NERVTAG: Immunity certification update, 4 February 2021
Published 5 July 2021
Summary
1. Natural infection provides protection against reinfection (any PCR positive) for around 7 out of 10 people for a period of at least 5 to 6 months (see figure). [footnote 1]
2. Immunisation with a single standard dose of the AstraZeneca vaccine provides protection against infection (any PCR positive) for around 7 out of 10 people for at least 90 days. Data on any PCR positive infection (symptomatic or asymptomatic) after 1 dose are not available for other vaccines. [footnote 2]
3. Both natural infection and vaccination provide a high level (at least 77%) of protection against symptomatic and severe disease.
4. For both natural infection and vaccination, there is an interval of around 2 weeks after exposure before immunity is established.
5. All quoted estimates of protection are against viruses that are similar to the one causing the primary infection and against viruses with similar spike antigen as are included in the vaccines. Protection could be lower for antigenically different virus variants.
6. The prevalence of infection in the community will have an important impact on the effectiveness of certification.
7. For illustrative purposes only, with current levels of infection (about 325 per 100,000 per day), then in a population of 100,000 people with an immune certificate then as many as 100 (325 * 0.31 (see figure)) could be PCR positive, of which an estimated 80 would be asymptomatic and 20 symptomatic. Whereas, with levels of prevalence seen in the summer (about 3 per 100,000), then in a population of 100,000 people with an immune certificate less than 1 would actually be PCR positive.
8. Cases of asymptomatic (re)infection occurring in people with natural or vaccine derived immunity may be less infectious if they have lower viral loads than immunologically naïve infected but asymptomatic people comparison of PCR cycle threshold values in asymptomatic reinfection compared to asymptomatic primary infection are needed to assess if this is the case. [footnote 3] , [footnote 4]
9. The practicalities of certifying immunity are challenging. Immune correlates of protection are not yet established. Studies of reinfection have used anti-spike or anti-nucleocapsid IgG assays to define seropositive or seronegative status. In practice, such assays are not currently available at the scale needed for widespread immunity certification. [footnote 5]
10. Duration of immunity is not well characterised beyond 6 months after natural infection or beyond 3 months following vaccination, so the duration of any certification is also a challenge.
Implications
11. Immunity certification could be used as an adjunct to other measures to control transmission and or to enable relaxation of certain measures, but it must be recognised that it is an imperfect tool and a risk-based approach should be adopted.
12. The reliability of any immunity certificate will be reduced if virus variants with significant antigen escape are circulating.
13. Since protection against (re)infection is not 100%, immunity certification should not permit the relaxation or avoidance of self-isolation and testing if symptoms develop.
14. Since natural infection and immunisation provide a high level of (albeit not 100%) protection against severe disease, immunity certification could be used to ease restrictions that are intended to protect the individual themselves (not others).
15. When levels of infection in the community are high and/or the consequences of onwards transmission are high (such as in care homes), a combination of immunity certification and infection testing may be preferable to either in measure in isolation.
16. When levels of infection in the community are low, immunity certification may be preferable to antigen-based screening, which will have a low yield and high false positive rate.
17. Whether immunity certification alone would perform better, equally well, or worse than other approaches such as Lateral Flow Device (LFD) screening alone cannot be ascertained as the effectiveness of other approaches in reducing transmission is not known.
18. The performance and practicality of immunity certificates should be formally evaluated.
Background
19. Sterilising immunity means that a person is protected against both infection and illness. Therefore, as well as being themselves protected from illness they cannot be a source of infection for others.
20. Non-sterilising immunity means that a person can still get infected but not become ill. Therefore, although themselves protected from illness, they may still be able to become infected, shed virus and be a source of infection for others.
