EMHP wastewater monitoring of SARS-CoV-2 in England: 1 June to 7 February 2022
Published 24 February 2022
Data showing the concentration of SARS-CoV-2 RNA and variants of concern (VOCs) detected in wastewater by the Environmental Monitoring for Health Protection (EMHP) wastewater monitoring programme.
Background on the EMHP wastewater monitoring programme
People infected with coronavirus (COVID-19) shed the virus during daily activities such as going to the toilet and blowing their nose. The virus enters the sewer system through sinks, drains and toilets. Fragments of SARS-CoV-2 (the virus that causes COVID-19) can be detected in samples of wastewater (untreated sewage).
The EMHP programme is led by the UK Health Security Agency (UKHSA) and run in partnership with the Department for Environment, Food and Rural Affairs (Defra), the Environment Agency (EA), the Centre for Environment, Fisheries and Aquaculture Science (Cefas), academia, and water companies. It provides coverage of approximately 40 million people across England.
The EMHP team coordinates with programmes in the devolved administrations to provide UK-wide wastewater monitoring. The programme tests sewage for fragments of SARS-CoV-2 RNA. Untreated influent samples are taken from approximately 294 Sewage Treatment Works (STWs) in England, 3 times a week (see figures 1 to 9).
EMHP also samples from sewer network sites (manholes). Untreated influent samples are taken from approximately 197 sewer network sites in England 4 times a week. Data from sewer network sites is not included in the concentration data in this publication as these sites generally fall within STWs catchments.
Data from sewer network sites is included in the Omicron detection data in this publication. Further detail about EMHP wastewater coverage can be found in the publication Wastewater testing coverage data for the EMHP programme.
The programme helps identify where the virus is circulating in England as well as detecting mutations of the virus, including those associated with known VOCs, and variants under investigation (VUIs). Findings are reported to national decision makers and local stakeholders on a regular basis, helping to inform national strategy and localised action.
Wastewater analysis has the benefit of detecting the virus regardless of whether people have symptoms or whether they are tested. Wastewater monitoring complements other testing programmes and public health actions to help protect against the threat of new variants.
As the threat of variants has emerged, the programme has played a key role in the detection of mutations of the virus, including those associated with known VOCs and VUIs.
This is achieved through genomic sequencing of wastewater samples, to provide an indication of where VOCs and VUIs may be present across England. Findings are reported to national and local stakeholders on a regular basis, helping to inform decision making.
Wastewater variant detection data (Omicron variant only) included in the Investigation of SARS-CoV-2 variants: technical briefings is now available in this month’s publication as experimental statistics. We are exploring the feasibility of adding more variant detection data in future releases.
We continue to develop our approach for identifying variants and mutations in wastewater. The data presented in this publication is generated by non-accredited research laboratories and should be considered experimental.
Formal validation of wastewater monitoring as a variant surveillance system is underway. EMHP advises caution when directly comparing data from STWs and concentration of SARS-CoV-2 RNA in wastewater with clinical testing data.
Wastewater detections of a variant are reported as either confirmed or possible, based on the number of signature or unique single nucleotide polymorphisms (SNPs), and co-occurrence on the same amplicon. This describes the level of confidence EMHP has in the specificity of the detection; ‘confirmed’ status is assigned when there is a high degree of confidence the variant was present in the wastewater sample, ‘possible’ is assigned where there is some evidence that the variant was present.
These definitions are different to the clinical case definitions and the two should not be compared. Lack of a positive wastewater sequencing result is not necessarily confirmation that no variant cases reside within a given geography, due to factors such as rainfall dilution impacting RNA recovery.
Interpretation of all data in this publication should be made with careful consideration of the points in the Uncertainty, data quality and revisions section.
EMHP sampling sites
EMHP samples at sewer network sites (manholes in the street which generally serve a local area) and STWs (which generally serve wider areas such as a city or town). Associated with every STWs and sewer network site is a catchment, the geographical area from which wastewater flows into the sampling location.
EMHP SARS-CoV-2 RNA concentrations data from sewer network sites is not included in this publication as these sites generally fall within STW catchments. In addition, EMHP change sampling strategy and locations of sewer network sites to meet epidemiological priorities (for example to support local public health responses).
Further detail about EMHP wastewater coverage can be found in the publication Wastewater testing coverage data for the EMHP programme.
Figures 1 to 9 show the location of STWs that EMHP samples at in each region.
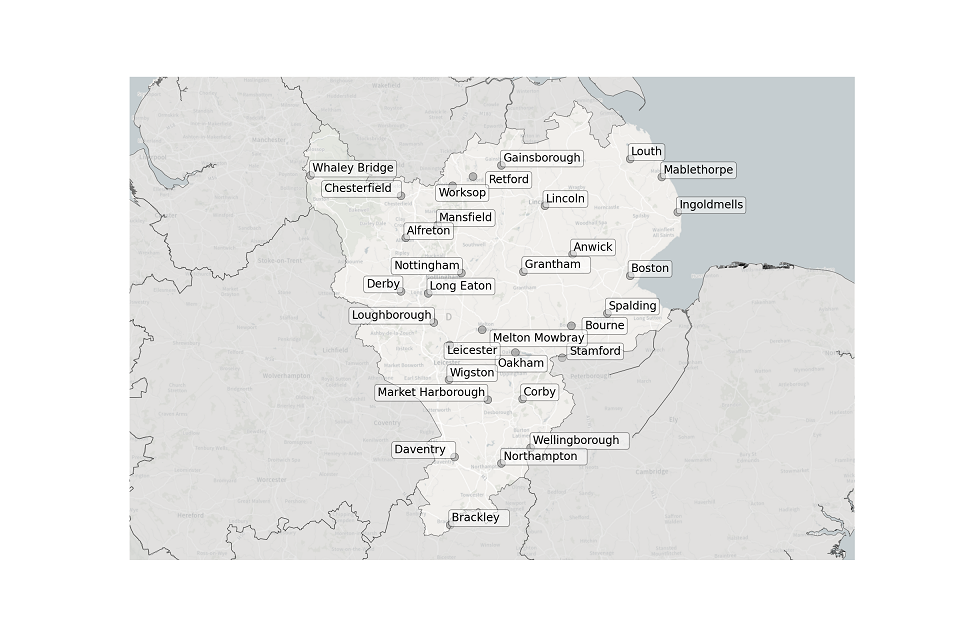
Figure 1. Map showing the location of STWs sampled in the East Midlands.
Location of STWs sampled in the East Midlands:
- Alfreton
- Anwick
- Boston
- Bourne
- Brackley
- Chesterfield
- Corby
- Daventry
- Derby
- Gainsborough
- Grantham
- Ingoldmells
- Leicester
- Lincoln
- Long Eaton
- Loughborough
- Louth
- Mablethorpe
- Market Harborough
- Mansfield
- Melton Mowbray
- Northampton
- Nottingham
- Oakham
- Retford
- Spalding
- Stamford
- Wellingborough
- Whaley Bridge
- Wigston
- Worksop
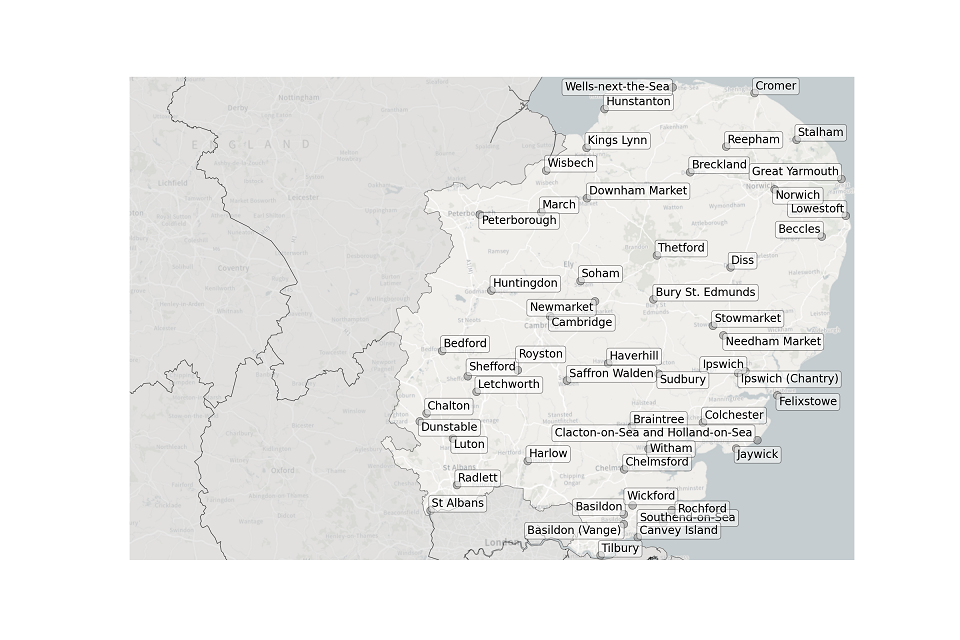
Figure 2. Map showing the location of STWs sampled in the East of England.
