Regulatory Horizons Council: the Governance of Engineering Biology
Published 16 January 2025
Executive summary
Engineering biology (EB) is an overarching, agile and rapidly evolving technology platform with the potential to spawn transformative, or disruptive, innovations across a broad range of sectors of the economy. Its increasingly sophisticated range of genetic technologies, including genetic modification, synthetic biology, and genome editing, are enabling the creation of a new bioeconomy with the potential to disrupt or displace incumbent industries. The McKinsey Global Institute claims that “as much as 60 percent of the physical inputs to the global economy could, in principle, be produced biologically.”[footnote 1]
Based on our interactions with stakeholders, we concluded that our most useful course of action would be to address the question, “How can we best manage overall governance of the complex array of products arising from the EB platform,” making recommendations on the most important issues and systemic interactions to be considered in providing future answers on a sector-by-sector basis or on the basis of novel classes of product. These recommendations are therefore intended to provide the framework for future decisions on EB governance, to be undertaken on a sectoral or market basis or based on common sets of properties.
This report therefore addresses the challenges surrounding the effective governance of products of this broad range of enterprises. For regulators, there is an understandable desire for simplicity, to capture as many areas of application as possible under a single regulatory system; but that approach can result in the unnecessary failure of innovative developments that would otherwise contribute to growth of the UK economy, meeting Net Zero policy commitments or curing or preventing a disease. The challenge is to find an optimal balance between simplicity in governance systems and regulatory design, and their ability to handle a range of innovation scales and complexities.
This report underscores the need for a dynamic, adaptive governance framework to support translation of EB products into markets and sets out recommendations for government that will help the UK to harness the potential of EB, contributing to economic growth, environmental sustainability, and public health.
Recommendations to government
Recommendation 1: Engineering biology products should be governed from the earliest stages of development based on their properties as they emerge at different points along a value chain (including balancing potential benefits and hazards) and not based on the platform technology from which they originate.
Recommendation 2: Innovators should ensure that regulators, standards bodies, metrology organisations and policy makers have a good systemic understanding of the innovative potential and properties of EB products and the uncertainties surrounding them at different development stages. The Department for Science Innovation and Technology (DSIT) should own the process of commissioning this information and disseminating it to the wider EB regulatory landscape through, for example, the already-established Engineering Biology Regulators’ Network (EBRN) or via a new broader, product/market focused Industrial Biotechnology Regulators’ Network (IBRN). This could be coordinated by the most relevant trade body in each case, or where multiple trade bodies exist, by a nominated group. A direct ‘in confidence’ route could be established alongside this to enable businesses to share commercially sensitive information directly with regulators.
Recommendation 3: Regulators, standards bodies and policy makers should work together (via the Engineering Biology Regulators’ Network or another route such as a product/market focused IBRN) to optimise EB governance decisions based on: (i) information provided based on recommendation 2; (ii) the principles of proportionality (to the benefits and hazards of EB products) and adaptation (to innovative governance requirements); and (iii) the creative use of standards and guidelines, in sequence or in parallel with legally-based regulations, depending on the circumstances.
Recommendation 4.1: In addition to planned biosecurity-related communications among those involved in policy making, research and development of EB products, there needs to be a linked, parallel, public-facing strategy and narrative, designed to communicate the background and reasons for biosecurity-related governance to a general, non-specialist audience.
Recommendation 4.2: The Biosecurity Leadership Council should consider the need to ensure that the latest government thinking on pro-innovation regulation, as implemented through the Regulatory Innovation Office (RIO), and as embodied in Recommendations 2 and 3, is considered and integrated into future plans for biosecurity governance.
Recommendation 5: In the context of the Convention on Biological Diversity (CBD) Nagoya Protocol and the new DSI multilateral benefit sharing mechanism, including the Cali Fund, ensure that the implementation of Access and Benefit Sharing Agreements aligns with the needs of the sector.[footnote 2] The Regulatory Horizons Council can work closely with Defra and the Department for Business and Trade to support the design and delivery of industry engagement over the first quarter of 2025. This will be to ensure, as far as possible, that the implementation of the Nagoya Protocol and the DSI benefit sharing mechanism is compatible with, and supports, the overall governance approach recommended in this report.
Recommendation 6: Across all sectors of the economy, including IB, as part of the implementation of a pro-innovation governance approach, companies should be encouraged to undertake a formal commitment to responsible innovation.
1. Background
The Regulatory Horizons Council (RHC) is an independent expert committee established in 2019 to identify the implications of technological innovation and provide government with impartial advice on the regulatory reforms required to support its rapid and safe introduction.[footnote 3]
The RHC was commissioned to undertake a review of the regulation of engineering biology sectors as part of the Department for Science, Innovation and Technology’s ‘Sector Vision’ (December 2023). Following the commission, we brought together the engineering biology sector regulators for a workshop to explore initial issues and hear their perspectives on the current governance system and how it is performing. We also reviewed responses to DSIT’s 2023 Engineering Biology Call for Evidence and conducted further structured interviews with 10 stakeholders from business and academia, before subsequently sense testing our proposals with members of that group.
Definitions
Engineering Biology (EB) and Industrial Biotechnology (IB)
These terms are often used interchangeably.[footnote 4] In other cases, EB is more closely linked to synthetic biology as a process of pushing the boundaries of biotechnology-related sciences,[footnote 5] including making customised genomes.[footnote 6]
In this report, we are making the following distinctions:
-
Engineering biology is a platform technology, pushing the boundaries of biotechnology and related sciences, including making customised genomes, acting as a platform from which to deliver transformative innovative transformative innovative products across a broad range of sectors of the economy.
-
Industrial Biotechnology covers the range of manufacturing processes, mainly involving large scale fermentation, across all sectors involved in the exploitation of these opportunities, to deliver end products to a market.
Regulation and Governance
In this report, the term ‘regulation’ is used to describe legally based regulatory systems. The term ‘governance’ refers to both regulation (‘hard law’) and standards, guidance, and policies (‘soft law’), covering the overall means of exercising authority over the nature and application of products developed under the heading of EB. Likewise, the term ‘regulatory system’ describes a system based only or primarily on regulation, and ‘governance system’ describes a system based on a mixture of standards, guidance, policy and regulation.
2. Introduction
Engineering biology (EB) is one of the ‘great technologies’ that have been identified in successive UK innovation strategies as transformative for the economy and worthy of state support in various forms.[footnote 7] It is an example of an overarching, agile and rapidly evolving technology platform with the potential to spawn transformative, or disruptive, innovations across a broad range of sectors of the economy, rather than being envisaged as a single monolithic sector. It shares these properties with other major disruptive technology super-platforms like artificial intelligence (AI) and quantum technologies, and indeed EB product developments are often enhanced by the use of AI. Relevant business sectors include commodity chemicals (detergents, flavours, perfumes, cosmetics, plastics, dyestuffs, fuel, building materials, fibres and fabrics); plant- and animal-based food and feedstocks; complex biomolecules (drugs, diagnostics, vaccines, pesticides); and cell-based products (single-cell proteins for food and feed, cell cultivated meat); mostly manufactured using industrial biotechnology (IB) fermentation but also involving plant and animal production systems.
This report focuses on the needs of the majority of EB/IB developments, where products are manufactured using living organisms, but the end-products on the market are non-living. There are cases where live end-products are marketed (e.g. bioremediation using micro-organisms, vaccines) but these would need to be considered as part of a further report based on this one, dealing with these special circumstances.
Many EB developments have the potential to be disruptive of the business models of incumbent companies and the more disruptive the innovation, the more challenging it will be to manage the governance of products based on EB. The governance systems we choose will determine which products and services are able to be developed, how profitable they will be and also how societally and environmentally beneficial, which sectors of the economy will be involved, and which nations and regions are able to participate in their production. For each sector involved there will be governance systems already in place, leading to different sets of challenges and requirements for adaptation when or if future products are assigned to them, in many cases requiring the setting up of new governance systems or major re-setting of old ones. This complexity brings associated governance-related challenges that are defying attempts at simple solutions, and some will delay or prevent altogether the development of potentially transformative innovative products.
In our previous report on the regulation of Genetic Technologies, we considered how EB-related techniques will have a major impact on global crop production.[footnote 8] This report focuses on the potentially even greater impact of EB products being developed in industrial biotechnology sectors, using living organisms rather than fossil fuels, and reducing greenhouse gas emissions.