21. This paper does not address behavioural, ethical, legal, or operational issues related to immune certification. Immune responses following natural SARS-CoV-2 Infection
22. One to 2 weeks following documented SARS-CoV-2 infection, more than 90% of people, including the elderly, have SARS-CoV-2 antibodies detectable in their serum. [footnote 6], [footnote 1], [footnote 7], [footnote 8], [footnote 9]
23. Antibody levels tend to be higher in people who have suffered more severe disease, but antibodies do develop following asymptomatic SARS-CoV-2 infection or mild COVID-19. This is similar to MERS-coronavirus, where severity of infection is linked to antibody longevity. [footnote 10], [footnote 11]
24. Antibodies begin to appear within 5 to 6 days of symptoms and are detectable for at least 6 months and probably 8 months or more. [footnote 1], [footnote 7], [footnote 11], [footnote 12]
25. Following documented SARS-CoV-2 infection, cell mediated immunity also develops and is detectable for at least 6 months. [footnote 13], [footnote 14]
26. Antibody waning following infection with seasonal coronaviruses can result in reinfections. Reinfections with seasonal coronaviruses might also be due to some antigenic evolution. [footnote 15], [footnote 16], [footnote 17]
Animal studies on SARS-CoV-2 immunity
27. In animal models, the presence of neutralising antibodies as a result of prior infection is associated with protection from disease and infection (sterilising immunity). [footnote 18], [footnote 19], [footnote 20]
28. Studies in hamsters and macaques indicate that passively administered antibodies are sufficient to suppress viral replication in both the upper and lower respiratory tract. [footnote 21],[footnote 22]
29. Depletion of CD8 T cells shows that cellular immunity can contribute to protection against SARS-CoV-2 re-challenge in convalescent macaques with waning antibody titres. [footnote 22]
Observational data on immunity following natural SARS-CoV-2 infection
30. A meta-analysis of studies with more than 3-month follow-up following an antibody test and regular antigen testing, investigated the predictive value of baseline antibodies in preventing subsequent development of PCR confirmed nasal infection. Where possible, analyses were stratified by whether the infection was symptomatic (with typical COVID symptoms), asymptomatic, or with symptoms that do not meet the COVID-19 case definition (of cough, fever or loss of or altered sense of smell or taste) and all infections combined.
31. The following studies were included:
- a PHE study of infection rates in one nursing home (PHE Nursing home study) [footnote 23]
- a study of Healthcare workers in Oxford (Oxford HCW) [footnote 5]
- the national SIREN study of Health Care Workers (SIREN) [footnote 24]
- a study of residents and staff in a national chain of nursing homes (VIVALDI) [footnote 25]
- a cohort study of Scottish Health and Social Care workers (Scottish HCW) [footnote 26]
32. The figure below shows the forest plot for the Relative Risks derived from these studies. These relative risks assume equivalent person follow up time in those with and without baseline antibodies.
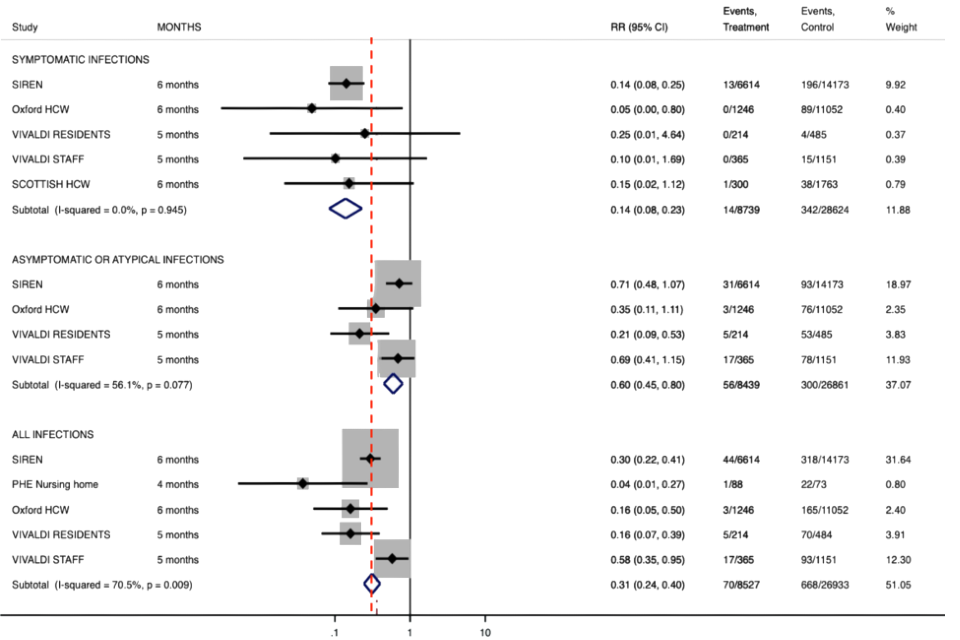