Location of STWs sampled in the East of England:
- Basildon
- Basildon (Vange)
- Beccles
- Bedford
- Braintree
- Breckland
- Bury St Edmunds
- Cambridge
- Chalton
- Chelmsford
- Colchester
- Cromer
- Diss
- Downham Market
- Felixstowe
- Great Yarmouth
- Harlow
- Haverhill
- Hunstanton
- Huntingdon
- Ipswich
- Ipswich (Chantry)
- Jaywick
- Kings Lynn
- Letchworth
- Lowestoft
- Luton
- March
- Needham Market
- Newmarket
- Norwich
- Peterborough
- Radlett
- Reepham
- Rochford
- Royston
- Saffron Walden
- Shefford
- Soham
- St Albans
- Stalham
- Stowmarket
- Sudbury
- Southend-on-Sea
- Thetford
- Tilbury
- Wells-next-the-Sea
- Wickford
- Wisbech
- Witham
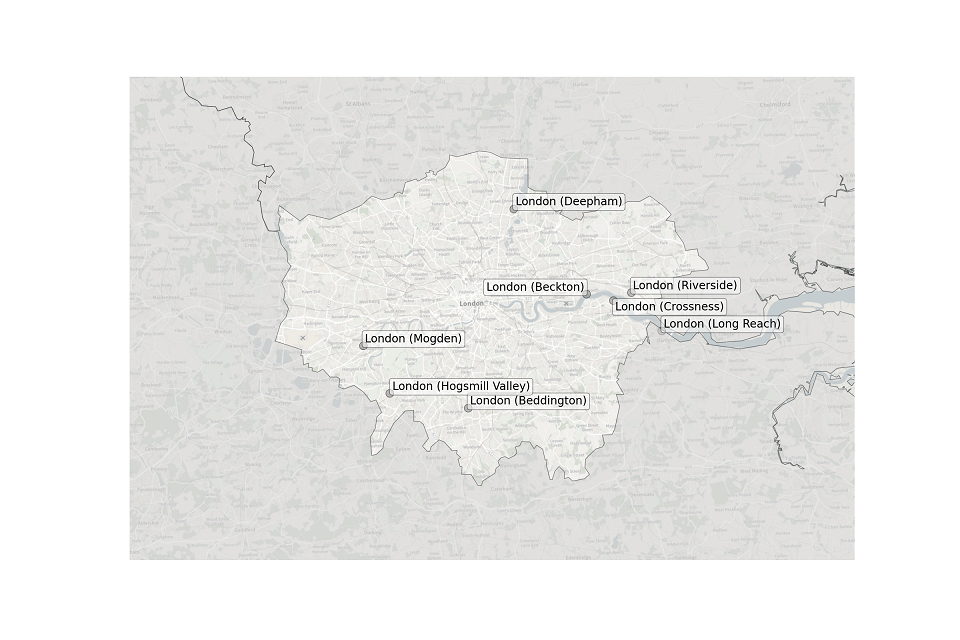
Figure 3. Map showing the location of STWs sampled in London.
Location of STWs sampled in London:
- Beckton
- Beddington
- Crossness
- Deepham
- Hogsmill Valley
- Long Reach
- Mogden
- Riverside
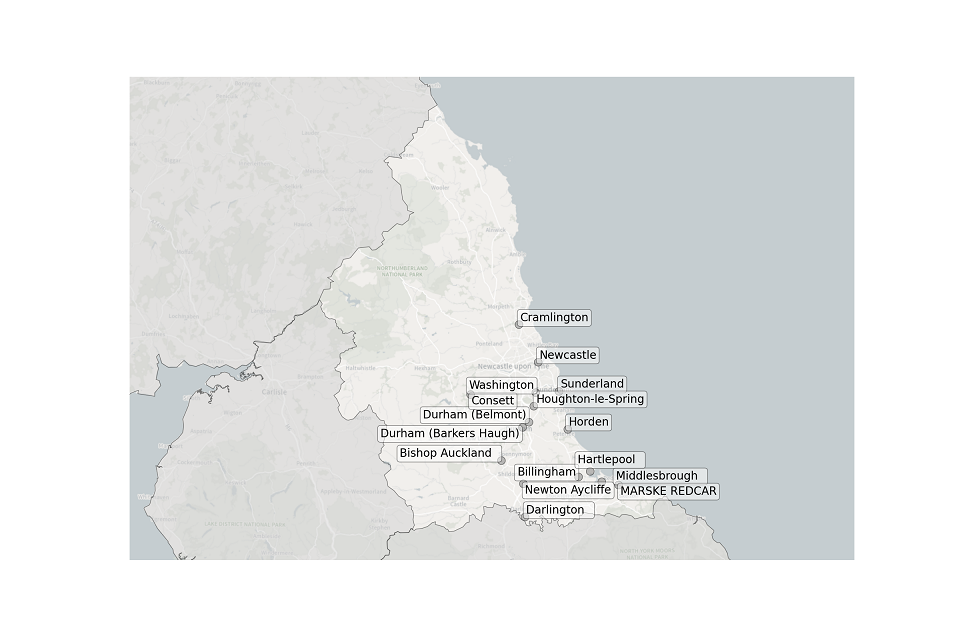
Figure 4. Map showing the location of STWs sampled in the North East.
Location of STWs sampled in the North East:
- Billingham
- Bishop Auckland
- Consett
- Cramlington
- Darlington
- Durham (Barkers Haugh)
- Durham (Belmont)
- Hartlepool
- Horden
- Houghton-le-Spring
- Marske Redcar
- Middlesbrough
- Newcastle
- Newton Aycliffe
- Sunderland
- Washington
Figure 5. Map showing the location of STWs sampled in the North West.
Location of STWs sampled in the North West:
- Barnoldswick
- Barrow-in-Furness
- Birkenhead
- Blackburn
- Bolton
- Bromborough
- Burnley
- Bury
- Carlisle
- Clitheroe
- Congleton
- Crewe
- Ellesmere Port
- Fleetwood
- Huyton and Prescot
- Hyde
- Hyndburn
- Kendal
- Lancaster
- Leigh
- Liverpool (Sandon)
- Macclesfield
- Maghull
- Northwich
- Penrith
- Preston
- Rochdale
- Rossendale
- Skelmersdale
- St. Helens
- Stockport
- Walton-Le-Dale
- Warrington
- Wigan
- Wirral
- Workington
Figure 6. Map showing the location of STWs sampled in the South East.
Location of STWs sampled in the South East:
- Andover
- Alton
- Ashford
- Aylesbury
- Banbury
- Basingstoke
- Bexhill
- Bicester
- Bordon
- Buckingham
- Burgess Hill
- Camberley
- Canterbury
- Chatham
- Chesham
- Chichester
- Crawley
- Didcot
- Dover and Folkestone
- Eastleigh
- Fareham and Gosport
- Guildford
- Hailsham
- Hythe
- Isle of Wight
- Lewes
- Littlehampton and Bognor
- Lymington and New Milton
- Maidstone and Aylesford
- Milton Keynes
- Newbury
- New Forest
- Oxford
- Portsmouth and Havant
- Ramsgate, Sandwich and Deal
- Reading
- Reigate
- Scaynes Hill
- Sittingbourne
- Slough
- Southampton
- Tonbridge
- Tunbridge Wells
- Witney
- Woking
- Worthing
- Wycombe

Figure 7. Map showing the location of STWs sampled in the South West.