Based on our interactions with stakeholders, we concluded that our most useful course of action would be to address the overall question “How can we best manage overall governance of the complex array of products arising from the EB platform,” making recommendations on the most important issues and systemic interactions to be considered in providing future answers on a sector-by-sector basis or on the basis of novel classes of product.
This report therefore addresses the challenges surrounding the effective governance of products of this broad range of enterprises. For regulators, there is an understandable desire for simplicity, to capture as many areas of application as possible under a single regulatory system; but that approach can result in the unnecessary failure of innovative developments that would otherwise contribute to growth of the UK economy, meeting Net Zero policy commitments or curing or preventing a disease. The challenge is to find an optimal balance between simplicity in governance systems and regulatory design, and their ability to handle a range of innovation scales and complexities.
2.1 The global capacity and reach of Engineering Biology
Engineering biology, acting as a technology platform with the potential to deliver transformatively innovative products, could alter radically the business models and value chains of a very broad range of industry sectors. Its increasingly sophisticated range of genetic technologies, including genetic modification, synthetic biology, and genome editing, are enabling the creation of a new bioeconomy with the potential to disrupt or displace incumbent industries. The McKinsey Global Institute claims that “as much as 60 percent of the physical inputs to the global economy could, in principle, be produced biologically.”[footnote 1]
The benefits of EB innovations will be delivered mainly through the IB sectors of the bioeconomy as noted above, reducing the climate impact of these sectors, addressing global challenges around climate change, food security, biodiversity, sustainable energy generation and pollution clean-up. Almost all respondents to a 2024 poll of executives from 1100 corporate organisations believed that bio-solutions will significantly disrupt their industry, and half of those believed this change will come within five years.[footnote 9] IB delivery mechanisms include mainly fermentation-based manufacture and also crop, plant or animal-based production of food, feed and fibres. Given this scale of operation, EB’s potential economic impact could be worth trillions of dollars to the global economy and the UK is well placed to capitalise on this opportunity. It sits behind only the US and China for fundraising with over £5.2 billion raised by firms between 2017 to 2022 alongside a strong commitment to R&D, foundational research, and infrastructure.[footnote 10] We have renowned research institutions, and strong talents, skills and industry across the range of converging technologies at the forefront of EB developments. However, these technology-based advances will depend on successful market uptake which, in turn, will require a creative, smarter governance approach.[footnote 11]
2.2 Synergies between AI and EB
There are important synergistic interactions between the AI and the EB innovation platforms:
-
AI contributes, from early research stages through translational development, to the discovery of new tools, techniques, and product-related opportunities, extending the capabilities of the EB platform, scaling up and speeding up the development of new products;
-
AI enables the development of measurement and modelling tools to support implementation of standards involved in EB governance; and
-
AI can play a role in the intelligent creation and management of information contributing to understanding the nature of potential benefits and hazards arising from EB products and how they can be mitigated, for example by adapting innovation processes, and avoiding the need for specific governance actions.
Alongside these synergistic interactions between EB and AI, governance-related problems can arise when combining two very different innovation-related traditions with different challenges and different expected timescales for development.
2.3 Contribution of engineering biology products to national policies and goals
Harnessing the economic opportunities of EB and growing the UK bioeconomy will contribute to the government’s ‘kickstarting growth’ mission, making Britain a clean energy superpower, and building a more resilient National Health Service. Success there will spill over into other priority policy areas, for example, presenting significant opportunities to advance national policy goals such as food security, Net Zero, the UN Sustainable Development Goals, and developing a circular bioeconomy.
Given the pervasive nature of its product range, its potential to replace fossil fuels as the starting material for production processes, and the generally greater thermal efficiency and lack of pollution from these processes, cell-based manufacturing could reduce carbon emissions while also creating more resilient supply chains for UK industry. EB also has the potential to play a role in mitigating the impact of climate change through replacing some fossil fuels; harnessing production process emissions in industry, construction and agriculture by reusing ruminant and waste by-products; bio-based product substitution for emissions-intensive industries; direct environmental sequestration of greenhouse gases outside of industrial systems.[footnote 12]
2.4 Principles, governance processes and practical consequences
The following principles should play a central role in guiding governance for innovative EB technologies:
-
Proportionality in considering the risks and benefits of products;
-
Adaptability to their emerging properties;
-
Balance in responding to the interests and values of a wide range of stakeholders;
-
Characterised by responsibility in the behaviour of companies and stakeholders.
These principles point to the value of regulating EB products on the basis of their properties, potential risks and benefits, rather than on the basis of the technologies used to produce them, as advised in Recommendation 1.
2.5 Report structure
Section 3 describes a systemic approach to the governance of products arising from major technology platforms like EB, focusing on the separate perspectives of innovators and governance-related bodies and the need to integrate both perspectives in governance decision making. Section 4 considers issues that extend beyond the proposed product-based governance approach for EB-related developments, and Section 5 considers how the recommendations in this report would act as a framework for further sector-based governance of EB products and could be applied to other major innovation platforms.
3. A systemic approach to governing the products of engineering biology
3.1 Overview
A systemic approach takes account of the system components, how they interact with one another to deliver a desired endpoint and the nature and location of the boundary that divides the system from its environment. This approach is justified where the range, complexity and uncertainty of interacting issues cannot be addressed within the scope of conventional discipline-based analysis, although discipline-based insights will often be necessary to understand a system or to guide its behaviour. Another important aspect of systemic analyses is the need to take account of, or integrate, several different perspectives in coming to decisions, and understanding which system elements will be relevant to these decisions.
The RHC Report on Genetic Technologies considered crop-based products designed to be grown in an open environment (deliberate release) and, at the time of publication, coming under the EU regulatory system, described as ‘process-based’, i.e. governed in the early development stages according to the nature of the innovative platform technology from which they originate (genetic modification (GM)), rather than according to the properties (potential benefits and risks) of the products being developed.[footnote 8] GM regulation is one of the most extreme cases where a supra-national governance system is inhibiting development of innovative products that are widely regarded as equivalent to conventionally bred crops in other countries. That report argues the case for regulating such products on the basis of their potential benefits and hazards and not the platform from which they originate. A similar conclusion was reached in the RHC Quantum report.[footnote 13]
The term “regulate the product, not the process”, expressed as a stark alternative, is difficult to operationalise as the production process will often influence the nature of a product’s benefits and risks. It should be seen as a shorthand phrase, covering the more complex reality: for disruptive innovations, the production process should be considered insofar as it influences the properties, benefits and hazards inherent in the end product, but it should not be used as a basis for regulation that slows down or stops early stage development of all products derived from that process, as has been the case with EU regulation of GM and related technologies. The Proportionate and Adaptive Governance of Innovative Technologies (PAGIT) Report recommends that, in early development stages, up to technology readiness level (TRL) ~6, standards and guidelines should be used to govern the development of an innovative product range, given their greater flexibility and adaptability in response to changes in understanding of product properties and capabilities (see figure 3).[footnote 14]
The EU regulatory system for deliberate release applies where the end-products (crops) will be introduced live into the natural environment. IB is generally a contained, fermentation-based production process, also subject to regulatory requirements that are based on the nature of the innovation platform from which they originate. The relevant regulatory instrument is the Genetically Modified Organisms (Contained Use) Regulations 2014, and the Health and Safety Executive (HSE) is the competent authority, in collaboration with the Department for Environment, Food & Rural Affairs (Defra). The legislation generally requires manufacturing to be carried out at containment level 1 (the lowest level) which has not been inhibiting of product development in contained use. However, some pressure groups are beginning to advocate that products manufactured under contained conditions should be governed in the same way as those involving deliberate release in the EU, a course of action for which there would be no scientific, risk-based justification.[footnote 15] Recommendation 1 emphasises the view that non-living EB end-products, manufactured under safe, contained conditions, however achieved, should not be governed primarily through a system based on the production process.
Recommendation 1
Engineering biology products should be governed from the earliest stages of development based on their properties as they emerge at different points along a value chain (including balancing potential benefits and hazards) and not based on the platform technology from which they originate.
Delivering this recommendation will require increased collaboration among all the relevant governance bodies, as emphasised in Recommendations 2 and 3.