Symptomatic infections
| Study | Months | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|---|
| SIREN | 6 | 0.14 (0.08, 0.25) | 13/6614 | 196/14173 | 9.92 |
| Oxford HCW | 6 | 0.05 (0.00, 0.80) | 0/1246 | 89/11052 | 0.40 |
| VIVALDI RESIDENTS | 5 | 0.25 (0.01, 4.64) | 0/214 | 4/485 | 0.37 |
| VIVALDI STAFF | 5 | 0.10 (0.01, 1.69) | 0/365 | 15/1151 | 0.39 |
| SCOTTISH HCW | 6 | 0.15 (0.02, 1.12) | 1/300 | 38/1763 | 0.79 |
| Subtotal (I-squared = 0.0%, p = 0.945) | 0.14 (0.08, 0.23) | 14/8739 | 342/28624 | 11.88 |
Asymptomatic or atypical infections
| Study | Months | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|---|
| SIREN | 6 | 0.71 (0.48, 1.07) | 31/6614 | 93/14173 | 18.97 |
| Oxford HCW | 6 | 0.35 (0.11, 1.11) | 3/1246 | 76/11052 | 2.35 |
| VIVALDI RESIDENTS | 5 | 0.21 (0.09, 0.53) | 5/214 | 53/485 | 3.83 |
| VIVALDI STAFF | 5 | 0.69 (0.41, 1.15) | 17/365 | 78/1151 | 11.93 |
| Subtotal (I-squared = 56.1%, p = 0.077) | 0.60 (0.45, 0.80) | 56/8439 | 300/26861 | 37.07 |
All infections
| Study | Months | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|---|
| SIREN | 6 | 0.30 (0.22, 0.41) | 44/6614 | 318/14173 | 31.64 |
| PHE Nursing home | 4 | 0.04 (0.01, 0.27) | 1/88 | 22/73 | 0.80 |
| Oxford HCW | 6 | 0.16 (0.05, 0.50) | 3/1246 | 165/11052 | 2.40 |
| VIVALDI RESIDENTS | 5 | 0.16 (0.07, 0.39) | 5/214 | 70/484 | 3.91 |
| VIVALDI STAFF | 5 | 0.58 (0.35, 0.95) | 17/365 | 93/1151 | 12.30 |
| Subtotal (I-squared = 70.5%, p = 0.009) | 0.31 (0.24, 0.40) | 70/8527 | 668/26933 | 51.05 |
33. Protective effectiveness of baseline antibodies against infection was calculated as 1-RR*100.
- the pooled estimate of the protective effectiveness against symptomatic PCR confirmed infection was 86% (95% CI 77% to 92%)
- the pooled estimate of the protective effectiveness against asymptomatic or atypical PCR confirmed infections was 40% (95% CI 20% to 55%)
- the pooled estimate of protective effectiveness against all PCR confirmed infections (regardless of symptoms) was 69% (95% CI 60% to 76%)
34. Despite the widespread and continued circulation of the virus in many countries, the worldwide number of confirmed reinfections is very low. COVID-19 reinfection tracker.
35. Human challenge experiments performed at the Common Cold unit showed that individuals who became re-infected on challenge with a seasonal coronavirus 1 year after their first challenge shed less virus and for shorter duration, implying transmission risk may be lower on reinfection. [footnote 22]
36. In summary;
- although baseline antibodies derived through natural infection are strongly associated with protection against symptomatic infection over a period of at least 6 months, protection against asymptomatic nasal infection is substantially less
- further work is needed to establish whether viral load in the respiratory mucosa is reduced in those developing infection despite baseline antibodies, and whether transmission of infection occurs in those who are PCR positive but show evidence of immunity
Immunity following SARS-CoV-2 vaccination
37. Six weeks after the second vaccine dose, human clinical trial data are reporting protective efficacy against disease of up to 95%.