Location of STWs sampled in the South West:
- Barnstaple
- Bath
- Bideford
- Blandford Forum
- Bodmin Sc Well
- Bournemouth (Central)
- Bridport
- Bristol
- Camborne
- Chard
- Cheltenham
- Chippenham
- Cirencester
- Clevedon and Nailsea
- Ernesettle and Saltash
- Exmouth
- Falmouth
- Gloucester
- Helston
- Liskeard
- Lydney
- Menagwins
- Minehead
- Newquay
- Newton Abbot
- Par
- Plymouth (Camels Head)
- Plymouth
- Plympton
- Salisbury
- Shaftesbury
- Shepton Mallet
- Sidmouth
- St Ives and Penzance
- Stroud
- Swanage
- Swindon
- Taunton
- Tavistock
- Tiverton
- Torquay
- Trowbridge
- Wellington
- Weston-super-Mare
- Weymouth
- Yeovil
Figure 8. Map showing the location of STWs sampled in the West Midlands.
Location of STWs sampled in the West Midlands:
- Barston
- Brancote
- Checkley
- Birmingham (Coleshill)
- Birmingham (Minworth)
- Burton on Trent
- Coventry
- Evesham
- Kidderminster
- Leek
- Ludlow
- Malvern
- Market Drayton
- Nuneaton
- Oswestry
- Rugby
- Spernal
- Stoke-on-Trent
- Stourbridge and Halesowen
- Telford
- Telford South
- Walsall
- Warwick
- Wolverhampton
- Worcester
Figure 9. Map showing the location of STWs sampled in Yorkshire and the Humber.
Location of STWs sampled in Yorkshire and the Humber:
- Barnsley
- Barton-upon-Humber
- Beverley
- Bingley
- Bradford
- Bridlington
- Castleford
- Colburn
- Dewsbury
- Doncaster (Thorne)
- Doncaster (Sandall)
- Driffield
- Grimsby
- Halifax
- Harrogate North
- Harrogate South
- Hemsworth and South Elmsall
- Huddersfield
- Hull
- Keighley
- Leeds
- Malton
- Mexborough and Conisbrough
- Northallerton
- Pontefract
- Scarborough
- Scunthorpe
- Sheffield (Blackburn Meadows)
- Sheffield (Woodhouse Mill)
- Wakefield
- York
Concentration of SARS-CoV-2 RNA in wastewater samples
EMHP wastewater concentration data from 1 June 2021 to 7 February 2022 is available in the accompanying spreadsheet. The spreadsheet includes data from previous months’ publications, along with the latest data from January and February.
The most recent 4 weeks of data are visualised in figures 10 and 11; England concentration map. Note that STWs are omitted from figures 10 and 11 if no samples were taken during the week.
In the week beginning 11 January, 198 of 271 STWs (73%) had a decrease in average concentration levels compared to the previous week. In the week beginning 18 January, 132 of 293 STWs (45%) had lower average concentration than the preceding week.
In the week beginning 25 January, 143 of 293 STWs (49%) had a decrease in average concentration relative to the previous week. In the week beginning 1 February, 190 out of 292 STWs (65%) showed a decrease in average concentrations compared to the previous week. Interpretation should be made with careful consideration of the points in the Uncertainty, data quality and revisions section below.
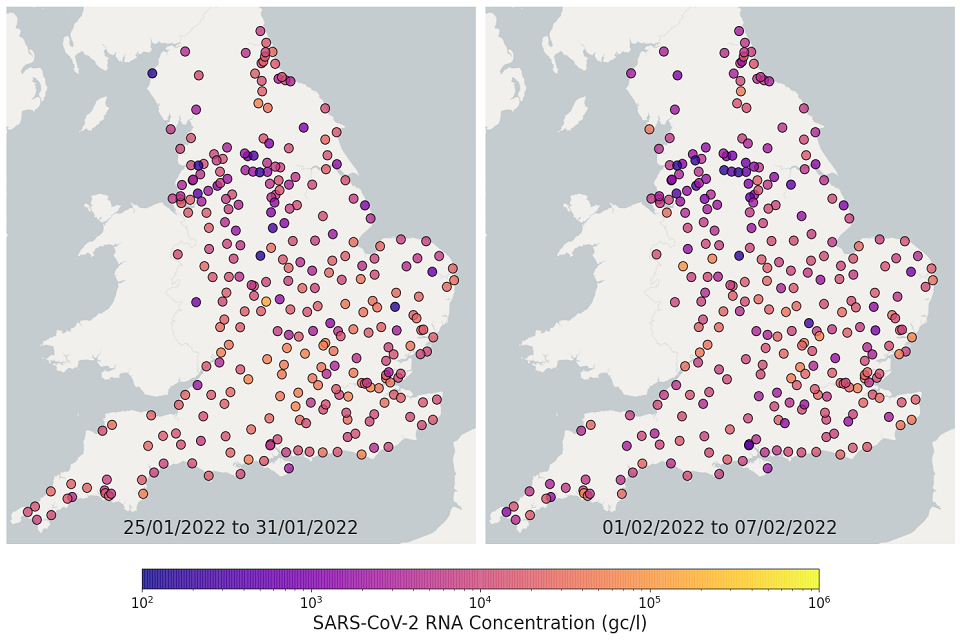
Figure 10. England concentration map: map showing the location of STWs sampled. Colour indicates the weekly-average SARS-CoV-2 RNA concentration (gene copies per litre) in the last 2 weeks. See regional maps for the names of each STWs.
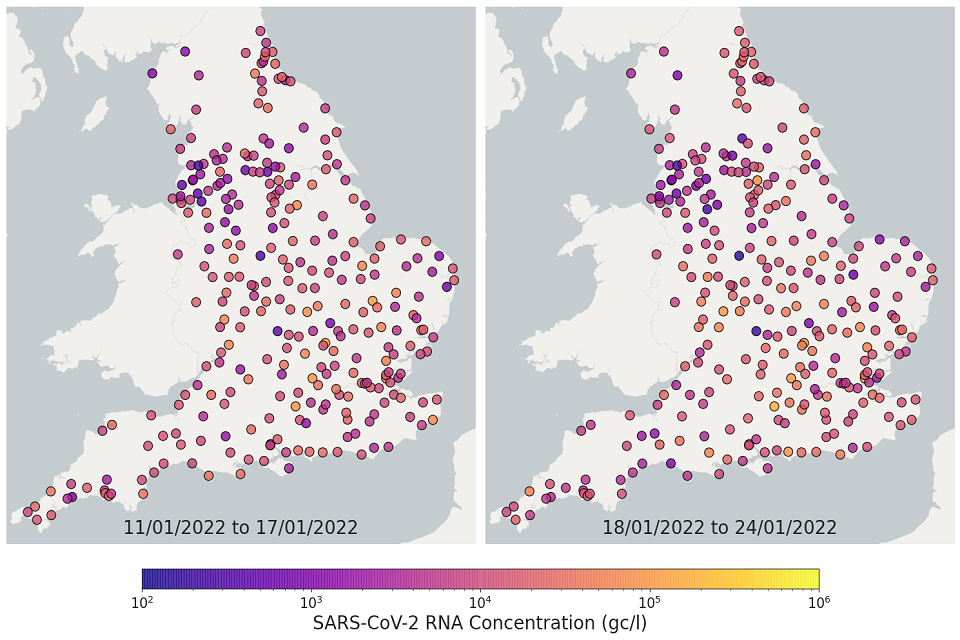
Figure 11. England concentration map: map showing the location of STWs sampled. Colour indicates the weekly-average SARS-CoV-2 RNA concentration (gene copies per litre) in the first 2 weeks. See regional maps for the names of each STWs.