3.2 Integrating the perspectives of innovators and governance-related actors
3.2.1 The innovators’ perspective
Given the scale and range of EB products and their potential to contribute to mitigating climate change and improving biodiversity, a systemic approach is needed to guide decision makers on the important factors to be considered in devising future governance systems. Proportionate and adaptive governance of EB products (Section 3.2.2) will require governance actors (standards bodies, metrology organisations and regulators) to have a detailed understanding of the innovators’ knowledge base, including future business models and value chains (Figure 1), and expectations on how these are likely to change as understanding of the properties of the product and its capabilities improves over time. This information is part of the expected skills base of an innovator, but it is a new requirement to expect governance-related actors, particularly regulators, to take account of it in their governance decision making.
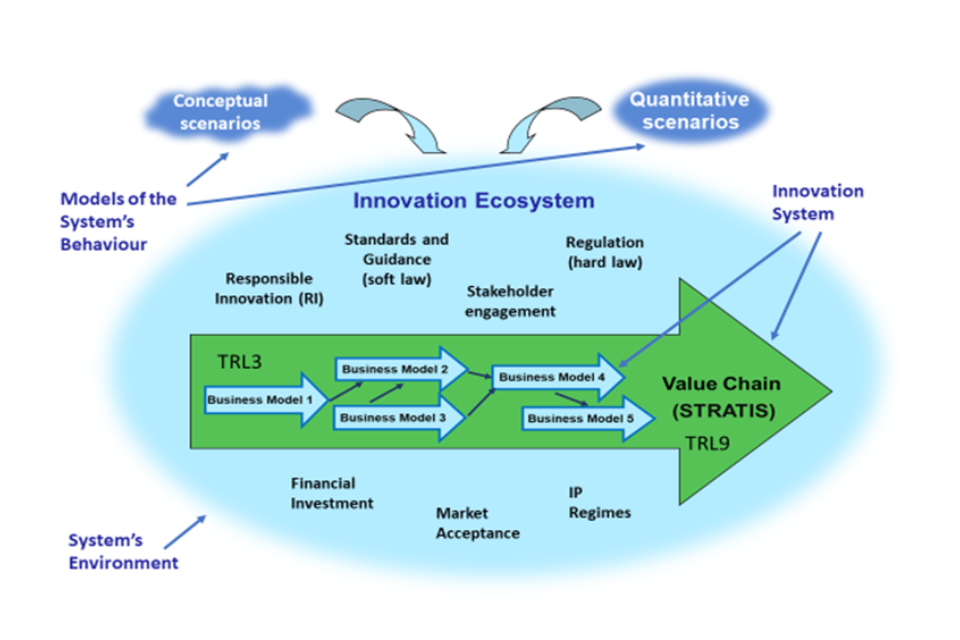
Figure 1, Strategic Analysis of Advanced Technology Innovation Systems (STRATIS).
This information will need to be provided by innovators and, particularly for large companies, it should be part of their routine management planning. However, some of it may be regarded as confidential, requiring skill in getting the message across without divulging confidential information. In the early stages of product development, the path to the end product may be very uncertain and companies often have to pivot unexpectedly and change their business model. They may also have to do this because of an existing regulation which is inappropriate for the product being developed, something that happens quite frequently for disruptive innovation. The factors to be considered as part of this procedure will be sector specific so this report is emphasising that this contribution to governance decision making will need to become a standard operating procedure as part of the process of reforming the governance of innovative technologies, providing a framework on which to build future sector-specific models.
On their route to market, EB products will often progress through several companies with different business models and different contributions to an overall value chain. As the innovative product moves along the value chain and at the same time through a sequence of technology readiness levels (TRLs),[footnote 16] improved understanding of its properties, hazards and opportunities will lead to changed economic expectations for its future roles and markets and changed expectations about future governance requirements. Thus, different regulatory systems may be brought into consideration as a product moves along TRLs in Figure 1 from TRL3 (proof-of-concept) to TRL9 (market readiness) or moves from one type of company to another (business models 1 – 5 in Figure 1) along a value chain. Consider, for example, using EB and IB to manufacture single cell protein (SCP) for animal feed, based on gas fermentation using hydrogen and carbon dioxide (Figure 2). Successfully delivering the SCP is only the beginning. It will then need to be tested to ensure that it meets the regulatory requirements for animal feed, then sold to animal feed producers who will sell it on to fish or chicken farmers; who will sell it on to food processors and/or supermarkets; who will sell it to the public. If the governance system unnecessarily halts or slows down development along this value chain, then the value of all the earlier investment in the product is lost or at least diminished. This is the reason why we need urgently to adapt our governance systems to meet the needs of innovative technologies, rather than expecting the technologies to be adapted to the requirements of pre-existing governance systems.
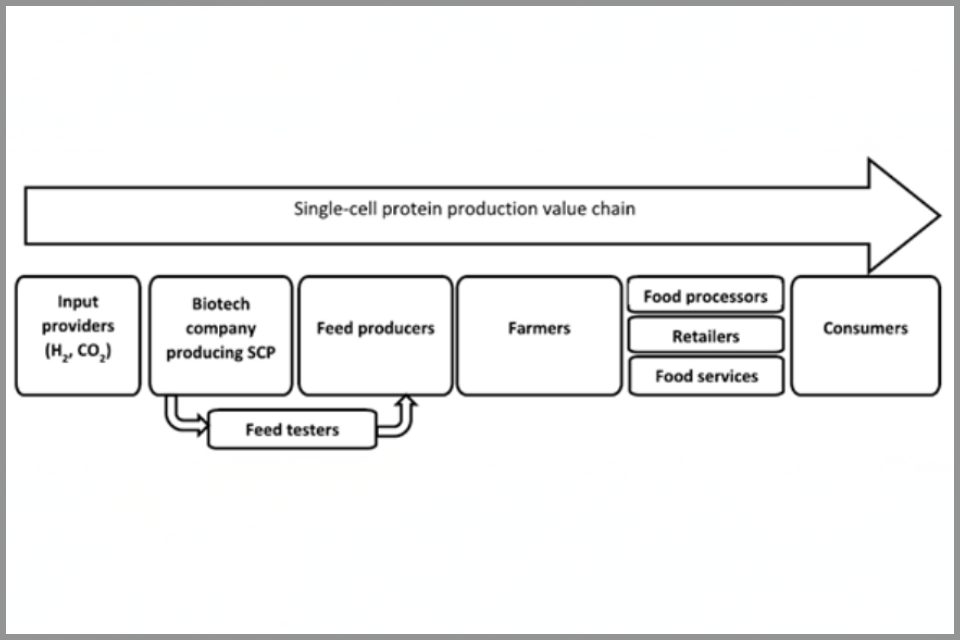
Figure 2, illustrative value chain for single-cell protein production. Image licensed under CC BY 4.0, see footnote 18 for full attribution.
The choice of governance approach, including the balance between regulations and standards, will need to be informed by an understanding of the extent to which the technology is disruptive, and for which companies in a projected value chain it will be most disruptive, as explained in Section 3.2.1.[footnote 17] An important prerequisite for managing governance systems to accommodate the needs of specific categories of innovation will be understanding how to optimise governance choices across all stages of product development and, if necessary, managing the changes needed to adapt specific governance systems to the needs of disruptively innovative products.
Particularly where a product is disruptively innovative, the expected governance approach may include requirements that do not fit well with the products’ properties, potentially eroding or eliminating its expected benefits or otherwise inhibiting its future potential. Identifying any such circumstances as early as possible in the development of a range of products could lead to creative, more adaptive relationships between innovators and governance actors, making a significant difference to the overall innovation capacity of a sector.
Recommendation 2
Innovators should ensure that regulators, standards bodies, metrology organisations and policy makers have a good systemic understanding of the innovative potential and properties of EB products and the uncertainties surrounding them at different development stages. DSIT should own the process of commissioning this information and disseminating it to the wider EB regulatory landscape through, for example, the already-established Engineering Biology Regulators’ Network (EBRN) or via a new broader, product/market focused IB Regulators’ Network (IBRN). This could be coordinated by the most relevant trade body in each case, or where multiple trade bodies exist, by a nominated group. A direct ‘in confidence’ route could be established alongside this to enable businesses to share commercially sensitive information directly with regulators.