38. To our knowledge only the Oxford AstraZeneca vaccine trials have undertaken routine swabbing to detect asymptomatic infection. They report a protective efficacy against infection of 49.3% (7.4% to 72.2%) for the low dose/standard dose regimen and 2.0% (95%CI –50.7% to 36.2%) for the recipients of 2 standard doses. Confidence intervals are very wide. There is an overall reduction in PCR positivity of 54.1% (95%CI 44.7% to 61.9%) across both regimens, though there is no indication of Ct values to indicate the likelihood of onwards transmission from infected vaccinees. [footnote 2]
39. Animal (non-human primate, NHP) models show that some vaccines protect against disease but not all confer sterilising immunity. It should be noted that the challenge dose and route are quite different in animal studies (likely higher challenge dose) that for naturally acquired infection of humans.
- NHPs given the highest dose of Moderna vaccine, equivalent to that used in humans, did show sterilising immunity upon challenge [footnote 27]
- There is also evidence from NHP studies that the Pfizer BioNtech vaccine induced sterilising immunity upon challenge, with viral RNA detected in nasal swabs on Day 1 after challenge and not in swabs obtained on Day 3 or subsequently. [footnote 28]
- There is also good evidence that the Jansen Ad26 vaccine provides sterilising immunity in NHPs. [footnote 29]
- In animal studies the ChAdOx vaccine protected against disease but did not produce sterilizing immunity. The amount of viral RNA measured in the nose of vaccinated animals who became infected upon challenge was not different from naïve (non-vaccinated) animals. [footnote 30] However, the duration and level of viral replication in the lower respiratory tract was attenuated. A possible explanation is that the vaccine induced a strong IgG response that affects virus replication in the lung, but not a mucosal IgA response that would control viral load in the upper respiratory tract. Also, the ChAdOx study involved a very high- dose challenge (TCID50 2.6 x 106 ) to both upper and lower respiratory tract and did not replicate a realistic challenge scenario in human volunteers.
40. Emerging data suggest high levels of protection after a single vaccine dose.
- For the AstraZeneca vaccine, short term (<90 day) vaccine efficacy against symptomatic disease >21 days after a single dose was 76% (95%CI 59 – 86). Short term (<90 day) vaccine efficacy against asymptomatic infection (identified by routine swabbing) was 16% (95% -88 – 62), with very wide confidence intervals. Overall cases of any PCR+ were reduced by 67% (95%CI 49%, 78%) after a single standard dose vaccine. [footnote 2]
- For the Pfizer vaccine, vaccine efficacy against symptomatic disease between days 15 and 21 after the first dose was estimated at 89% (95% CI 52 to 97%). [footnote 31]
- Real-world data from a cohort of 503,875 individuals in Israel showed a vaccine effectiveness against infection of 51.4% (95%CI -7.2 -78.0) in Pfizer vaccinees during days 13 to 24 following immunisation with a single dose, compared to days 1 to 12. In total, 3,098 incident cases of PCR-confirmed SARS-CoV-2 infection were identified during the follow up period, with 2,484 occurring during the first follow up period (days 1 to 12) and 614 during the second (days 13 to 24). Cases were identified by PCR testing, available to participants irrespective of symptoms, but there was no routine swabbing. [footnote 32]
- Janssen’s COVID-19 vaccine candidate showed 66% effectiveness against moderate to severe disease, 28 days after a single vaccination. [footnote 33]
- As protective efficacy is higher following 2 vaccine doses, the risk of incorrectly labelling an individual as ‘immune’ would be lower if 2 doses of vaccine had been received.
41. At time of writing, only limited data are available on the duration of protective immunity induced by any SARS-CoV-2 vaccines (for either 1 or 2 doses). There is also limited information on the durability of measured immune responses in peripheral blood and about the best interval between priming and booster doses.