Regional average concentration of SARS-CoV-2 RNA in wastewater
In the latest data (11 January to 7 February) the concentration data showed signs of a downward trend in most regions. Concentration levels in the North East, North West and Yorkshire and the Humber were below national average.
There is high variability in concentration between individual STWs, nationally. Interpretation should be made with careful consideration of the points in the Uncertainty, data quality, and revisions section.
Figures 12 to 20 show the 7-day rolling average SARS-CoV-2 concentration detected in wastewater for each region in England, together with the national average of SARS-CoV-2 concentration detected in wastewater and individual site data.
Samples where no SARS-CoV-2 was detected are assigned the value 160 (gene copies per litre) which is the Theoretical Limit of Detection (tLOD). Data is weighted by catchment population to account for varying catchment sizes. For further information, see Methodology section below.
Note: there was limited wastewater sampling over the Christmas period, therefore trends are not shown for the period 21 December 2021 to 28 December 2021
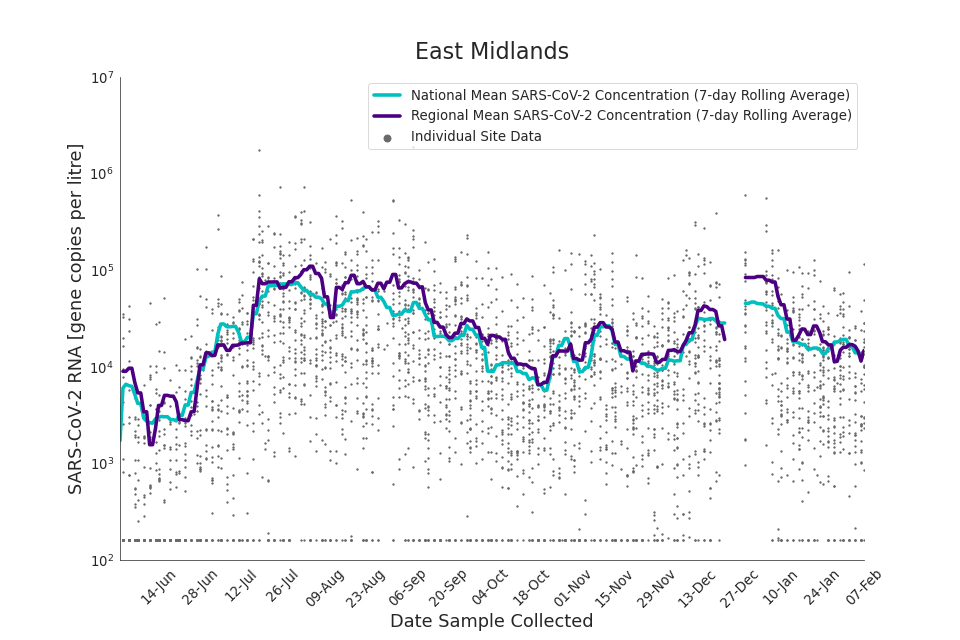
Figure 12. East Midlands 7-day rolling average SARS-CoV-2 RNA concentration
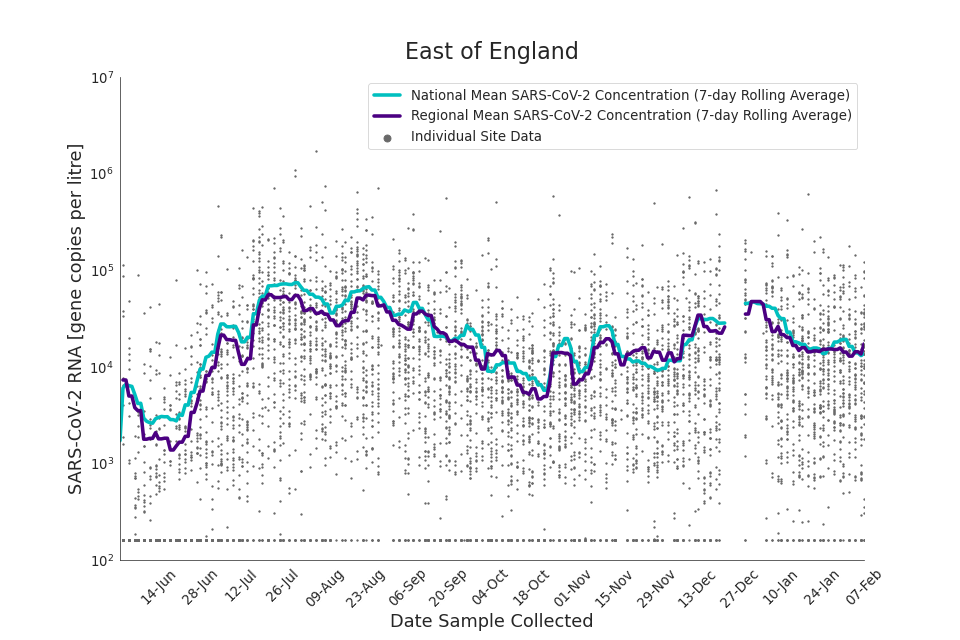
Figure 13. East of England 7-day rolling average SARS-CoV-2 RNA concentration
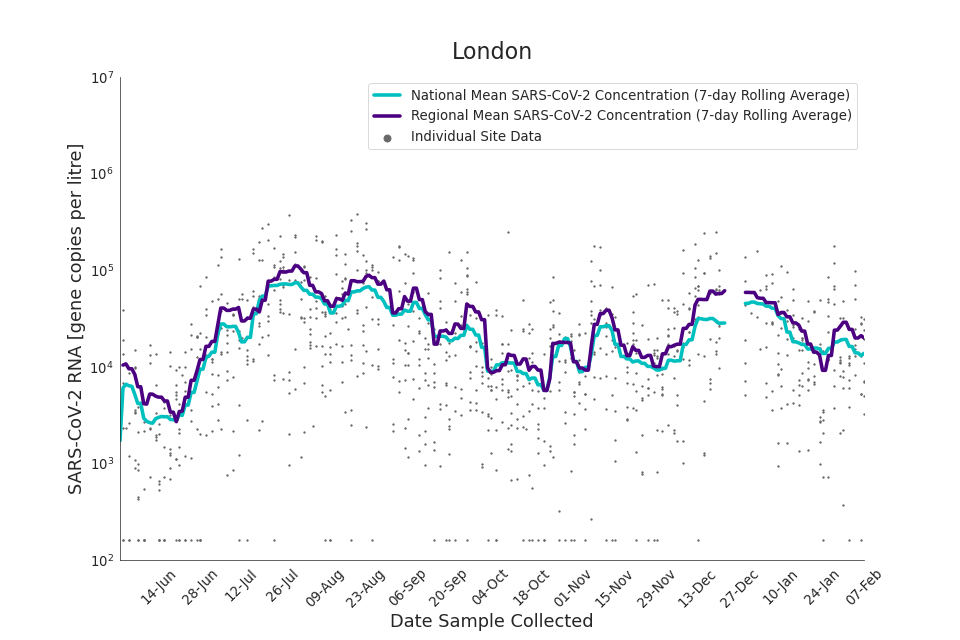
Figure 14. London 7-day rolling average SARS-CoV-2 RNA concentration
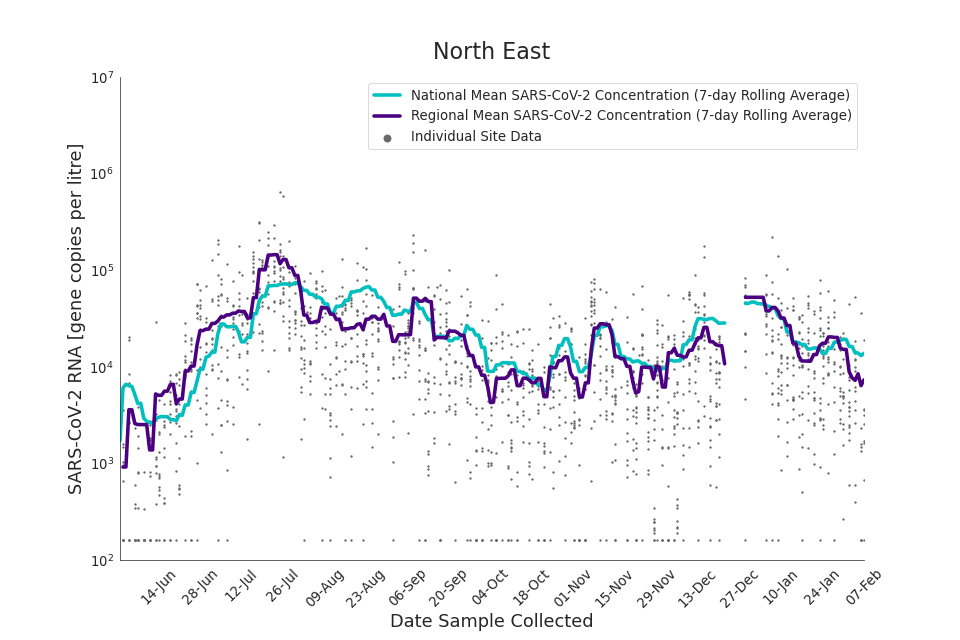
Figure 15. North East 7-day rolling average SARS-CoV-2 RNA concentration
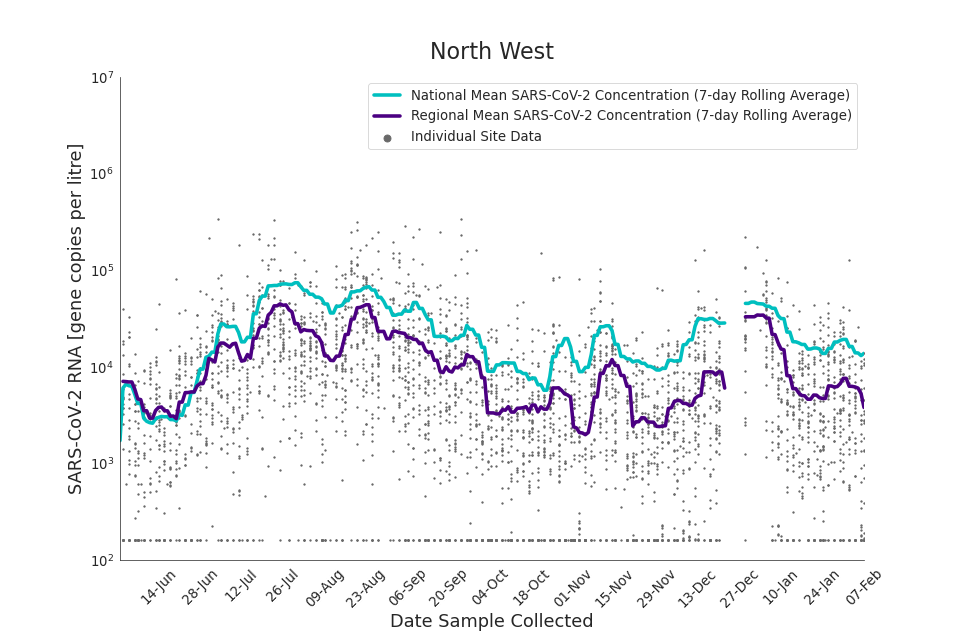
Figure 16. North West 7-day rolling average SARS-CoV-2 RNA concentration
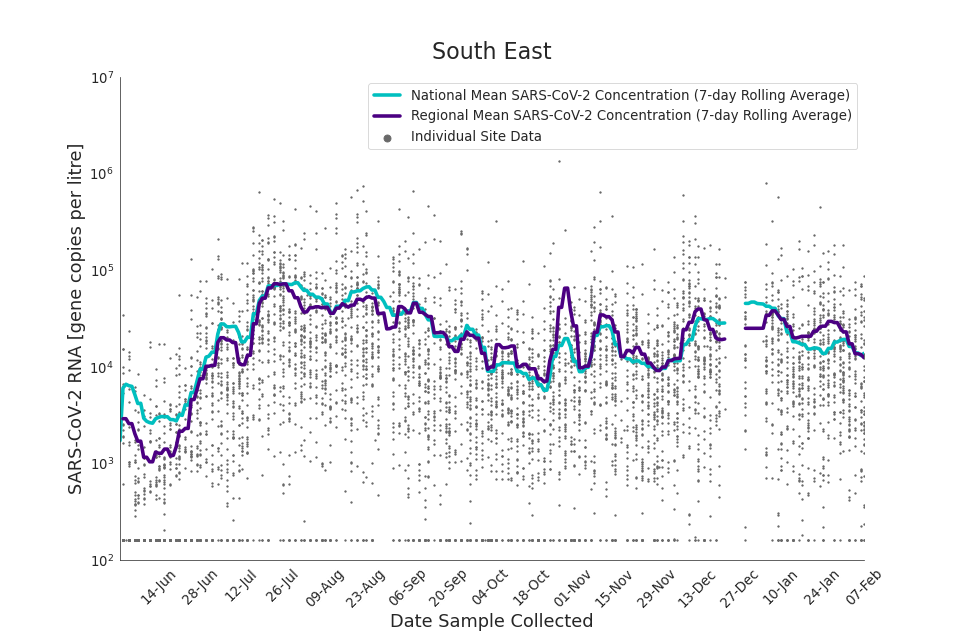
Figure 17. South East 7-day rolling average SARS-CoV-2 RNA concentration
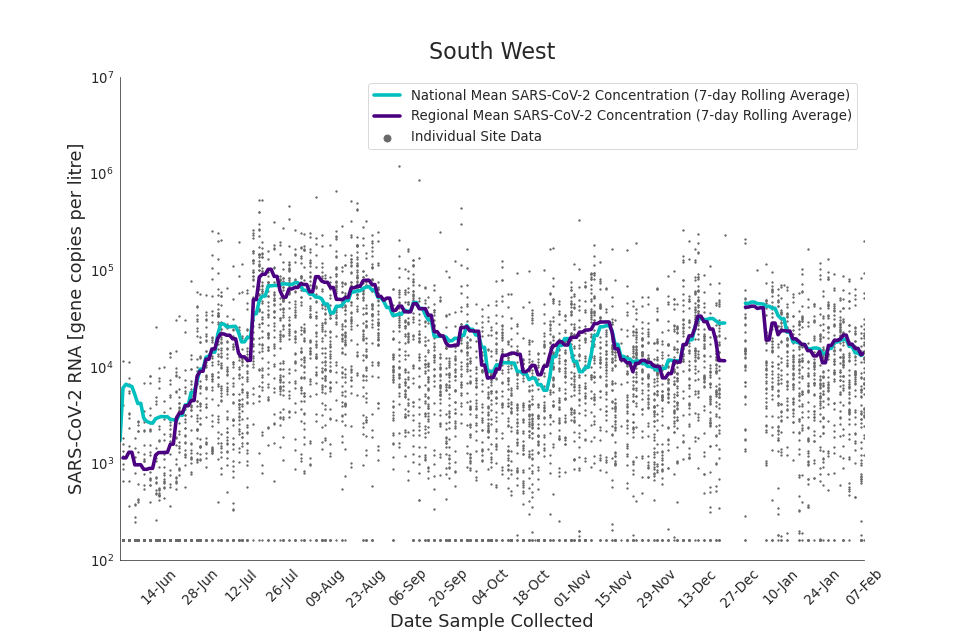
Figure 18. South West 7-day rolling average SARS-CoV-2 RNA concentration
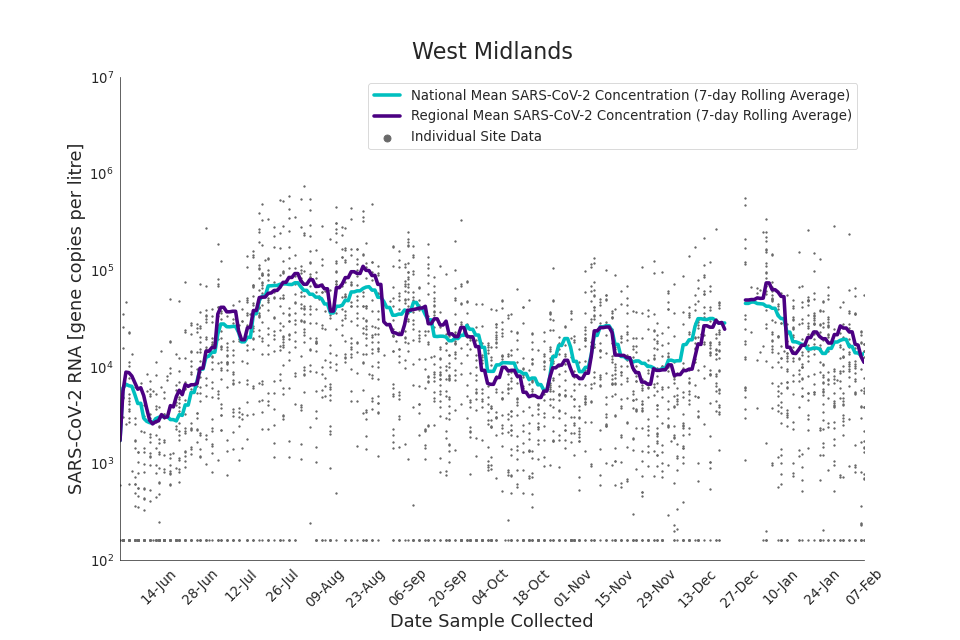
Figure 19. West Midlands 7-day rolling average SARS-CoV-2 RNA concentration
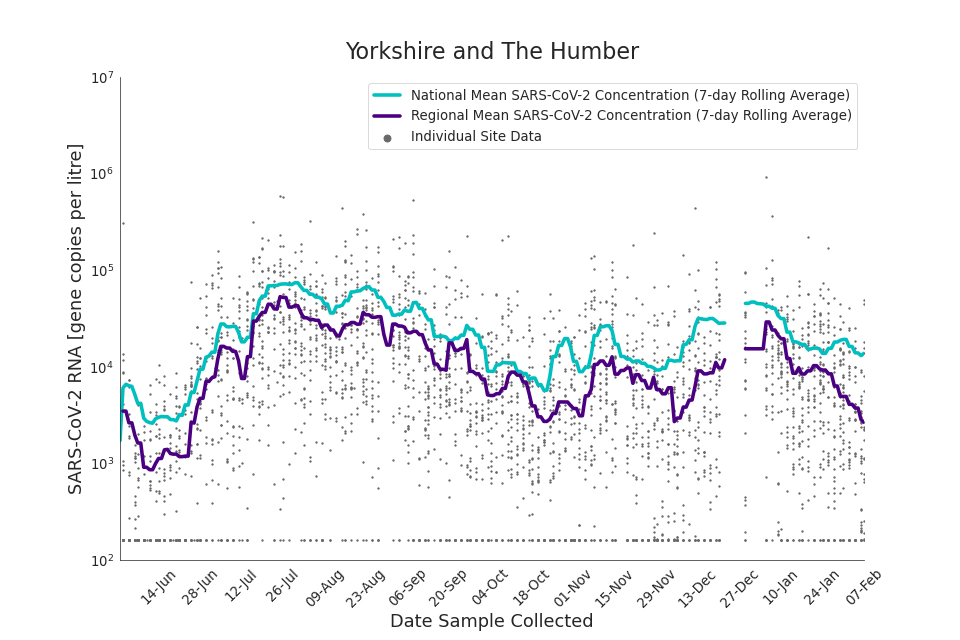
Figure 20. Yorkshire and the Humber 7-day rolling average SARS-CoV-2 RNA concentration
Wastewater variant detections
Omicron detections 22 November 2021 to 30 January 2022