This would involve the preparation of dossiers for innovative products, refreshed at intervals up to TRL 6 when there is expected to be a more stable understanding of the final properties of a product or of a group of similar products. This Company Innovation Dossier (CID) should include the information specified below. Many organisations already generate such data, some of which is included in their annual reports to shareholders, and so we would anticipate the overall burden of this being limited, and proportionate to the downstream benefits of improved governance, felt by companies. The CID should be designed to ensure that governance-related decision makers understand where and how current and expected future governance systems will support or unnecessarily hinder the delivery of innovative products and to contribute to the design of fully systemic governance arrangements:
-
The expected business models of the companies developing the product; and the value chains within which they will be located; also, their evolution along TRLs as products go through sequential stages of development, including the business sectors involved and their current governance regimes, any gaps in the value chain that will need to be bridged, and whether there are existing markets or a market will need to be created (Figure 3);
-
This dossier should be updated at intervals depending on the pace of development of the product(s), noting the opening up or closing down of future opportunities and any changes in the related governance questions and challenges,[footnote 18] and what kinds of decision will be supportive of the development of useful innovations;
-
The nature, likelihood and extent of the anticipated benefits and potential hazards of the product range;
-
How disruptive the innovations will be and for which companies/sectors in the value chain they will be most disruptive.[footnote 19]
In gathering information for the CID, innovators may become aware of cases where technological innovation could play a direct role in governance system adaptation – a product could be modified to change its properties so that it will no longer need to meet the requirements of a specific regulation or standard. However, such actions should not be undertaken at the expense of delivering the potential disruptive innovation. This may depend on early detection of the opportunity, at TRL~3, so that adaptation can take place while the innovation pathway is still open to modification.
3.2.2 The governance perspective
In the innovation ecosystem in Figure 1 are the governance-related decision makers who will be involved in interacting with innovators to manage optimally the future governance of EB products, as described from the governance perspective in Figure 3.
In the UK, the safety of new products, for people and the environment, is governed mainly through legally-based regulatory systems that were first set up in the 1950s, supported by standards and guidance Some of these are directed to a specific sector or set of sectors: drugs, diagnostics and medical devices (Medicines and Healthcare products Regulatory Agency); foods and animal feed (Food Standards Agency); chemicals (REACH); consumer goods and alternative fuels (Office for Product Safety and Standards). Some governance systems are directed towards specific hazard areas: health and safety of workers (Health and Safety Executive); environmental protection (Environment Agency). This complex array of wide-ranging governance instruments is sometimes ill-adapted to deal with the products of today’s EB technologies,[footnote 20] and the PAGIT framework points to the creative use of standards and guidance to introduce more flexibility and adaptability into legally based regulatory systems. Recent initiatives, like the establishment of an EBRN are welcome but may not be sufficient to address what are fundamental challenges in the system. Particularly for disruptive or transformative new technologies, these pre-existing governance systems are often damaging to innovation, depending on the extent to which their requirements fit with the properties of new product ranges. The primary decision, on the governance or regulatory system to which a set of innovative EB products should be assigned, is probably the most challenging to their future development.
Harder to evidence is the impact of this situation on the decisions of innovators and investors, who often decide to drop work on a particular set of innovative products in anticipation of their being governed in future through a system known to be onerous, expensive and time-consuming. This can be a much more insidious factor, acting as a drag on innovation potential.
The decision on where and how to capture new EB products within the remit of a particular governance system will determine whether new industries can coalesce around an innovative technology area, define its future shape, and determine the scale of its contribution to national economies, as well as ensuring safety, quality and efficacy. A poor choice of regulatory precedent, or delay in making such a choice through lack of a decision framework, are common reasons for EB product failure.[footnote 21] The suggestions in Recommendation 2 are designed to ensure that regulators, standards bodies and policy makers have access to the information needed to make good governance decisions at this systemic level.
Industrial biotechnology fermentation processes that involve modified micro-organisms, developed using genetic modification, gene editing or synthetic biology are treated from a governance point of view as ‘contained use’ and the manufacturing process is subject to the Genetically Modified Organisms (Contained Use) Regulations 2014. Depending on the modified organism used and the modifications made to it, this generally requires level 1 containment which is not regarded by companies as unreasonable or excessively onerous. Living products of IB based on any genetic technology will be regarded as a GMO and, depending on their downstream use they should follow the ‘contained use’ or deliberate release’ pathways. Advocacy groups/stakeholders may argue for non-living products of genetic technologies, developed by ‘contained use’ manufacturing processes, to be treated in the same way as GMOs under ‘deliberate release’ and engage in media campaigns against the products of IB. Several companies, large and small have indicated during our engagement that using genetic modification or gene editing to improve the manufacturing capabilities of micro-organisms would make their products more competitive, but that they are refraining from doing this to avoid such an outcome.
Figure 3 shows how the information about the properties of innovative EB products, as they evolve at different TRLs and along a value chain, provided as part of the CID, can contribute to governance-related decisions: building in the principles of proportionality and adaptation to different degrees and at different TRLs, depending on the extent to which the innovation is disruptive, and considering creative roles for standards and guidelines in enabling any required adaptation of regulations or standards.
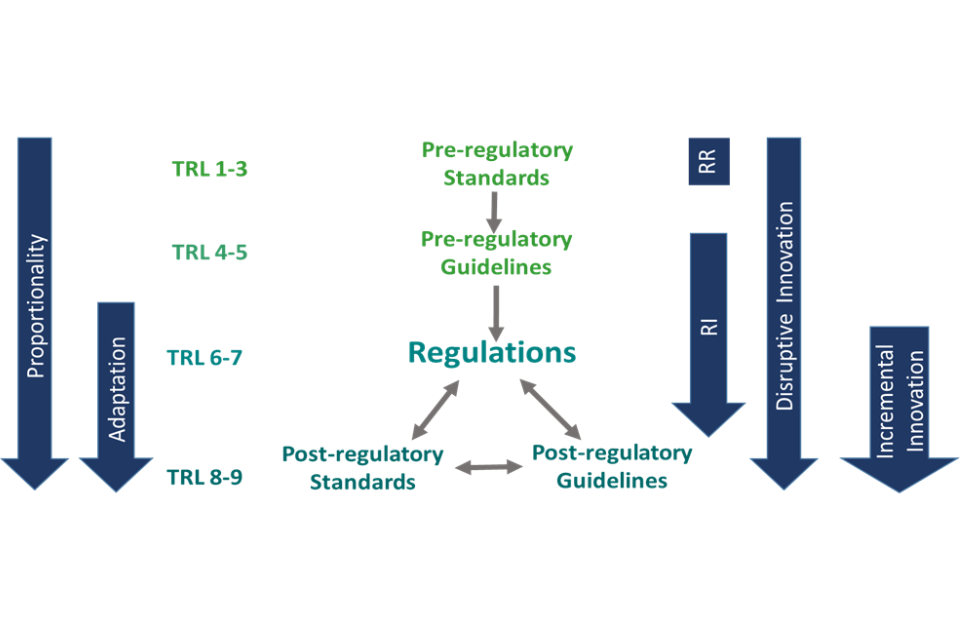
Figure 3, Proportionate and adaptive governance of innovative technologies (PAGIT) Framework.
Recommendation 3.
Regulators, standards bodies and policy makers should work together (via the Engineering Biology Regulators’ Network or another route such as a product/market focused IBRN) to optimise EB governance decisions based on: (i) information provided based on recommendation 2; (ii) the principles of proportionality (to the benefits and hazards of EB products) and adaptation (to innovative governance requirements); and (iii) the creative use of standards and guidelines, in sequence or in parallel with legally-based regulations, depending on the circumstances.
Where innovative EB products will be most disruptive, there are likely to be areas where adaptation of existing regulations or standards will be needed to accommodate the novel properties of the product.[footnote 21] Adaptation choices should build on the knowledge and expectations of regulators and standards bodies, informed by the innovators’ understanding of the properties of the product as it moves along the TRL scale and becomes incorporated into value chains in different sectors with different governance regimes. The PAGIT Framework provides guidance on where, when and how to adapt governance systems to the needs of disruptively innovative technologies, taking the following factors into account.[footnote 22]
1) The location of the product on the TRL scale and in the expected value chain (particularly the sectoral governance regimes likely to be encountered);
2) The use of the TRL scale to develop understanding of the relationships between companies participating in a value chain (based on their business models), and to guide the timing and direction of governance decisions when choosing an appropriate governance precedent for innovative products;
3) The expected properties (potential benefits and hazards) of the product and the level of certainty about these outcomes (depending on TRL stage);
4) How disruptive of current business models the product is likely to be and for which business models in a value chain it will be most disruptive;
5) The roles of current standards and guidelines in governing products in the expected future regime and, where necessary, how they could be adapted to enable innovation to proceed more smoothly, making sure to remain aligned with the positions of international standards bodies for trade-related reasons:
-
Pre-regulatory (behavioural) standards and guidelines can be used to delay until TRL6 making a decision on the need to require legally based regulation, by which time there will be more certainty about the properties of products and their benefits and hazards, potentially enabling continuing governance beyond TRL6 using only standards and guidelines;
-
Post-regulatory standards and guidance can be used to remove a regulatory blockage for a particular class of innovative products, disruptive or incremental, particularly where they have been developed to deliver health or environmental benefits;[footnote 23]
-
Meeting the requirements of a particular standard can be used to provide evidence of equivalence for a product in meeting a regulatory requirement (i.e. replacing a legally based regulation which is hard to adapt with a more readily adaptive standard).