42. In summary;
- SARS-CoV-2 vaccines can provide a high level of protection against disease
- SARS-CoV-2 2 vaccines may provide protection against infection, but data is incomplete
- the duration of immunity provided by SARS-CoV-2 vaccines is not yet known
Testing to certify immunity
43. Testing options for issuing time-limited certificates following documented infection include a positive RT-PCR test, lateral flow device test, or antibody test, or any combination of these. The potential limitations of these approaches are discussed.
44. RT-PCR is the most sensitive and specific available test for acute SARS-CoV-2 infection. Since more than 95% of individuals who have been infected by SARS-CoV-2 mount an immune response, one could assume there is a high probability that a person who has a laboratory confirmed RT-PCR positive result will have some immunity. Due to the sensitivity of PCR, many who have recovered from COVID-19 and are no longer infectious may remain PCR positive for a month or more.
45. Confirmation of acute infection (antigen) by lateral flow devices (LFDs) is less specific and less sensitive than RT-PCR. This is of particular concern when used in asymptomatic testing as the positive predictive value (and hence the proportion of those testing positive who are false positives) is highly dependent on both specificity and the prevalence of infection in those tested. Thus, use of lateral flow results from mass population asymptomatic screening to issue immunity certificates would lead to many being issued certificates when they are not immune.
46. Presence of antibody in serum. There is a good correlation between neutralising antibodies to SARS-CoV-2 and protection against disease. There is also good correlation between individual antibodies and plasma virus neutralisation activity. However, there is as yet no standardisation of antibody assays and no validated antibody concentration that correlates with protection. In addition, a single positive antibody test does not provide information on the timing of infection and therefore the duration of protection cannot be inferred.
47. A combination of a RT-PCR positive test and subsequently the presence of antibody in serum (indicating that an individual has both been infected and developed antibodies) would give greater assurance that the individual is immune to symptomatic reinfection.
48. If there is a need to differentiate infected from vaccinated individuals, then the choice of antibody assay is important.
49. There are several approaches to measuring antibodies but the main limitation is the lack of correlates of protection. The studies presented in the figure above used a simple seropositive or seronegative categorisation. Use of ELISA requires significant laboratory capacity and may not be feasible for the issuance of immunity certificates. Other possible techniques include high throughput immune assays or point of care tests, noting there are many caveats about test performance
50. Measurement of antibody will only establish the level of anti-S antibody in the plasma at the time of the test. If IgG levels wane, it is still possible that memory S specific plasma cells are present and upon re-infection could rapidly proliferate. The concentration of RBD antibodies that correlates with protection is not yet established but emerging data suggest a neutralising titres of around 1:30 may correlate with protection. Also, the kinetics of RBD antibody decay are not fully understood. This makes it difficult to determine what RBD antibody concentration is considered protective and for what period of time that protection holds.
51. T-cells may also contribute to disease control or protection and the RBD assay won’t detect these. Assays for use at scale for these other immune parameters are not available. Therefore, the certificate might be ‘removed’ from a person even though they are still protected. However, this cautious approach may be prudent since in all human coronaviruses (seasonal and severe) there is strong evidence of reinfection in some individuals due to both waning immunity and likely strain variation.
Impact of new emerging variants
52. The impact of the emergence of new SARS-CoV-2 variants such as variant of concern Alpha (formerly known as B.1.1.7) and variant of concern Beta (formerly known as B.1.351) on immunity certification and risk of re-infection is not yet clear.
53. There is currently no evidence that variant of concern Alpha (formerly known as B.1.1.7) is associated with antigenic escape from naturally, monoclonal, or vaccine acquired immunity [footnote 34], [footnote 35], [footnote 36]
54. There is some evidence to suggest that variant of concern Beta (formerly known as B.1.351) is associated with antigenic escape from naturally, monoclonal, and vaccine acquired immunity, though more data is needed [footnote 37], [footnote 38], [footnote 39], [footnote 40], [footnote 41]
55. SARS-CoV-2 accrues genetic changes that could lead to evasion of immunity that developed in response to infection with an earlier virus variant. Therefore, if SARS-CoV- 2 variants emerge that evade existing immunity, immunity certificates due to prior infections could no longer be valid.
- A mechanism needs to be determined as to how immunity certification could be updated or withdrawn depending on emergence of variants. This could be linked to vaccine updating.
