Global AMR Innovation Fund annual review: 2017 to 2019
Published 24 September 2020
Clearance checklist
- Quality assurance: DHSC Portfolio, Performance, Investment and Risk (PPRI) team sign off on 10 June 2019
- External assurance: independent body sign off on 14 June 2019
- Global Health Security (GHS) Programme Board sign-off on 25 November 2019
Acroymns in this document
| Acronym | What it means |
|---|---|
| AMR | antimicrobial resistance |
| BARDA | Biomedical Advanced Research and Development Authority (USA) |
| BBSRC | Biotechnology and Biological Sciences Research Council |
| BMBF | Federal Ministry of Education and Research (Germany) |
| CARB-X | Combatting Antibiotic Resistant Bacteria Accelerator |
| CMO | Chief Medical Officer |
| CONICET | National Scientific and Technical Research Council (Argentina) |
| DHSC | Department of Health and Social Care |
| EAB | Expert Advisory Board |
| FIND | Foundation for Innovative New Diagnostics |
| FY | financial year |
| GAMRIF | Global AMR Innovation Fund |
| GARDP | Global Antibiotic Research and Development Partnership |
| GHS | Global Health Security |
| HMT | Her Majesty’s Treasury |
| IDRC | International Development Research Centre |
| IUK | Innovate UK |
| LMIC | low and middle-income country |
| MRC | Medical Research Council |
| MoST | Ministry of Science and Technology (China) |
| MOU | memorandum of understanding |
| NERC | Natural Environment Research Council |
| ODA | Official Development Assistance |
| OECD | Organisation for Economic Cooperation and Development |
| PDP | product development partnership |
| R&D | research and development |
| S&A | stewardship and access |
| SR15 | Spending Review 2015 |
| TPP | target product profile |
| UKRI | UK Research and Innovation |
| WASH | water, sanitation and hygiene |
| WP | work packages |
Introduction
Outline of the Global Health Security programme
In the last Spending Review (SR15) the Department of Health and Social Care (DHSC) was allocated £477 million of UK Official Development Assistance (ODA) funding to develop Global Health Security (GHS) projects in and for low and middle-income countries (LMICs), with the aim of contributing to a “world safe and secure from infectious disease threats and promotion of Global Health as an international security priority”. This accounts for 34% of the total DHSC ODA funding for the SR15 spending period.
The programme is made up of 5 projects:
- Fleming Fund
- Global Antimicrobial Resistance Innovation Fund (GAMRIF)
- UK Public Health Rapid Support Team
- International Health Regulations Strengthening project
- UK Vaccine Network project
Through delivery of each of these projects the programme aims to support ODA-eligible countries to:
- prevent and reduce the likelihood of public emergencies such as disease outbreaks and antimicrobial resistance (AMR)
- detect health threats early to save lives
- provide rapid and effective response to health threats
Outline of Global AMR Innovation Fund (GAMRIF) in relation to the programme
GAMRIF is a £50 million fund that was established to invest in underfunded and neglected areas of early-stage innovative research and development (R&D) for AMR, for the benefit of people in LMICs. GAMRIF is a ‘One Health’ fund that invests in product development research across human, animal and environmental health. This fund supports high-quality research from around the world that has the potential to lead to tangible innovations that will help to prevent, detect and treat drug-resistant infections in resource-poor settings.
GAMRIF’s specific aims are to:
- establish international research partnerships and support research competitions that fund innovation and development of new technologies and interventions to tackle AMR
- leverage investment from other partners and donors to support sustainable financing in AMR R&D
- establish research partnerships using a One Health approach
- fund projects that will develop solutions specifically for LMICs, where the burden of AMR is greatest
Through achieving these objectives, GAMRIF advances the GHS programme aims to prevent and reduce the future burden of AMR in LMICs, while also supporting improved disease detection and response.