Applying the Wastewater Variant Genomic Case Definition (see Methodology), wastewater samples collected from sites across England from 22 November 2021 up to 30 January 2022 have been analysed for the presence of Omicron.
EMHP Omicron detection data is available in the accompanying spreadsheet. The spreadsheet includes a table showing the number of sequenced samples by region and the number of ‘confirmed’ and ‘possible’ Omicron detections. Note that data presented here is subject to change as methods are further developed.
Due to the additional time required to analyse wastewater detections versus concentrations, the latest detection data presented here is one week prior to the latest concentration data.
The most recent 4 weeks of detections data are visualised in figures 21 to 23. Starting 17 January, Omicron detections in this data are classified as Omicon-BA.1 or Omicron-BA.2 subtypes.
From 3 January up to 9 January, Omicron was detected in 79% of wastewater samples sequenced for SARS-CoV-2 mutations across England. From 10 January up to 16 January, Omicron was detected in 76% of wastewater samples sequenced for SARS-CoV-2 mutations across England.
In the week 17 January to 23 January, Omicron-BA.1 was detected in 76% of sequenced samples, while 14% of samples had detections of Omicron-BA.2. In the week 24 January to 30 January, Omicron-BA.1 was detected in 71% of sequenced samples, while 25% of samples had detections of Omicron-BA.2.
In the latest week, the North West and Yorkshire and the Humber had the lowest proportion of samples with Omicron-BA1 and Omicron-BA2 detections, corresponding to the trends seen in the concentrations data.
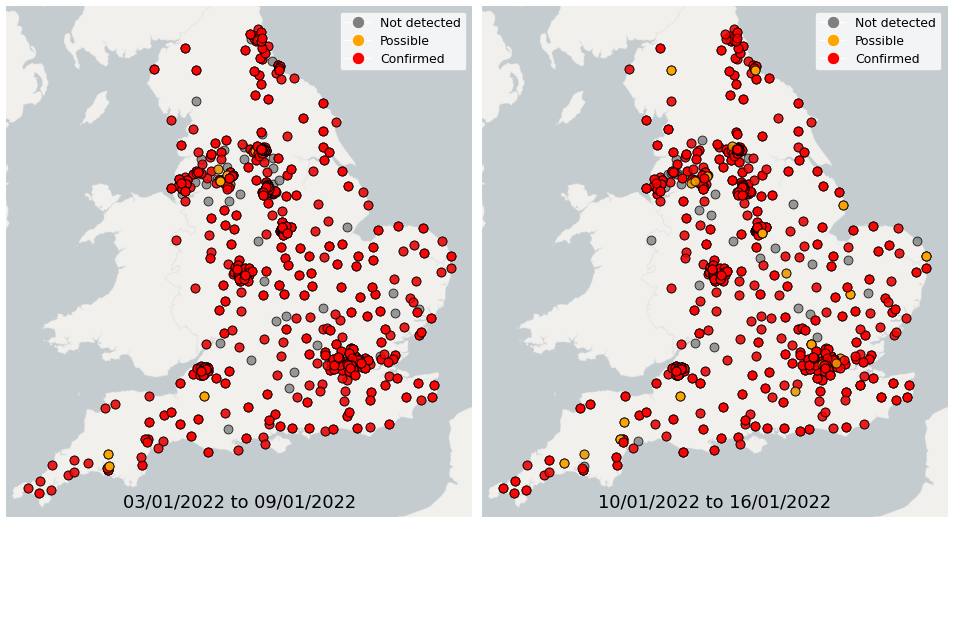
Figure 21. Confirmed and possible detections of Omicron VOC-21NOV-01 (B.1.1.529) in wastewater samples collected in England, data from 3 January 2022 to 16 January 2022
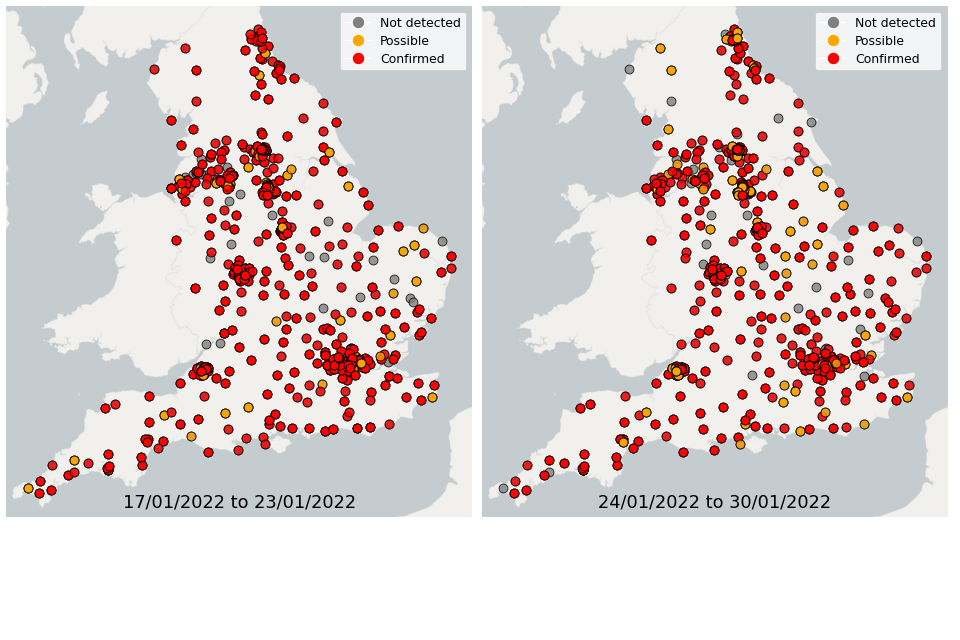
Figure 22. Confirmed and possible detections of Omicron-BA.1 (VUI-21NOV-01) in wastewater samples collected in England, data from 17 January to 30 January 2022
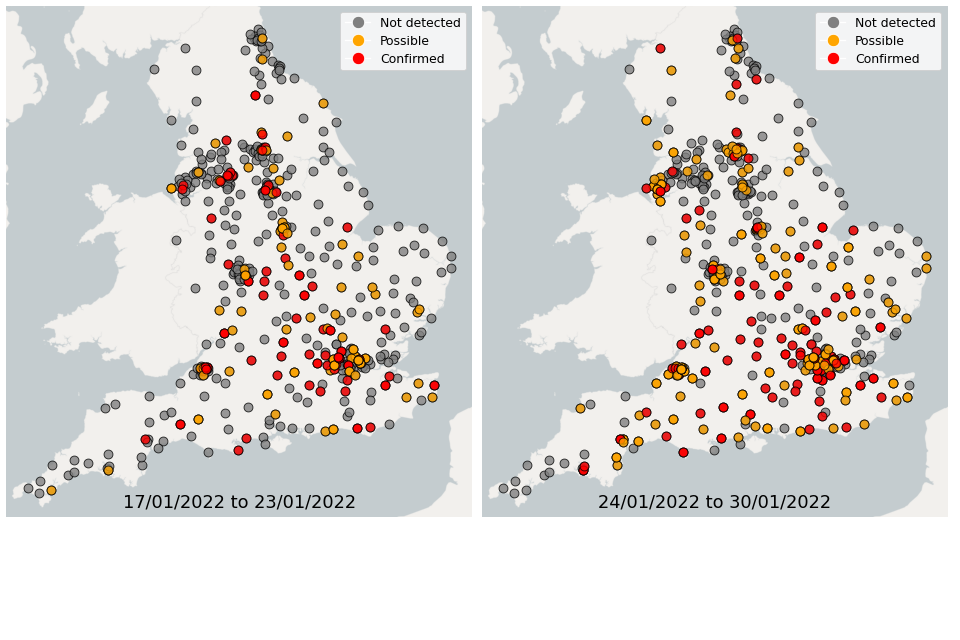
Figure 23. Confirmed and possible detections of Omicron-BA.2 (VUI-22JAN-01) in wastewater samples collected in England, data from 17 January to 30 January 2022
Methodology
About this data
SARS-CoV-2 RNA concentration