Recommendations for additional work
56. Cycle threshold values from RT-PCR positive results in the cohort studies are needed to assess if reinfection results in lower levels of virus replication, and therefore infectivity, than a primary infection.
57. Work is required to establish immune correlates of protection.
58. A pilot study of immune certification should be conducted.
-
J. Dan, J. Mateus, Y. Kato, K. Hastie, E. D. Yu and C. E. Faliti, ‘Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection,’ Science, vol.eabf4063, 2021. ↩ ↩2 ↩3
-
M. Voysey, S. A. C. Clemens, S. A. Madhi, L. Y. Weckx, P. M. Folegatti, P. K. Aley, B. Angus, V. L. Baillie, S. L. Barnabas and et al., ‘Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine,’ 2021. ↩ ↩2 ↩3
-
L. Y. Lee, S. Rozmanowski, M. Pang, A. Charlett, C. Anderson, G. J. Hughes, M. Barnard, L. Peto, R. Vipond, A. Sienkiewicz, S. Hopkins, j. Bell, D. W. Crook, N. Gent, S. A. Walker, D. W. Eyre and T. E. Peto, ‘An observational study of SARS-CoV-2 infectivity by viral load and demographic factors and the utility lateral flow devices to prevent transmission,’ preprint, 2021. ↩
-
M. Marks, P. Millat-Martinez, D. Ouchi, C. H. Roberts, A. Alemany, M. Corbacho-Monne, M. Ubals, A. Tobias, C. Tebe, E. Ballana, Q. Bassat and et al., ‘Transmission of COVID-19 in 282 clusters in Catalonia, Spain:,’ The Lancet Infectious Diseases, vol. Published online, 2021. ↩
-
S. F. Lumley, D. O’Donnell, N. E. Stoesser, P. C. Matthews, A. Howarth, S. B. Hatch, B. D. Marsden, S. Cox, T. James, F. Warren, L. J. Peck and et al., ‘Antibodies to SARS-CoV-2 are associated with protection against,’ MedRxiv [preprint], 2020. ↩ ↩2
-
H. Harvala, M. L. Robb, N. Watkins, S. Ijaz, S. Dicks and M. Patel, ‘Convalescent plasma therapy for the treatment of patients with COVID-19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels,’ Transfus Med, vol. Online ahead of print, 2020. ↩
-
D. F. Gudbjartssson, G. L. Norddahl, P. Melsted, K. Gunnarsdottir, H. Holm, E. Eythorsson and A. Arnthorsson, ‘Humoral Immune Response to SARS-CoV-2 in Iceland,’ NEJM, vol. 383, pp. 1724-1734, 2020. ↩ ↩2
-
S. N. Ladhani, A. Jeffrey-Smith, M. Patel, R. Janarthanan, J. Fok, E. Crawley-Boevey, A. Vusirikala, E. F. R. De Olano and M. S. Perez, ‘High prevalence of SARS-CoV-2 antibodies in care homes affected by,’ EClinicalMedicine, 2020. ↩
-
J. Seow, C. Graham, B. Merrick, S. Acors, S. Pickering, K. J. Steel and O. Hemmings, ‘Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans,’ Nature Microbiology, vol. 5, pp. 1598-1607, 202. ↩
-
A. N. Alshukairi, I. Khalid, W. Ahmed, A. M. Dada, D. T. Bayumi, L. S. Malic and S. Althawadi, ‘Antibody Response and Disease Severity in Healthcare Worker MERS Survivors,’ Emerg Infect Dis, vol. 22, no. 6, pp. 1113-115, 2016. ↩
-
L. Grandjean, A. Saso, A. T. Ortiz, T. Lam, J. Hatcher, R. Thistlethwayte, M. Harris, T. Best, M. Johnson and H. Wagstaffe, ‘Long-Term Persistence of Spike Antibody and Predictive Modeling of Antibody Dynamics Following Infection with SARS-CoV-2,’ medRxiv [preprint], 2020. ↩ ↩2
-