GAMRIF portfolio at a glance
GAMRIF responds to a recommendation from the independent 2016 AMR Review chaired by Lord O’Neill, which advocated for a global innovation fund to provide early-stage research funding in neglected areas of AMR R&D. In 2016, DHSC convened an Expert Advisory Group (EAB) to advise on the scientific scope and direction for the GAMRIF funds. Previously, £10 million had been committed to a UK-China bilateral research partnership after securing a bilateral agreement with the Ministry of Science and Technology (MoST) in China, which reinforced the mutual interest of the UK and China in combatting AMR. The scope for this UK-China funding stream was shaped by a bilateral panel of scientific experts. The EAB shaped the scope for the remaining £40 million, aligning where appropriate with the decisions made for the UK-China partnership. It recommended the following thematic areas to be addressed:
- therapeutics in humans
- prophylactics and interventions in human and animals
- the environment, transmission, and water, sanitation and hygiene (WASH)
- diagnostics
- stewardship
Using the advice from the EAB, as well as oversight from the Chief Medical Officer (CMO) for England, the below work packages were developed and partnership arrangements with delivery partners agreed. The EAB determined that additional funds would have been required to appropriately address stewardship, so for this reason stewardship was paused as a distinct work package.
In 2018 to 2019, GAMRIF was also involved in repurposing a programme-wide underspend of ODA funds to contribute to global health security objectives with value for money. This has led to a total of £57 million being committed to date.
As of March 2019, the GAMRIF portfolio includes the following work packages:
Work package 1 (WP1): UK-China - innovation and collaboration to tackle AMR
This is a bilateral partnership between DHSC and MoST to support innovation focusing on unique areas of AMR R&D in human and animal health. Projects are made from a consortia of academic and research partners in both countries. This is co-funded by China and delivered on behalf of GAMRIF by Innovate UK (IUK).
Work package 2 (WP2): accelerating antibacterial innovation with combatting antibiotic-resistant bacteria accelerator (CARB-X)
This investment supports the best scientific research around the world to develop new vaccines and alternative innovations to antibiotics that can be used to prevent and treat drug-resistant bacterial infections in humans. This is delivered on behalf of GAMRIF by CARB-X.
Work package 3 (WP3): InnoVet-AMR - innovative veterinary solutions for AMR with the International Development Research Centre (IDRC)
This investment supports the best scientific research around the world to develop new vaccines and alternative innovations to antibiotics to fight AMR in livestock and aquaculture production. This is co-funded and delivered on behalf of GAMRIF by IDRC.
Work package 4 (WP4): UK-Argentina - tools to tackle AMR in the environment
This is a bilateral partnership between the UK and Argentinean governments to support the best scientific research that will tackle AMR in agriculture and the impact on the environment. This is co-funded by Argentina’s National Scientific and Technical Research Council (CONICET) and delivered on behalf of GAMRIF by the Biotechnology and Biological Sciences Research Council (BBSRC), with support from the Natural Environment Research Council (NERC).
Work package 5 (WP5): innovation in AMR diagnostic tools with the Foundation for Innovative New Diagnostics (FIND)
This investment supports 2 projects. First, a project to develop technological methods to connect point-of-care data to larger database and surveillance systems. Second, a project to develop new diagnostic technologies for drug-resistant gonorrhoea. This is delivered by FIND.
Work package 6 (WP6): new antibiotic treatment for drug-resistant gonorrhoea with the Global Antibiotic Research and Development Partnership (GARDP)
This investment supports the development of a new antibiotic for drug-resistant gonorrhoea. This is delivered by GARDP.
Work package 7 (WP7): vaccine innovation with the BactiVac Network
This investment supports the best research to accelerate the development of vaccines for AMR in humans and animals. This is delivered on behalf of GAMRIF by the BactiVac Network.
Context of this annual review
This is the first annual review for the GAMRIF project and covers the 2017 to 2018 and 2018 to 2019 financial years.
While GAMRIF was created in the 2016 to 2017 financial year, the first year was largely dedicated to strategic conversations to establish the overall purpose of the fund and to explore different potential partnerships. Therefore, 2016 to 2017 has not been included in this review as the objective and method for fund delivery had yet to be established, the business case had yet to be approved and no spend was disbursed.
Activities in 2017 to 2018 were part of the fund’s inception phase and included work to identify delivery partners and work to formalise agreements.
The 2018 to 2019 financial year included project initiation, including finalising agreements with delivery partners and launching the research competitions.