EMHP sample wastewater from STWs throughout England. Samples are analysed for the concentration of SARS-CoV-2 by quantifying the number of copies of the nucleocapsid gene (N1).
Samples are transported to Environment Agency laboratory for quantification of the N1 gene using a process called Reverse Transcriptase Polymerase Chain Reaction. The data reported here is the number of SARS-CoV-2 N1 gene copies per litre of wastewater (shortened to gene copies per litre throughout this document).
Generally, the more people with COVID-19 in the community the more viral RNA will be shed into wastewater. Therefore, the concentration in wastewater is indicative of the prevalence of COVID-19 in the community.
Samples are analysed for the concentration of SARS-CoV-2 RNA and concentration is adjusted for flow (see Uncertainty, data quality, and revisions). Typically, 4 samples per week are analysed from each STW. However, occasionally fewer samples will be collected and/or analysed. This can be due to several reasons such as EMHP altering sampling strategy because of testing capacity constraints, changing epidemiological priorities and current local response activities.
Weekly Concentration Table and Daily Concentration Table in the accompanying spreadsheet show the concentration of SARS-CoV-2 RNA in wastewater samples (gene copies per litre). Note that there is sensitivity associated with the analysis of wastewater; the Limit of Detection (LOD) is the minimum concentration of SARS-CoV-2 RNA that can be reliably detected.
Data tables contain the values ‘tLOD’ where analysis of the sample did not detect presence of SARS-CoV-2 RNA. For the purposes of averaging data to produce weekly averages in the Weekly Concentration Table and Figures 10 and 11, EMHP assigns a concentration of 160 gene copies/litre to all ‘tLOD’ samples. This concentration is the Theoretical Limit of Detection and is calculated by the Environment Agency.
Note that there is uncertainty in this calculation and continuing work to refine it, please see the Uncertainty, data quality, and revisions section for further information.
To calculate region averages, data from each STW was weighted by catchment population. To estimate the population of STWs, the catchment areas of STWs are matched to lower layer super output areas (LSOAs).