Arguably, the UK’s future prosperity, and its ability to meet climate change and biodiversity-related objectives, will require successful and rapid deployment of innovative products based on EB and IB technologies. These recommendations could make a significant contribution to ensuring that our future governance systems support these objectives and that we are able to develop the products that will enable us to do so on an economically viable timescale.
4. Beyond product-based governance
For any innovative technology platform, like EB, there will be some areas of governance that extend beyond the space defined by products and their properties. In the case of EB this includes biosecurity issues related to the dual use nature of the underlying technologies - genetic modification, synthetic biology, and engineering biology - requiring pre-emptive action at very early stages in the TRL scale. Also, the Nagoya Protocol (NP) to the UN Convention on Biological Diversity (CBD) is an important element of the EB governance system that is intended to enable nations that are curators of biologically diverse regions to have access to, and to benefit from, products and services derived from these genetic resources. However, the NP is proving difficult for companies and countries to implement and is stopping development of potentially useful products. Finally, increasingly, companies and sectors involved in EB will be expected to innovate responsibly and to demonstrate, across all their innovation related activities, that they are doing so.
4.1 Biosecurity issues
Significant attention is being given to bio-security related issues, for example in the US National Academy of Sciences, UK Biosecurity Leadership Council, OECD Global Forum on Technology Expert Focus Group on Synthetic Biology.[footnote 24] The UK Biological Security Strategy refers particularly to biological threats with catastrophic impacts, such as a pandemic, a terrorist attack, or antimicrobial resistance, and it also points to the role of EB in combating these threats.[footnote 25] Within the scope of this report, we focus on the ways in which biosecurity issues are brought to the attention of public stakeholders, along with the governance-related remedies proposed to deal with them, and how they could have long lasting implications for the future viability of industry sectors based on genetic technologies. This is particularly important because, as emphasised in the UK Biological Security Strategy, healthy innovation within the EB-related sectors will be critical to our ability to cope with the outcomes of a bio-threat, whether natural or engineered. Furthermore, the environmental, health, and climate risks of not enabling the adoption of EB products, and continuing to use petrochemicals in the value chain, are significant in themselves. Recommendations 4.1 and 4.2 therefore address the very big question “How can we best enable the beneficial applications of engineering biology and at the same time guard against potential biosecurity-related outcomes?”
Both these recommendations have the potential to impact fundamentally on the future potential of the products of genetic technologies either through their secondary impact on public and stakeholder perceptions or by speeding up or slowing down the pace of innovation.
Recommendation 4.1 - Biosecurity communication-related issues
In addition to planned biosecurity-related communications among those involved in policy making, research and development of EB products, there needs to be a linked, parallel, public-facing strategy and narrative, designed to communicate the background and reasons for biosecurity-related governance to a general, non-specialist audience.
For example, the below could be considered for inclusion in a narrative for a public-facing communication strategy:
-
Lay out the broad range of beneficial products being developed to meet human needs and desires and to meet important policy objectives – food security, better health, Net Zero, improved biodiversity.
-
Note that supporting innovation in these areas will lead to an increase in the range of useful products and to speeding up their delivery, contributing to green growth.
-
Note that government is also developing better, smarter governance processes for the resulting products to ensure safe, affordable and speedy production.
-
Emphasise that the processes of synthetic biology, genetic engineering and gene editing, supplemented by AI, are inherently neutral tools and most of the resulting products will be beneficial and useful. However, there is also a possibility of harmful applications, creating novel organisms that could cause diseases harmful to people, animals or plants.
-
Explain the UK government’s strategy for meeting the biosecurity challenge as part of an international collaboration.
-
Balance the possibility of human-induced threats from products based on new genetic technologies against the more likely scenario that we will experience such challenges from naturally occurring evolution to create new pathogens affecting human, animal or crop health, with potentially devastating consequences. If or when that occurs, new genetic technologies will be the main source of solutions to the problem, enabling the development of the necessary diagnostics and treatments (vaccines and drugs) as happened in the Covid pandemic.
Recommendation 4.2 - Biosecurity governance-related issues
The Biosecurity Leadership Council should consider the need to ensure that the latest government thinking on pro-innovation regulation, as implemented through the Regulatory Innovation Office (RIO), and as embodied in Recommendations 2 and 3, is considered and integrated into future plans for biosecurity governance.
The 2024 ‘UK Screening guidance on synthetic nucleic acids for users and providers’ is an example where this could be helpful, and the UK’s ‘National Quality Infrastructure’ could be an important component of the delivery mechanism.[footnote 26]
4.2 Nagoya Protocol (NP)
In our discussions with stakeholders, one regulatory instrument stood out as presenting problems for the majority of those we interviewed, the Nagoya Protocol (NP), which provides a legal framework for access and benefit sharing from the use of genetic resources in a fair and equitable way.[footnote 27] Put simply, it aims to ensure that where genetic resources have been taken from a provider country by researchers or businesses, the country of origin receives a share of any benefits arising from their use. Access to genetic resources is essential to innovation in engineering biology, and having well-functioning mechanisms in place that enable benefit sharing without hindering science, research and innovation is essential. However, 20th Century regulatory systems (including the Nagoya Protocol) are proving very difficult to adapt to the needs of existing and new genetic technologies, starting at the very earliest research stages and continuing through to manufacturing and scale-up.
Stakeholders highlighted a general cooling effect on activities that fall under the Protocol with researchers and companies undertaking research appearing to be avoiding projects that are likely to fall within its scope.[footnote 28] The Department for Environment, Food & Rural Affairs (Defra) is responsible for the UK’s policy and implementation in relation to the Nagoya Protocol, with the Office for Product Safety and Standards (OPSS) being responsible for monitoring and enforcement through the Nagoya Protocol (Compliance) Regulations 2015.[footnote 29] Some stakeholders shared a view that OPSS’s early implementation of the NP was perceived to have been heavy handed.
Given that the UK’s implementation of the Protocol has been set out in assimilated EU Law, it was called to our attention that the UK now has latitude to update the guidance around the protocol to give more certainty to innovators and companies that they are complying with national and international requirements.
A particular case exemplifying issues with the Protocol, involving a PhD candidate at the John Innes Centre, was highlighted to us. Researchers wanted to access resources from the Meliaceae family of tropical trees to investigate the limonoid biosynthetic pathways specific to these trees and useful in insect pest control. The researchers sought a species within the scope of the NP and were able to leverage an existing relationship with partners at a Vietnamese university via a Royal Society International Partnership Award. The NP requires two documents to be negotiated between providers and users: Mutually Agreed Terms (MAT) and Prior Informed Consent (PIC), which outline the benefits being shared and proposed use of materials and permission to sample them. However, the process of acquiring these agreements broke down after three years of negotiations. The researcher noted that factors such as language barriers, legal ambiguities, lack of awareness, and institutional hurdles, as well as the newness of the Protocol, all played a part. Resultantly, the project accessed an alternative, less optimal species and the material was procured according to EU rules whereby material can be sourced from countries that do not exercise their sovereign rights over genetic resources or where the material was present in that country before the Protocol came into force.
Another issue under the umbrella of Access and Benefit sharing is that of Digital Sequence Information (DSI) on genetic resources. DSI is taken up under the Convention on Biological Diversity, to which the Protocol is a supplementary agreement. Governance of Digital Sequence Information (DSI) has been a particularly contentious issue over the past ten years. There is still no agreed definition of the term, but it is generally taken to cover plant and animal information that has been digitally interpreted and uploaded to an online database. Unlike the tangible nature of material previously covered by the NP, DSI concerns digital data about the genetic (nucleic acid and protein sequence) makeup of organisms. Where a gene sequence has been designed de novo (rather than copied from an organism’s genome), there may be no basis for knowing which species in the wild, if any, have those sequences in their genomes. Therefore, issues with DSI may become more pertinent with the development of generative AI tools and other forms of computational biology that open the design space for engineering biology.