Outline summary of project’s last year annual review
This is the first annual review for the GAMRIF project and therefore there is no summary available for previous years.
Key successes 2017 to 2019
The GAMRIF EAB was established in November 2016 and includes 12 experts from human and animal health, as well as a range of disciplines from economics to R&D. The EAB comes together from academia, private industries and philanthropic organisations. From November 2016 to July 2017, the EAB engaged in a direction-setting process for the fund, starting broad with a high-level prioritisation exercise, and ending with specifically recommending a short list of topics. This included target outcomes and appropriate resource allocation of GAMRIF funds amongst the work packages.
GAMRIF has worked alongside delivery partners to support several key events with the research community to set the scope for research calls, or to create bilateral research partnering opportunities. This included a partnering meeting with researchers in a mission to China (November 2017), the Wilton Park Workshop to inform the scope for the InnoVet-AMR partnership (April 2018), and a partnering meeting with researchers to Argentina (September 2018).
Seven agreements with delivery partners have been completed and signed, covering 8 different research programmes. These agreements commit the original £50 million allocated to GAMRIF as well as an additional £7 million in commitments, largely from repurposing activities that maintain global health security objectives.
To date, GAMRIF has leveraged an additional £41 million for AMR R&D from other donors, which provide complementary funding for research within GAMRIF’s portfolio.
Four research competitions have been initiated and completed (that is, within WP1, WP3, WP4 and WP7), with all individual research projects selected and contracting underway by the end of FY 2018 to 2019. An additional competition is currently underway within WP7. Competitions under WP2 are ongoing on a rolling basis, with contracting underway. An overview of competitions has been included in Annex A.
Forty-nine research projects across human (44%), animal (47%) and environmental (9%) health have been selected. Target innovations include: therapeutics (antibiotics and alternatives to antibiotics); prophylactics (vaccines and alternatives to antibiotics); growth promoter/animal feed solutions (alternatives to antibiotics); diagnostic and surveillance tools; farm waste management; and farm practice.
The GAMRIF team maintains strong cross-government working relationships, and frequently engages with the Department for International Development (DFID), the Department for Environment, Food & Rural Affairs (Defra), the Veterinary Medicines Directorate (VMD), the Department for Business, Energy & Industrial Strategy (BEIS), the Foreign and Commonwealth Office (FCO), the Science and Innovation Network (SIN) and UK Research and Innovation (UKRI). These relationships help GAMRIF and the GHS programme to leverage the expertise and advice of officials in other government departments, while ensuring that the fund’s work isn’t duplicative. These relationships also ensure continuous learning from the experience of other investments (for example, the Newton Fund at BEIS, or the Research and Evidence Division in DFID).
Project management
GAMRIF began as a fairly high-risk project during the set-up phase; however, after agreements were finalised and the project moved into implementation, the risk greatly decreased. As a result, risk ratings are shown below both for the duration of this review (overall average), and the risk rating at the end of this annual review period. This division was recommended by the GHS Programme Board, and reflects the substantial progress made in GAMRIF.
Average risk rating during the annual review period (1 April 2017 to 31 March 2019): Amber (Medium)
Risk rating at the end of the Annual Review period (31 March 2019): Amber/Green (Medium/Low)
Evidence of managing the delivery of project
| Q1 | Q2 | Q3 | Q4 | RAG rating throughout the reporting year | |
|---|---|---|---|---|---|
| 2017 to 2018 | A | A | A/R | A/R | A/R |
| 2018 to 2019 | A/R | A | A/G | A/G | A/G |
Note: The RAG trend is shown in Annex A.
Overview of key delivery management approaches: key points
While GAMRIF began as a fairly high-risk project, receiving an average amber/red RAG rating over 2017 to 2018, the risk decreased substantially with the project ending the 2018 to 2019 financial year with a steady amber/green rating.
Review of RAG ratings in 2017 to 2018
It is helpful to note that the 2016 to 2017 financial year averaged an amber/red RAG rating and ended with this rating. This was downgraded to amber during the first 2 quarters of 2017 to 2018. Over these 2 quarters, substantial progress was made towards selecting and developing an agreement with the delivery partner for WP1 (that is, IUK). In March 2018, the fund also gained approval to proceed from HMT after submission of a 5-case business case. However, due to bilateral delays and challenges in selecting delivery partners for additional work packages, the programme risk increased to amber/red by the end of the financial year.