P. G. Choe, K.-H. Kim, C. K. Kang, H. J. Suh, E. Kang, S. Y. Lee, N. J. Kim, W. B. Park and M.-D. Oh, ‘Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection,’ Emerg Infect Dis, vol. 27, no. 3, 2020. ↩
-
T. Sekine, A. Perez-Potti, O. Rivera-Ballesteros, K. Stralin, J.-B. Gorin, A. Olsson, S. Llewellyn-Lacey, H. Kamal and G. Bogdanovic, ‘Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19,’ Cell, vol. 183, no. 1, pp. 158-168.e14, 2020. ↩
-
J. Zuo, A. Dowell, H. Pearce, K. Verma, H. Long, J. Begum, F. Aiano, Z. Amin-Chowdury, B. Hallis, L. Stapley, R. Borrow and E. Linley, ‘Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection,’ bioRxiv [preprint], 2020. ↩
-
A. W. Edridge, J. Kaczorowska, A. C. Hoste, M. Bakker, M. Klein, K. Loens, M. Jebbink, A. Matser, C. Kinsella, P. Rueda and M. Leven, ‘Seasonal coronavirus protective immunity is short-lasting,’ Nat. Med., vol. 26, no. 11, pp. 1691-1693, 2020. ↩
-
S. M. Kissler, C. Tedijanto, E. Golstein, Y. Grad and M. Lipsitch, ‘Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period,’ Science, vol. 368, no. 6493, pp. 860-868, 2020. ↩
-
R. Eguia, K. H. Crawford, T. Stevens-Ayers, L. Kelnohofer-Millevolte, A. L. Greninger, J. A. Englund, M. J. Boeckh and J. D. Bloom, ‘A human coronavirus evolves antigenically to escape antibody immunity,’ bioRxiv [preprint], 2020. ↩
-
W. Deng, L. Bao, J. Liu, C. Xiao, J. Liu, J. Xue, Q. Lv, F. Qi, H. Gao, P. Yu and Y. Xu, ‘Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques,’ Science, vol. 369, no. 6505, pp. 818-823, 2020. ↩
-
A. Chandrashekar, J. Liu, A. Martinot, K. McMahan, N. B. Mercado, L. Peter, L. H. Tostanoski, J. Yu, Z. Maliga, N. Michael, K. Busman-Sahey, M. Terry, L. M. Wrijil, S. Ducat and D. R. Martinez, ‘SARS-CoV-2 infection protects against rechallenge in rhesus macaques,’ Science, vol. 369, no. 6505, pp. 812-817, 2020. ↩
-
L. Bao, W. Deng, H. Gao, C. Xiao, J. Liu, J. Xue, Q. Lv, J. Liu, P. Yu, Y. Xu, F. Qi and Y. Qu, ‘Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2,’ bioRxiv [preprint], 2020. ↩
-
M. Imai, K. Iwatsuki-Horimoto, M. Hatta, S. Loeber, P. J. Halfmann, N. Nakajima, T. Watanabe, M. Ujie, K. Takahashi, M. Itso, S. Yamada and S. Fan, ‘Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development,’ Proc Natl Acad Sci USA, vol. 117, no. 28, pp. 16587-16595, 2020. ↩
-
K. McMahan, J. Yu, N. B. Mercado, C. Loos, L. H. Tostanoki, A. Chandrashekar, J. Liu, C. Atyeo, A. Zhu, E. A. Bondzie, G. Dagotto, M. S. Gebre and C. JAcob-Dolan, ‘Correlates of protection against SARS-CoV-2 in rhesus macaques,’ Nature, 2020. ↩ ↩2 ↩3
-
PHE, ‘Protection against SARS-CoV-2 Infection - PHE London Care Home Cohort Studies,’ Paper for NERVTAG 27/11/2020, 2020. ↩
-
V. Hall, S. Foulkes, A. Charlett, A. Atti, E. Monk, R. Simmons, E. Wellington and et al., ‘Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020,’ medRxiv [preprint], 2021. ↩
-
T. Palmer, A. Copas, M. Krutikov, L. Shallcross and et al., ‘VIVALDI Study - Prelimnary analaysis of infection rates,’ 2021. ↩
-
J. D. Chalmers, ‘The protective effect of SARS-CoV-2 antibodies in Scottish Healthcare Workers,’ Personal Communication, 2021. ↩