New modalities have now been agreed at the COP 16 meeting of the UN Convention on Biological Diversity, held at Cali in Colombia in November 2024. The decision on DSI and the associated Cali Fund, intended to enable benefit sharing for DSI, pave the way for an industry-supported fund that might be able to circumvent some of the points of disagreement from the past, e.g. getting agreement from a number of different countries on the sharing of the benefits, and providing clarity around approaches to benefit disbursement for users of DSI.[footnote 2]
It will be vital to ensure that implementation of both the NP and new DSI benefit sharing mechanism reflect the needs of the sector, and for the UK to continue to play an active role in shaping and reviewing international agreements. This is key to ensuring that access and benefit sharing can be as impactful as possible internationally without hindering science, research and innovation in the UK.
Recommendation 5:
In the context of the Convention on Biological Diversity (CBD) Nagoya Protocol and the new DSI multilateral benefit sharing mechanism, including the Cali Fund, ensure that the implementation of Access and Benefit Sharing Agreements aligns with the needs of the sector. The Regulatory Horizons Council can work closely with Defra and the Department for Business and Trade to support the design and delivery of industry engagement over the first quarter of 2025. This will be to ensure, as far as possible, that the implementation of the Nagoya Protocol and the DSI benefit sharing mechanism is compatible with, and supports, the overall governance approach recommended in this report.
4.3 Responsible Innovation
Companies are increasingly being required to demonstrate that they are behaving responsibly, from two different perspectives.
i) All companies are expected to comply with corporate social responsibility standards relevant to general company behaviour involving: accountability for impacts on society, the environment and the economy; transparency in decisions that impact on society and the environment; ethical behaviour; respect for stakeholder interests; respect for the rule of law; respect for international norms of behaviour; and respect for human rights.[footnote 30]
ii) Additional responsibility-related requirements will apply to companies involved in innovation, particularly where products are expected to be disruptive of company business models or markets. These requirements will vary depending on the nature of the product and will be expected to include: societal, environmental and health-related elements, both benefits and risks; regulatory elements; and value chain-related elements such as RI behaviour by other significant actors and trading partners. [footnote 31]
In both of the above situations there will be a need for companies to demonstrate Responsible Innovation, for example according to the requirements of British Standards Intuition (BSI) PAS 440 which provides companies with a framework by which to balance the potential benefits and harms of an innovative development and, where necessary, to take action to maximise the benefits and/or minimise the harms.[footnote 32] It also links RI to meeting the requirements of net zero government policies and the UN’s Sustainable Development Goals. Indeed, the Nuffield Council report on Biofuels: ethical issues recommends that, where products have the potential to contribute to such objectives, companies should be encouraged to innovate, as long as the investment will be financially attractive.[footnote 33]
Recommendation 6 – Demonstrating responsible Innovation
Across all sectors of the economy, including IB, as part of the implementation of a pro-innovation governance approach, companies should be encouraged to undertake a formal commitment to responsible innovation.
Responsible Innovation, e.g. according to the requirements of BSI PAS 440,[footnote 31] provides companies with a framework by which to balance the potential benefits and harms of an innovative development and, if necessary, to take action to maximise the benefits and/or minimise the harms. It can have the following benefits for companies:
-
long-term cost and risk reductions;
-
more resilient new product/service offerings to potential customers;
-
improvement of societal trust in the company and maintenance of social license to operate;
-
improved relations with investors and greater investor confidence in the company;
-
greater attractiveness as an employer;
-
better supply chain relationships;
-
improved reputation and brand value;
-
increased innovation capabilities;
-
improved ability to communicate the value of products and services to investors, companies, customers and citizens;
-
better relationships with governments, regulators and local communities; and
-
improved capacity for long term planning and sustainability.
5. Conclusions and future opportunities
Following receipt of this commission and undertaking preparatory stakeholder consultation, the picture that emerged was one of very significant innovation potential across a broad range of sectors, in many cases with governance related factors inhibiting future development of products for an equally broad range of reasons. Developing an EB report based on the individual cases brought to our attention in the consultation would have left large numbers of issues unaddressed and would not have significantly advanced our national capability to deliver proportionate and adaptive governance of EB products as a whole. Also, given our recent experience in writing the report on Regulating Quantum Technology Applications, we were beginning to see commonalities across the governance requirements for products emerging from these disruptive super-platforms.[footnote 13] We concluded that the interests of pro-innovation governance of innovative technologies in the UK would be best served by formalising a framework to act as a basis for future governance of EB products, contributing to early recognition of opportunities and problems likely to emerge during the course of development, and advising decision makers on how to respond to them.
The approach proposed here is intended to lead to better governance of all products of engineering biology by outlining a framework that helps decision makers to understand what factors to pay attention to and how these factors will interact with one another, either to support or to block particular courses of action. We suggest that it could also be considered as a basis for the future governance of other disruptive super-platforms such as AI, quantum technologies and robotics. The purpose of the PAGIT approach and the other approaches recommended here is to make the overall governance system more agile and adaptive, thereby reducing the time and cost of meeting governance obligations by companies and coincidentally also for regulators and standards bodies.
There is an increasing appreciation, among innovators and across society as a whole, of the extent to which we will need to rely on innovative technologies to contribute to meeting national and international policy goals related to climate change and biodiversity loss. Given the urgency of these issues, ensuring proportionate and adaptive governance approaches that can optimise the delivery time, particularly for the disruptive/transformative innovations that will potentially have the greatest impact, the approach proposed here could improve our ability to meet our international commitments on these issues.
Annex
Annex A: Background research and stakeholder consultation
We undertook comprehensive desk research in the first stages of this report. Seeking to minimise duplication, we drew from previous efforts looking at aspects of EB by the Council for Science and Technology;[footnote 11] the Government Chief Science Advisor (GCSA),[footnote 34] the Engineering Biology Leadership Council,[footnote 35] and DSIT’s Call for Evidence on engineering biology. We also excluded agricultural and medical applications from scope on the basis that the former has been examined in the RHC’s report on Genetic Technologies,[footnote 8] and the latter is subject to distinctive regulatory regimes under the Medicines and Healthcare products Regulatory Agency (MHRA).
To gather insights from stakeholders, we undertook 10 formal interviews, several informal conversations and held a workshop with regulators and innovators. The workshop covered areas such as, keeping pace with innovation in the sector and systems considerations for engineering biology governance.
The varied views gained at this early stage gave an insight into the complexity of the governance environment, given the broad range of sectors and the variety of properties inherent in different product ranges. Rather than dealing one-by-one with issues raised by stakeholders, we chose to use our analysis to consider how governance systems can be adapted to deal with the complexity presented by disruptive super-platforms (or even hyper-platforms) like EB.
Below we outline key themes raised by stakeholders. Quotes from stakeholders have not been validated by regulators. They illustrate how regulation is perceived by the interviewee.
Engagement with regulators and regulatory pathways
Several stakeholders highlighted positive interactions with regulatory bodies despite challenges. However, they expounded the need for better collaboration between industry and regulators, and believe regulators need to engage innovators at an earlier stage to understand what is being developed. Challenges with regulators rose up around the impact of regulatory processes on time and resources, particularly for startups. One made the point that, ‘‘companies spend two years trying to get a product on the market, and then the answer is no. So that’s why they’re all going to Singapore.”
The lack of clear regulatory pathways was also raised multiples times, an issue that was found to be compounded by what was regarded as need for better coordination between government departments involved with regulation and regulators. Multiple pathways could also result in a variability of regulatory requirements based on product claims. One stakeholder critiqued the case-by-case regulatory approach, advocating for a more streamlined process based on product safety. It was noted on several occasions that it was easier to look internationally in the first place. In areas such as plant-based gene editing, it was easier to get regulatory approval in the United States and that the US Food and Drug Administration were prepared to accept newer technologies when assessing the safety questions. Several comments referred to companies that had produced innovations in the UK but simply chose to enter the US market.
Market shaping
The role of regulation to help shape markets to transition to bio-based products was also raised with us. Companies may need to be incentivised to move to bio-based production over the longer term. Regulation was not seen as the only driver to achieving this and government procurement was another route by which EB products could be pulled through, along with subsidies or taxes. For example, there was discussion on whether bio-based materials should fall under plastic tax regulations, and the incumbent advantage this gives petrochemical-based products.