During 2017 to 2018, project risks were primarily 2-fold. One was the inherent challenge of setting up bilateral research calls. This was largely mitigated through increased communications with IUK and engagement with staff at the British Embassy in Beijing to support the UK-China bilateral call. The second main risk was associated with setting up future research projects in neglected research areas, as it was found that delivery partner options were limited, or there was limited appetite at the time for some research themes (for example, environment and AMR). This was addressed by mapping a wide range of delivery options to understand possible methods to achieve the fund’s priorities.
For areas with limited interest, the team reshaped the approach to fund basic science with a strong link to policy outcomes to ensure translation to meaningful policy products for the local contexts. Taking the advice of the EAB, funds were also balanced appropriately amongst thematic priorities considering the needs of the research themes, as well as the ability to spend based on the capacity of respective global research communities to absorb the GAMRIF funds. For example, £5 million was allocated to the environment work package where there were fewer delivery partners globally, whereas £20 million was allocated for vaccines and alternatives to antibiotics for humans (which contributes to expensive drug development processes). By the end of the year, 6 work packages were established to deliver the GAMRIF priorities.
Review of RAG ratings in 2018 to 2019
Significant progress was made in the 2018 to 2019 financial year to finalise agreements with selected delivery partners. This included seeking ministerial approval, undertaking due diligence assessments, and developing a second bilateral agreement (that is, with Argentina). The risk rating was downgraded in the second quarter when funding allocation was finalised, partnerships were announced and the WP1 call for proposals was launched. This coincided with GAMRIF moving from the inception phase to the implementation phase. By the third quarter, all research calls had been opened,[footnote 1] and the project risk was downgraded to amber/green. This risk rating has been maintained despite internal resourcing shortages in the second half of the 2018 to 2019 financial year, where the team ran vacancies in both the G7 and the HEO posts.
Over 2018 to 2019, the most consistent risk was due to delays in aligning countries to administer the 2 bilateral calls. This was mitigated through regular dialogue with relevant government ministries, either directly or through the local British Embassy. This also meant hiring a local member of staff in the Beijing Embassy to engage in discussions where appropriate with the Chinese MoST.
In summary, this review of project status RAG ratings from April 2017 to March 2019 reflects the challenges of setting up a globally-facing R&D fund, with particular delays due to bilateral calls and research calls in neglected research areas. However, the reduced risk rating reflects that after appropriate set up time, project delivery can become stable.
Evidence of meeting milestones/deliverables: key points
GAMRIF has achieved several key milestones during the initial set-up phase, as well as during the start of the implementation phase. Milestones and project progress have been outlined in the GAMRIF project Logical Framework (‘LogFrame’).This was developed in late 2018 and was submitted and approved by the GHS Programme Board in January 2019. The LogFrame is treated as a living document and targets and indicators may be adjusted as the programme develops. The Annual Reviews will continue to be a good ‘snapshot’ of changes required for the LogFrame over the lifetime of GAMRIF.
Table 1 below includes the narrative progress against indicators that have been delivered in GAMRIF Year 1, 2 and 3, as per targets set out in the LogFrame.
It is also worth noting 2 substantial milestones that were achieved for DHSC during the first 3 years of GAMRIF, which relate to bilateral country agreements. This includes 1 signed bilateral agreement with the Chinese government through the MoST in 2016, which was a required commitment for WP1. Additionally, in 2018 DHSC signed a bilateral agreement with the Argentinean Ministry of Health to underpin the work on WP4. These were major milestones for the GAMIRF project, but also supported the UK’s international diplomatic ties, most notably during the Argentinean G20 Presidency in 2018.
