequent increase in cases in the care home (odds ratio (OR) 1.24, 95% CI 1.01 to 1.52). In the adjusted analysis, the association was not statistically significant (OR 0.99, 95% CI 0.82 to 1.90). Care home size was the characteristic most strongly associated with an increased risk of an outbreak (for the largest fifth of homes compared to the smallest fifth, adjusted OR 26.3 (95% CI 12.9 to 53.8).
Swansea University Medical School Hawkes process model
The study population is the same as described and referenced above.[footnote 14] Analysis used a Hawkes process model which allows an estimate of the effect of a hospital discharge on the rate of spread of infection, compared to the ‘background’ rate of introduction from other sources, modelled by comparing the association with outbreak intensification of a laboratory confirmed case in a resident, with the association with outbreak intensification of a hospital discharge whose infection status is unknown.
The findings were that a hospital discharge was statistically significantly associated with subsequent outbreak intensification but the effect was small compared to the association of a known positive case. One hospital discharge was estimated to have the same effect on intensity as 0.018 cases. An alternative interpretation is that 1.8% of hospital discharges were actually infected. Associations with hospital discharge were smaller than associations with care home size, suggesting the highest risk of introduction came from interaction with the community.
Summary
The PHW and PHS analyses use the same method on different populations with similarly constructed but not identical datasets, to examine if hospital discharge was associated with the start of care home outbreaks. The findings are consistent, in that neither finds statistically significant associations between hospital discharge and the start of COVID-19 outbreaks in care homes once other care home characteristics are accounted for, and in particular care home size which is consistently found to be statistically significantly and so very strongly associated with the presence of an outbreak. However, the estimated hazard ratio (HR) for hospital discharge in both analyses was greater than one and confidence intervals relatively wide, meaning that neither analysis can exclude a small but important association with discharges (although any plausible association with hospital discharge is very small compared to the observed association with care home size).
The Swansea University analyses examine Welsh data using different methods to examine whether hospital discharge is associated with a subsequent intensification of an outbreak (which is important since a care home ‘outbreak’ can be due to multiple introductions rather than a single point source). The first analysis using multilevel logistic modelling found no association between hospital discharge and subsequent outbreak intensity. The second analysis using a Hawkes process model found evidence that hospital discharge was associated with an increase in subsequent outbreak intensity, but the effect was small compared to the association with a known case in the care home, and compared to associations with care home size.
International evidence for outbreak causation
There are a few case studies where admissions to care homes of people discharged from hospital without COVID-19 testing has been identified as a possible route of infection, but these studies do not provide a robust way to estimate the potential impact of hospital discharge policies due to risk of observational bias. For example, a case study of an outbreak in a long-term care facility in London for people with severe epilepsy reports that a resident was symptomatic and admitted to hospital on March 5, 2020 but was discharged four days later after testing negative. They then tested positive 2 weeks later on March 23 and were transferred back to hospital. Three more cases in the same unit (1 resident, 2 staff) were identified over the following 6 weeks, although a genomic investigation about the clustering of cases was not performed.[footnote 20]
At the beginning of the pandemic, countries such as Austria, Chile and Spain, and some states of the United States of America, also had a policy of discharging people from hospital to care homes without testing, with requirement that the provider found a way to isolate people recently discharged.[footnote 21] This was reversed as testing capacity increased and to respond to concern about the large number of deaths of care home residents and the lack of capacity within care homes to provide effective isolation. These approaches caused substantial alarm and controversy in many countries, for example in the United States.[footnote 22]
Limitations
This statement is not a formal systematic review nor is it intended to review the policy decisions. The considered studies are by national organisations or academic groups tasked by national organisations using routine data, analyses are published or pending peer review (and so taken from pre-print servers). Smaller studies working with care homes such as the UKHSA Easter 6 project with whole genomic sequencing found disease ingress to be a complex series of events and not linked to a single individual. All analyses of impact of hospital discharge are limited by 2 important features of the available data.
First, there were low and variable levels of testing in the care home population in wave 1, with variation between areas depending on testing capacity. Typically, symptomatic residents admitted to hospital were tested, but asymptomatic residents who were admitted for other reasons were not routinely tested before discharge in March and the first 3 weeks of April 2020. Typically, testing of symptomatic residents in the care home was restricted to the first few residents in March and early April 2020 (to identify an outbreak – this is normal practice for outbreaks of other infections in care homes historically). Subsequently in wave 1, testing of all symptomatic residents was recommended, but testing of all residents including those without symptoms residents was not routine until late summer and early autumn 2020, either as monthly whole home testing (for example in England) or as part of outbreak management (for example in Scotland).
Second, no UK country can easily and completely identify who is resident in care homes or who was discharged from hospital to care home. Identification of care home residents is typically done by matching a person’s GP registered address to a care home address. This is known to be a reasonable but not perfect method, because of mismatch failures and because people with short stays in care homes (for example, temporary placements) do not usually change their GP address. Identification of discharge from hospital to care home either uses hospital discharge coding (which is also not always accurate) or address matching.
Given under-ascertainment (incomplete testing in wave 1) an outbreak is defined pragmatically in these analyses as one case equals an outbreak. This is different from other settings and diseases where convention is at least 2 cases, but for COVID-19 one case equals outbreak is a pragmatic definition in public health practice in wave 1 because they usually got rapidly larger. Where it exists, then genomic evidence (albeit selected as big outbreaks) suggests that the actual outbreak was larger than the clinical testing found. Under-ascertainment also means it was not possible to monitor PPE usage systematically or other sources of infection.