-
K. S. Corbett, B. Flynn, K. E. Foulds, J. R. Francica, S. Boyoglu-Barnum, A. P. Werner, B. Flach, S. O’Connell and K. W. Bock, ‘Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates,’ NEJM, vol. 383, pp. 1544- 1555, 2020. ↩
-
A. B. Vogel, I. Kanevsky, Y. Che, K. A. Swanson, A. Muik, M. Vormehr, L. M. Kranz, K. C. Walzer, S. Hein, A. Guler, J. Loschko and M. S. Maddur, ‘A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates,’ bioRxiv [preprint], 2020. ↩
-
N. B. Mercado, R. Zahn, F. Wegmann, C. Loos, A. Chadrashekar, J. Yu, J. Liu, L. Peter, K. McMahan, L. H. Tostanoski, X. He and D. R. Martinez, ‘Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques,’ Nature, vol. 586, pp. 583-588, 2020. ↩
-
N. van Doremalen, T. Lambe, A. Spencer, S. Belij-Rammerstorfer, J. N. Purushotham, J. R. Port, V. A. Avanzato, T. Bushmaker, A. Flaxman, M. Ulaszewska, F. Feldman and E. R. Allen, ‘ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques,’ Nature, no. 586, pp. 578-582, 2020. ↩
-
Joint Committee on Vaccination and Immunisation (JCVI), ‘Optimising the COVID-19 vaccination programme for maximum short-term impact,’ 2020. ↩
-
G. Chodick, L. Tene, T. Patalon, S. Gazit, A. Ben Tov, D. Cohen and K. Muhsen, ‘The effectiveness of the first dose of Bnt162b2 vaccine in reducing SARS-CoV-2 Infection in 13-24 days after immunization: real world evidence,’ medRxiv [preprint], 2021. ↩
-
Johnson&Johnson, ‘Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial,’ 2021. ↩
-
Public Health England, ‘Investigation of novel SARS-CoV-2 Variant of Concern 202012/01: Technical briefing 2,’ 2020. ↩
-
N. G. Davies, R. C. Barnard, C. I. Jarvis, A. J. Kucharski, J. Munday, C. A. Pearson, T. W. Russell, D. C. Tully and S. Abbott, ‘Estimated transmissibility and severity,’ 2020. ↩
-
A. Muik, A.-K. Wallisch, B. Sanger, K. A. Swanson, J. Muhl, W. Chen, C. Hui and R. arkar, ‘Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera,’ bioRxiv [preprint], 2021. ↩
-
A. J. Greaney, A. N. Loes, K. H. Crawford, T. N. Starr, K. D. Malone, H. Y. Chu and J. D. Bloom, ‘Comprehensive mapping of mutations to the SARS-CoV-2 receptor- binding domain that affect recognition by polyclonal human serum antibodies,’ bioRxiv [preprint], 2021. ↩
-
A. Baum, B. O. Fulton, E. Wloga, R. Copin, K. E. Pascal, V. Russo, S. Giordano, K. Lanza, N. Negron, M. Ni, Y. Wei, G. S. Atwal and A. J. Murphy, ‘Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies,’ Science, vol. 369, no. 6506, pp. 1014-1018, 2020. ↩
-
Eli Lilly and Company, ‘Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Bamlanivimab,’ 2020. ↩
-
Z. Wang, F. Schmidt, Y. Weisblum, F. Muecksch, C. O. Banres, S. Finkin, D. Schaefer- Babajew, M. Cipolla, C. Gaebler, J. A. Lieberman and Z. Yang, ‘mRNA vaccine- elicited antibodies to SARS-CoV-2 and circulating variants,’ bioRxiv [preprint], 2021. ↩
-
C. K. Wibmer, F. Ayres, T. Hermanus, M. Madzivhandila, P. Kgagudi, B. Lambsen, M. Vermeulen, K. van den Berg, T. Roussow and M. Boswell, ‘SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma,’ bioRxiv [preprint], 2021. ↩