Catchment areas were provided by the 9 water companies serving England. See the EMHP publication Wastewater testing coverage data for the EMHP programme for a detailed description of this method. Regional and national averages were calculated by taking the 7 day rolling arithmetic mean. Figures are displayed on a logarithmic scale for ease of interpretation.
We are exploring the feasibility of adding more data in future releases, such as concentration data from network sites.
SARS-CoV-2 variant detection
The wastewater routine analysis is to look for the presence of pre-defined sets of SNPs that identify known VOCs, VUIs and signals in monitoring.
It is possible to look for mutations associated with variants in the wastewater, but detection of variants can be transient and the correlation between population prevalence and wastewater variant detection has not been established for Omicron.
Wastewater monitoring remains under development and is currently considered as supplementary data in variant monitoring.
A proportion of samples are sent to Liverpool, Nottingham and Exeter Universities Sequencing facilities each week for genomic sequencing, results are analysed by EMHP for the presence of known VOCs and VUIs officially recognised by UKHSA.
Wastewater detections of a variant are reported as either confirmed or possible, based on the number of signature or unique SNPs, and co-occurrence on the same amplicon. This describes the level of confidence EMHP has in the specificity of the detection; ‘confirmed’ status is assigned when there is a high degree of confidence the variant is present, ‘possible’ is assigned where there is some evidence that the variant is present. These definitions are different to the clinical case definitions and the two should not be compared.
Lack of a positive sequencing result is not necessarily confirmation that no variant cases reside within a given geography, due to factors such as rainfall dilution impacting RNA recovery. The wastewater variant genomic case definition used for detections in this publication is detailed below. Note that this definition is under review and subject to change.
Wastewater variant genomic case definition
B.1.1.529-BA.1 (VUI-21NOV-01) OMICRON-BA.1 (updated 25 January 2022)
B.1.1.529-BA.1 lineage has 1 synonymous and 16 non-synonymous signature SNPs of which 11 are unique amongst known VOC and VUI. Co-occurrence of signature mutations occurs on 2 amplicons for Nimagen protocol*.