The quality of data varies between the different UK countries (for example, Wales has invested significantly in how public services record addresses which will improve Welsh data for this kind of analysis), and there is variation in the effort put into improving data quality for the different analyses described above (for example, the Scottish analysis involved considerable effort to identify who was actually discharged from hospital to care home in wave 1 including identification of temporary residents and manual checking of hospital records where discharge destination was uncertain).
None of these problems is fixable retrospectively, meaning that all the analyses done are based on data that are less than ideal.
Declarations of interest by members of the SCWG
The participants reporting an interest in studies or activities associated with the content of this report are:
-
SCWG participants Bruce Guthrie, Rich Fry, Dimple Chudasama and Laura Shallcross were co-authors of major studies cited in this consensus statement
-
Adam Gordon is an expert witness for the claimants in the case for Dr Cathy Gardner and Fay Harris against the Secretary of State for Health and Social Care, NHS Commissioning Board (NHS England) and Public Health England
-
Liz Jones has absented herself from this consensus statement
-
Éamonn O’Moore is an UKHSA employee and has provided and reviewed evidence by Government Legal Department and submitted witness statements in relation to the Gardner case
-
Jackie Cassell is employed at the Brighton and Sussex Medical School, but has been seconded to UKSHA on a part-time basis since 1 September 2021
-
Tom Finnie, Martyn Regan, Hannah Williams and Dimple Chudasama are UKHSA employees
-
Shallcross L, Burke D, Abbott O and others, Factors associated with SARS-CoV-2 infection and outbreaks in long-term care facilities in England: a national cross-sectional survey, The Lancet Healthy Longevity ↩
-
Genomic epidemiology of COVID-19 in care homes in the east of England ↩
-
Large-scale sequencing of SARS-CoV-2 genomes from one region allows detailed epidemiology and enables local outbreak management ↩
-
The role of viral genomics in understanding COVID-19 outbreaks in long-term care facilities ↩
-
Deaths involving COVID-19 in the care sector, England and Wales – Office for National Statistics. The average for 2015 to 2019 provides a comparison of the number of deaths expected per week in a usual (non-pandemic) year. This comparison provides a frame of reference but it should be noted that occupancy will have fallen after wave 1, which will affect comparability for wave 2. ↩
-
Morciano M, Stokes J, Kontopantelis E, Hall I, Turner AJ, 2021 Excess mortality for care home residents during the first 23 weeks of the COVID-19 pandemic in England: a national cohort study, BMC Med 19, 71 (2021) ↩
-
Tang S, Sanchez Perez M, Saavedra-Campos M and others. ‘Mass testing after a single suspected or confirmed case of COVID-19 in London care homes, April to May 2020: implications for policy and practice,’ Age and Ageing 2021;50:649-56. ↩
-
Tang S, Sanchez Perez M, Saavedra-Campos M and others. ‘Mass testing after a single suspected or confirmed case of COVID-19 in London care homes, April to May 2020: implications for policy and practice,’ Age and Ageing 2021;50:649-56. ↩
-
Tang S, Sanchez Perez M, Saavedra-Campos M and others. ‘Mass testing after a single suspected or confirmed case of COVID-19 in London care homes, April to May 2020: implications for policy and practice,’ Age and Ageing 2021;50:649-56. ↩
-
The role of viral genomics in understanding COVID-19 outbreaks in long-term care facilities ↩
-
Byrd W, Salcher-Konrad M (2021), Evidence summary: what research is there linking hospital discharges to COVID-19 outbreaks in long-term care facilities?, International Long-Term Care Policy Network, CPEC-LSE, 22 October 2021 ↩
-
Genomic epidemiology of COVID-19 in care homes in the east of England ↩
-
COVID-19: assessment of hospital-associated SARS-CoV-2 infection and care home outbreaks ↩
-
Statistical report: Discharges from NHSScotland hospitals to care homes, and methodology ↩
-
Clinical analysis of discharge patterns from HSC hospitals in Northern Ireland ↩
-
Risk factors for outbreaks of COVID-19 in care homes following hospital discharge: a national cohort analysis ↩
-
Statistical report: Discharges from NHSScotland hospitals to care homes, and methodology ↩
-
Intensity of COVID-19 in care homes following hospital discharge in the early stages of the UK epidemic, Age and Ageing, Oxford Academic ↩
-
Balestrini S, Koepp MJ, Gandhi S, Rickman HM, Shin GY, Houlihan CF and others. Clinical outcomes of COVID-19 in long-term care facilities for people with epilepsy, Epilepsy and Behavior, 107602 ↩
-
Comas-Herrera A, Marczak J, Byrd W, Lorenz-Dant K, Patel D, Pharoah D (editors) and long-term care (LTC) contributors. LTC COVID International living report on COVID-19 and long-term care. LTC COVID, Care Policy and Evaluation Centre, London School of Economics and Political Science ↩
-
Van Houtven C, Miller K, Gorges R, Campbell H, Dawson W, McHugh J, McGarry B, Gilmartin R, Boucher N, Kaufman B, Chisholm L, Beltran S, Fashaw S, Wang X, Reneau O, Chun A, Jacobs J, Abrahamson K, Unroe K, Bishop C, Arling G, Kelly S, Werner RM, Konetzka RT and Norton EC, State policy responses to COVID-19 in nursing homes, Journal of Long-Term Care, 2021, pages 264 to 282 ↩