Public engagement
As part of this report, we held a workshop with innovators and regulators where participants emphasised the use of learnings from other emerging technologies to better understand potential public perceptions of future engineering biology developments. They highlighted the importance of understanding both risks and benefit perceptions, the role of trust in organisations and institutions, and the need to understand the views of various ‘publics’. Interviewees also stressed the importance of engaging the public and educating them about the benefits and safety of engineering biology to gain their support and trust, noting that “being bold and transparent about the benefits and motivations behind engineering biology is essential for public acceptance.” Without this, the public could react negatively to the development of these technologies, particularly as EB consumer products come on to the market. A proactive approach to this should cover multiple levels of engagement and be honest in outlining the benefits and risks of the technology as one of the possible solutions to policy problems.
“Old rules”
Several interviewees underscored ways in which current rules and definitions didn’t fit the state of technological development. One interview spoke of company which produced an organism produced through EB with edited genes and faced delays due to the process used. The team ultimately used a selective assay approach instead and resulted in a product with a nearly identical genome sequence, yet a year was added to the process. What were resultantly two extremely similar organisms were classified entirely differently under current regulations. In some instances, direct edits to genes could be more precise than through older methods, but the treatment of innovative techniques is based on with rules that “made sense in the 80s or 90s where there was a couple of methods” used for such purposes. Additionally, issues around definitions were raised during our workshop around engineering biology processes such as ‘bioengineering’, ‘genetic engineering’ and whether it matters exactly what technique was used. It was suggested that demonstrating product safety could be achieved by considering its equivalence to existing natural/conventionally produced products. However, it was acknowledged that this raises further questions around determining thresholds for equivalence, and that ‘natural’ does not necessarily equate to ‘safe’. A challenge was raised about ensuring that regulators take a consistently proportionate approach to regulating products of different categories. Several stakeholders noted the potential use of databases and tools such as AI to define safe organisms.
Deliberate release and contained use
Challenges were also raised around regulatory definitions such as contained use versus deliberate release. It was discussed that there remain uncertainties in the definition of contained use, and how it may apply to innovative new products such as biosensors. The need for clarity on aspects such as what are acceptable ‘premises’ (as there is a requirement to register premises for contained use manufacture) was also raised. During our workshop, attendees raised questions around how containment can be appropriately measured and demonstrated, and it was proposed that the UK system should consider multiple mechanisms of ensuring ‘containment’, which could be biological, such as kill switches, in alignment with regulatory thinking in other jurisdictions.
International alignment
The challenges of aligning UK regulations with international standards, were highlighted, particularly when it came to possible contamination of the supply chains with Europe. During our workshop, it was emphasised that we should support the global adoption of engineering biology by fostering international harmonisation of regulations. Considering standards, one interviewee mentioned the recent work coordinated by Imperial College London, the Engineering Biology Research Consortium, the National Institute of Standards and Technology, and the National University of Singapore around EB metrics and standards.[footnote 36]
Annex B: Summary note of the RHC workshop: Exploring the Future of Engineering Biology Regulation
On the 22nd of January 2024, the Regulatory Horizons Council (RHC) hosted a half-day workshop in partnership with the National Physical Laboratory (NPL), convening 30 stakeholders from regulators, government departments, standards and measurement bodies, and a select number of innovators. Nine different regulators and departments with engineering biology responsibilities were represented at the session.
The primary purpose of the workshop was to gather insights and expertise to inform the RHC’s independent review of engineering biology regulation, but the session also provided an opportunity for regulators to connect in-person and facilitated open dialogue on potential regulatory issues with innovators operating at the cutting edge of the field.
The key topics and questions addressed by the workshop were:
-
Which technological developments in the engineering biology sector will have the greatest regulatory implications in the next 5 years, and further beyond?
-
What are the most pressing regulatory challenges to resolve, and why?
-
How can the UK move towards a regulatory approach which is centred on the end products of engineering biology, as opposed to the particular technologies used to generate them? What could be the relative benefits and challenges of such an approach?
-
How can regulators best understand and respond to public perceptions of engineering biology applications, and what can we learn from other emerging technologies?
These questions were addressed through a combination of group discussions, breakout activities, and a survey sent in advance of the workshop. Whilst the focus of the workshop was on hearing the views of regulators, a small number of innovators and academics were invited to showcase cutting edge technological innovation and spark discussions.
1. Keeping Pace with Innovation
Given the RHC’s role in horizon scanning for emerging technological trends and their potential regulatory implications, the issue of keeping pace with innovation was a core part of the evidence gathering for the workshop.
In advance of the session, regulators shared their views on areas of technological development that are likely to have regulatory implications. Topics raised included: cell-cultivated food; the use of AI in engineering biology processes; the decreasing cost and miniaturisation of DNA sequencing equipment and increased computing speed; and the use of genetically modified organisms (GMOs). It was also noted that the field is progressing so rapidly that it can be difficult to predict and keep pace with new developments and how they may sit within current regulatory frameworks.
James Flewellen, Researcher at the University of Edinburgh, and John Waite, CEO of Phycobloom gave presentations on technological trends within their sectors and potential regulatory implications.
Specific issues that were discussed in the presentations and follow-up discussion include:
The need for clarification on definitions
It was raised that there is a need for better categorisation of biological products to reflect the variety of biological material that may be used or developed by the sector. For example, algae do not fall under any of the categories for the Animal and Plant Health Agency (APHA) import regulations.
Another area where challenges were raised around regulatory definitions was that of contained use versus deliberate release. It was discussed that there remain uncertainties in the definition of contained use, and how it may apply to innovative new products such as biosensors. The need for clarity on aspects such as what are acceptable ‘premises’ (as there is a requirement to register premises for contained use regulation) was raised as part of this. Attendees also raised questions around how containment can be appropriately measured and demonstrated, and it was raised that the UK system should consider multiple mechanisms of ensuring ‘containment’, which could be biological, such as kill switches, in alignment with regulatory thinking in other jurisdictions. HSE clarified that current contained-use regulations already allow for containment barriers which can be physical, chemical, biological, or a combination.
A wider concern was flagged during the presentation around definitions of engineering biology processes such as ‘bioengineering’, ‘genetic engineering’ and whether it matters exactly what technique was used. This issue was discussed in greater depth during the later part of the session looking at product- and process-based approaches to regulation.
Circular economy considerations for engineering biology products
It was also raised that there is a need for future thinking about the economic model for engineering biology feedstocks, as many feedstocks rely on waste products which are no longer ‘waste’ when they are in use.
In the field of biosensing, questions were raised around end-of-life considerations for these products. Options to separate out biological and electrical components were proposed as a way to facilitate re-use.
Challenges around the use of Artificial Intelligence (AI) in engineering biology processes
It was raised that there is a need to scale up ideas from AI, and robotics can provide a useful testing mechanism. The Centre for Process Innovation was cited as a helpful resource.
There were broad concerns about the availability of high-quality datasets for the use of AI in engineering biology, and it was suggested that data availability is currently limiting AI from delivering on its potential.
There was also a question around potential Nagoya protocol ramifications of generating novel proteins with AI that may be present in nature, but it was suggested that a larger challenge at the moment is that of intellectual property and assigning rights.
The need for strong ecosystem-level safety assurances
Phycobloom discussed the importance of developing strong safety standards within the industry, and how engineering biology can learn from good practice in the aviation industry.
International harmonisation as an enabler to innovation
Differences in attitudes between the UK and US were briefly raised. It was emphasised that we should support the global adoption of engineering biology technologies through fostering international harmonisation of regulations, but we may not want to exactly imitate certain jurisdictions’ approaches.
This was an area where it was recognised that regulation can act as a positive enabler to innovation.
Non-regulatory challenges
It was noted that there are a number of non-regulatory issues which impact on the ability to scale engineering biology innovation in the UK, these included scaleup/fermentation support and access to talent.
2. Exploring product- and process-based approaches to regulating engineering biology
The RHC previously recommended in its report on genetic technologies within the agri-food sector that: “regulatory scrutiny should focus on the product to be placed on the market and the balance between its risks and benefits, rather than the technology used to produce it.”
With the innovative possibilities brought by the development of engineering biology and its applicability to a wide range of sectors, this session’s aim was to explore the relative merits of product- and process-based regulation for three selected classes of products: 1) products used for food or feed; 2) products used for non-food/feed and 3) products that contain living cells.
Several key issues and themes were discussed:
End-use considerations
Participants discussed the importance of having a full understanding of the product’s end use, including who is using it and in what ways. Examples were given of products that may require additional considerations due to their potential use by young children.