Confirmed – ≥ 11 of 16 signature SNPs detected, ≥ 6 of 11 unique SNPs detected and co-occurrence detected OR If ≥ 16 signature SNPs and ≥ 6 unique SNPs are present, but with no co-occurrence confirmed presence can be assigned if some position of the co-occurring pairs are not covered.
Possible – ≥ 7 of 16 signature SNPs detected and ≥ 3 of 11 unique SNPs detected OR If < 7 signature SNPs and ≥ 3 unique SNPs are detected, but ≥ 6 SNPs are not covered possible presence can be assigned if those not covered are present across two dates from the same site, but in the same sequencing run.
Not detected – < 5 of 16 signature SNPs detected.
(*: amplicon_121 [22876-23119] positions 22898 and 23048, amplicon_139 [26476-26705] position 26530 and 26577.)
B.1.1.529-BA.2 (VUI-22JAN-01) OMICRON-BA.2
B.1.1.529-BA.1 lineage has 4 synonymous and 16 non-synonymous signature SNPs of which 12 are unique amongst known VOC and VUI. Co-occurrence of signature mutations occurs on 1 amplicon for Nimagen protocol*.
Confirmed – ≥ 13 of 20 signature SNPs detected, ≥ 6 of 12 unique SNPs detected and co-occurrence detected OR If ≥ 16 signature SNPs and ≥ 6 unique SNPs are present, but with no co-occurrence confirmed presence can be assigned if some position of the co-occurring pairs are not covered
Possible – ≥ 8 of 16 signature SNPs detected and ≥ 4 of 12 unique SNPs detected OR If < 8 signature SNPs and ≥ 4 unique SNPs are detected, but ≥ 6 SNPs are not covered possible presence can be assigned if those not covered are present across two dates from the same site, but in the same sequencing run.
Not detected – < 7 of 16 signature SNPs detected.
(*: amplicon_119 [22595-22837] positions 22679, 22688U,22775U and 22786U)
Data sources
Wastewater samples are collected by water companies and transported to an Environment Agency Laboratory. Concentration data is from Environment Agency analysis of wastewater samples.
Sequencing data is from Liverpool, Nottingham and Exeter Universities sequencing facilities. Variant detection’s data is from EMHP Genomics Analysis.
Figures in this publication show the location of STWs. These locations were obtained from the European Commission urban wastewater website. Note that names differ between those used in this document and the European Commission urban wastewater website, sites can be linked using the ‘STW Site Code’ in the attached data tables.
The figures and analysis presented will evolve over time to ensure the most relevant information is included and the needs of stakeholders are met. These figures will initially be published monthly, from June 2021, and we will continuously evaluate the frequency and date-range based on need and public interest.
How these figures can be used
These figures and data can be used to view the concentration of SARS-CoV-2 RNA in wastewater, which is indicative of prevalence in the community and an indication of where VOCs and VUIs may be present across England. Due to factors relating to data uncertainty, EMHP recommends careful interpretation of this data alongside other data sources such as Weekly Statistics for NHS Test and Trace (England).
Uncertainty, data quality, and revisions
The figures here are compiled by professional analysts and have been quality assured. However, the analytical pipeline to produce this data should still be considered experimental; it is subject to an ongoing quality assurance process. Any revisions to past publications will be in line with DHSC’s revision policy and highlighted in future publications accordingly.
SARS-CoV-2 RNA concentration
Since September 2021 the concentrations of SARS-CoV-2 RNA EMHP measured in wastewater appeared to diverge from reported clinical data. This trend is now not as pronounced as previously observed, nor is it as evident when considering the data over a longer timeframe.
EMHP are continuing to investigate the relationship between COVID-19 cases and wastewater and how this may be fluctuating over time. EMHP advises caution when directly comparing data from STWs and concentration of SARS-CoV-2 RNA in wastewater with clinical testing data. Interpretation should be made with careful consideration of the points below.
The sites from which EMHP sample can change on a regular basis depending on testing capacity, epidemiological priorities, and current local response activities. This can result in changes to specific STWs covered. This means the STW included in subsequent publications may change.
This publication shows STWs as single points. Every STW has an associated geographical catchment area (area from which wastewater flows into the sampling location). These catchments are not yet presented in these statistics publications. There is uncertainty surrounding catchment geography, as catchments vary in size and population covered, and in some cases an STW may not be located within the catchment it serves (for example, if wastewater is pumped to the STW). Therefore, the location of STW should be used as a general guide only.
Wastewater is an inherently variable material and can change greatly within and between days (foodstuffs being consumed; when washing machines are switched on and many other factors), the physical infrastructure and the type and levels of industrial wastes. It is generally more useful to consider trends over medium- and long-term periods of time, rather than between individual days.
There are several factors that can impact the quality of the signal recorded from wastewater and introduce uncertainty:
-
The concentration of SARS-CoV-2 RNA in wastewater may be impacted by weather, for example heavy rainfall wastewater may cause dilution. EMHP mitigates this by adjusting concentration to consider flow.
-
Whether samples are ‘composite’ or ‘grab’. Composite samples are where an autosampler (a machine which gathers wastewater at regular intervals over a set time period) gathers wastewater throughout the day. Grab samples are where a single sample is taken at one point in the day and are subject to greater variability; they are influenced by flow characteristics, time of day, and are more susceptible to outliers in the sewage. EMHP aims to mitigate this by sampling during times of peak load and ensuring the sample is collected mid-stream rather than close to the edges or bottom of the pipe.
-
Calculating the Limit of Detection (LOD; the minimum concentration of SARS-CoV-2 RNA that can be reliably detected in a wastewater sample) can be achieved by several methods. In this data we have used the Theoretical LOD as calculated by the Environment Agency. The Theoretical LOD uses several assumptions. Laboratory data is required to inform the more realistic Practical LOD. The Practical LOD for this analysis is not yet reported here. It is likely that the LOD used in producing these statistics will change in future publications. EMHP will clearly state when this occurs.
-
Although the exact value of the LOD is uncertain, it is unlikely to have a noticeable impact on the interpretation of this data. This is because the difference between Theoretical and Practical LOD is relatively small, with most impact at low levels of the target being measured.
-
As explained in the About this data section, typically 4 wastewater samples are taken at each STW per week. The average of all samples within the week is presented in the Weekly Concentration Table. Uncertainty occurs where fewer samples are taken. For example, if only one sample is taken during a week, that one sample defines the week average. This means weeks with fewer samples are more susceptible to variability and outliers. Sample Count Table shows how many samples were taken at an STW for each week in this release.
For the above reasons EMHP advises caution when directly comparing data from STWs and concentrations of SARS-CoV-2 RNA in wastewater with clinical testing data. Methodology and limitations will differ across data sources and this should be taken into account.
SARS-CoV-2 variant detection
The lack of a positive sequencing result is not confirmation that no variant cases reside within a given geography (given limitations of wastewater testing).
Environmental factors such as rainfall dilution can influence the detection of variants in wastewater. Therefore, low total detections on some days may not be reflective of low community infection levels.
Due to limitations of wastewater testing, EMHP cannot estimate the number of cases associated with a detection. In small catchments it is possible, but unlikely, the detection is due to a single individual. In larger catchments a detection is likely caused by a higher number of cases.
Figures 21 to 23 show the location of Omicron detections in STWs and sewer networks as single points. Sewer networks tend to be relatively small geographical areas. Please note that the underlying characteristics of city sewer networks can be complex. For example, there may be varying flow from other areas into the catchment, and/or the exact catchment geography may be uncertain. With this in mind, maps are presented as a general guide to the location of a detection.
It is possible that a detection in a catchment is contributed to by case(s) who do not reside in the catchment; interpretation with other epidemiological indicators, such as known clinical cases, is encouraged.
We continue to develop our approach for identifying variants and mutations in wastewater. The data presented in this publication is generated by non-accredited research laboratories and should be considered experimental. Formal validation of wastewater monitoring as a variant surveillance system is underway.
If you would like to give user feedback, please email testandtrace.statistics@dhsc.gov.uk