For certain applications, including construction, regulators raised the need for greater understanding of how a product might perform over time to be taken into account in regulatory decision-making. They also noted a role for regulation and/or standards in addressing questions on the monitoring and maintenance of eng-bio products, particularly where they contain living cells. It may be possible to adapt or extend an existing system to address this; whilst the technology is novel, the need for regulators to take account of the expected qualities of a product over its lifetime is not.
Regulator capability and capacity
Capability of scientists and engineers within regulators was raised as a broad challenge. It was suggested that there is an opportunity for greater sharing of expertise and capability between regulators, which is currently undertaken informally or through expensive consultancy models.
Regulators tended to agree that engagement with industry was valuable from a strategic perspective, in enabling them to understand their own future pipeline of work. However, they noted that engagement can be resource intensive and can take teams away from the day-to-day work of licencing and permissioning decisions (where a regulator operates on this basis). Therefore, it is important that regulators are able to maximise the usefulness of time spent engaging with industry.
Importance of understanding the production process to inform knowledge of the product
It was discussed that to have a full understanding of the product, it is often important to understand the process. A product-based regulatory approach should also be open to considering any specific hazards that might arise from the production process. For example, in the food sector the production process may lead to potential impurities in the product.
Equivalence and defining product ‘naturalness’
It was suggested that an important way of demonstrating product safety could be demonstrating a product’s equivalence to products already approved by the current governance system. However, this still leads to questions around determining thresholds for equivalence, and equating ‘natural’ with ‘safe’.
It was suggested that machine learning and AI could be a useful tool for determining product equivalence, and safety more broadly, but availability of data sources was cited as a limiting factor .
Regulatory consistency
A challenge was raised about ensuring that regulators take a consistently proportionate approach to regulating products of different categories. An example was raised of needing to ensure that regulations for human food are not perceived to be more lenient than that of pet feed.
3. Understanding public perceptions of engineering biology applications
Professor Nicholas Pidgeon presented on the use of learnings from other emerging technologies to better understand potential public perceptions of future engineering biology developments. Key topics raised included the importance of understanding both risk and benefit perceptions, the role of trust in organisations and institutions, and the need to understand the views of various ‘publics’. Professor Pidgeon cautioned against assuming that public perceptions of engineering biology applications will be analogous to those towards GMOs, which is an issue that was also raised in the recent Sciencewise report on public perceptions of engineering biology use in healthcare.[footnote 37]
Attendees reflected on the role of science communication, and the need to re-think approaches to delivering information in light of new forms of media. It was also suggested that science communication has typically been more centred towards early TRLs, and it will be important to communicate the benefits of technologies at the commercialisation stage.
A key theme of discussion was the need for a ‘mission centred’ approach to communicating the benefits of engineering biology. It was acknowledged that there have been several global changes since GMOs were first introduced, and the public may be more aware of the need to adopt technological solutions. That said, caution was raised around making grandiose claims that may not be realised.
-
The Bio Revolution: Innovations transforming economies, societies, and our lives, McKinsey Global Institute, (2020) ↩ ↩2
-
For more information on the Cali Fund, see: Taylor, L. (2024). Big win: global plan to pay for wildlife conservation emerges. Nature, 635, 264-5. ↩ ↩2
-
We have previously produced reports on the regulation of fusion energy; medical devices; genetic technologies (crops and farmed animals); drones; AI as a medical device; neurotechnology; hydrogen as a fuel for marine transport; the role of regulation in supporting startups and scaleups; quantum technologies; robotics and autonomous systems in agriculture; space; and ‘Closing the Gap’ between principles and practices for innovative regulation. ↩
-
For example, see: Heldt, D., Driving innovation in Industrial Biotechnology and Engineering Biology. ↩
-
Gallup, O., Ming, H. and Ellis, T., 2021. Ten future challenges for synthetic biology. Engineering Biology, 5(3), pp.51-59. ↩
-
Grue, B., What’s in a Name? Defining and Relating Synthetic Biology to Biomedical Engineering (2019). ↩
-
The phrase great technology dates from the BEIS Eight Great Technologies initiative: David Willetts (2023) ‘The Eight Great Technologies 10 years on’ Policy Exchange. ISBN: 978-1-910812. ↩
-
Regulatory Horizons Council, Report on Genetic Technologies (2021). ↩ ↩2 ↩3
-
Capgemini Research Institute, Unlocking the potential of engineering biology: the time is now (2024). ↩
-
Department for Science Innovation and Technology (DSIT), National vision for engineering biology (2023). ↩
-
Council for Science and Technology, Report on engineering biology: opportunities for the UK economy and national goals, (2023). ↩ ↩2
-
Symons, J., Engineering biology and climate change mitigation: Policy consideration, (2024). ↩
-
Regulatory Horizons Council, Regulating Quantum Technology Applications (2024). ↩ ↩2
-
Tait, J., Banda, G. and Watkins, A. (2017) Proportionate and Adaptive Governance of Innovative Technologies. Case Study: Responsible Governance of Innovative Technologies. Final Report. Innogen Institute Report to the British Standards Institution. ↩
-
Dederer, H-G and Hamburger, D. (2021) Are genome-edited micro-organisms covered by Directive 2009/41/EC? Journal of Law and the Biosciences, 1–27. ↩
-
EARTO (European Association of Research and Technology Organisations) (2014) The TRL scale as a research and innovating policy tool, EARTO recommendations. 30 April 2014. ↩
-
Disruptive (or transformative) innovation is based on new areas of research and development and leads to the creation of new modes of production and new markets, involving sectoral transformations and the displacement of incumbent companies, often with significant societal and economic benefits. There may be no existing business model on which companies can build, and a need to create new value chains, or new product roles in existing value chains, and there may be no obvious precedent for its governance. Incremental innovation fits well with current business models, generating competitive advantage and contributing to the economy, for example through more efficient use of resources. It does not lead to sectoral transformations and is likely to fit within a clear pre-existing regulatory framework. ↩
-
Monica Hoyos Flight, et al. (2024). Analysing Responsible Innovation along a value chain – a single-cell protein case study. Engineering Biology. Image licensed under CC BY 4.0. ↩
-
Tait, J and Wield, D. (2019) Policy Support for Disruptive Innovation in the Life Sciences. Technology Analysis and Strategic Management, 33:3, 307-319. ↩
-
While memoranda of understanding do exist between regulators in relation to specific issues the RHC believes the overall level of disconnect within the system remains unhelpful. ↩
-
Sundaram LS, Ajioka JW, Molloy JC. Synthetic biology regulation in Europe: containment, release and beyond. Synth Biol (Oxf). 2023 Apr 20;8(1):ysad009. ↩ ↩2
-
Tait, J. (2024) The PAGIT Framework: its role in the governance of UK technologies to drive greater innovation. British Standards Institution White Paper. ↩
-
Mittra, J., Bruce, A., Scannell, J.W. and Tait, J. (2019) Regulatory and market influences on innovation pathways for the development of new antimicrobial drugs. Technology Analysis and Strategic Management. ↩
-
For example, see: the UK’s Biosecurity Leadership Council; and Strategies for Identifying and Addressing Biodefense Vulnerabilities Posed by Synthetic Biology, US National Academy of Science ↩
-
DSIT, UK screening guidance on synthetic nucleic acids for users and providers (2024). ↩
-
The Nagoya Protocol on Access and Benefit-Sharing is a supplementary agreement to the Convention on Biological Diversity. For more information, see: The Nagoya Protocol on Access and Benefit-sharing, Convention on Biological Diversity. ↩
-
This may explain why OPSS and Defra’s post implementation review (2022) identified unusually high levels of compliance (at 100%). For information, see: The Nagoya Protocol Compliance Regulations Post Implementation Review ↩
-
British Standards Institution, Responsible Innovation – Guide, PAS 440 (2020). ↩ ↩2
-
Mittra, J., Bruce, A., Scannell, J.W. and Tait, J. (2019). Regulatory and market influences on innovation pathways for the development of new antimicrobial drugs. Technology Analysis and Strategic Management. ↩
-
Nuffield Council on Bioethics (2011). Biofuels: ethical issues, pp 77-79. ↩
-
Mclean, A., Pro-innovation Regulation of Technologies Review: Life Sciences (2023). ↩
-
Engineering Biology Research Consortium, Engineering Biology Metrics and Technical Standards for the Global Bioeconomy, (2024). ↩
-
Sciencewise, Public perceptions of engineering biology (2024). ↩