Table 1: progress against GAMRIF milestones and indicators in GAMRIF years 1, 2 and 3.
| Indicator | Year(s) measured | Target | Achieved |
|---|---|---|---|
| Outcome Indicator 2 Research projects are funded that specifically address ‘One Health’ issues in AMR through direct focus on topics within human, animal and environmental health |
Year 2 (2017 to 2018) |
1 human | Complete. When measured at the end of 2017 to 2018, the GAMRIF portfolio included: - 1 human health project - 1 animal and human health project |
| Outcome Indicator 2 Research projects are funded that specifically address ‘One Health’ issues in AMR through direct focus on topics within human, animal and environmental health |
Year 3 (2018 to 2019) |
≥ 18 human ≥ 10 animal ≥ 5 environmental |
Complete. When measured at the end of 2018 to 2019, the GAMRIF portfolio included: - 22 human health projects - 20 animal health projects - 5 environmental and animal health projects - 1 animal and human health project Note: This original target did not include assumptions about projects that would cross categories across the ‘One Health’ spectrum. This will need to be recorded in the future to capture the nature of ‘One Health’ projects but will be challenging to adjust targets accordingly. |
| Outcome Indicator 3 Findings are clearly communicated to global policy makers who work on AMR innovations for the direct benefit of LMICs, through non-academic forms of dissemination (for example, utilizing the forms of communication established by the Fleming Fund and the UK Science and Innovation Network) |
Year 3 (2018 to 2019) |
≥ 1 communication | In progress. The GAMRIF team had anticipated a report from the Wilton Park Workshop to be published during this financial year, which has been delayed. This is due to competing priorities, and because more time was needed to finalise the report than anticipated. New timelines have been set with partners on this report, and it is anticipated to be finalised internally in the coming months with publication expected in June 2019. |
| Output indicator 1.1 The amount of resources, both funding and in-kind, leveraged for AMR research and development with a benefit for people in LMICs. |
Year 3 (2018 to 2019) |
Approx. £11 million (approx. due to conversions) Milestone: Leverage in-kind resources from donors and partner organisations |
Complete. The agreement between IDRC during offered match funding (that is, CAD$1 for every £1), which was approximately £6 million at the time. Argentina also agreed to match the UK’s support of £5 million with in-kind support, upon signing the bilateral agreement. |
| Output indicator 1.2 Number of international-facing partnerships held between GAMRIF and other strategic partners that bring attention towards ‘One Health’ research and innovation in AMR globally, and political leverage for the UK |
Year 2 (2017 to 2018) |
1 new partnership | Complete. In December 2017, a MOU was signed between Innovate UK and DHSC for Innovate UK to deliver the UK-China project for the UK. |
| Output indicator 1.2 Number of international-facing partnerships held between GAMRIF and other strategic partners that bring attention towards ‘One Health’ research and innovation in AMR globally, and political leverage for the UK |
Year 3 (2018 to 2019) |
5 new partnerships Milestone: Partnerships are formed with a diverse array of actors in government and non-profit organisations |
Complete. The target of 5 was exceeded with 6 partnerships agreed during this financial year: - grant agreement signed with Boston University for CARB-X partnership (May 2018) - MOU signed with FIND for connectivity project (May 2018), this was later amended signed in Dec 2018 to include a STI diagnostics project (alongside a separate project between FIND and the GHS’s Fleming Fund) - MOU with DNDi for partnership with GARDP (May 2018) - Grant agreement signed with IDRC (June 2018) - MOU signed with BBSRC (June 2018) - Grant agreement signed with University of Birmingham for partnership with BactiVac Network (Jan 2019) These partnerships include government organisations (for example, UKRI), non-profits (for example, FIND), and crown corporations (IDRC). |
| Output indicator 1.3 Global meetings, discussions and other knowledge sharing opportunities are held and directly supported by GAMRIF, which bring together researchers from LMIC organisations |
Year 2 (2017 to 2018) |
≥ 20 LMIC research partners engaged Milestone: GAMRIF supports 1 international meeting with participants from LMIC research organisations |
Complete. Based on recorded information, approximately 81 researchers from LMICs were engaged in an international event: During this year, GAMRIF supported a partnering mission to China with Innovate UK in November 2017. During this mission, 42 research partners were engaged in a meeting in Beijing and 39 engaged at a meeting in Shanghai. GAMRIF also supported a ‘roadshow’ across the UK to increase awareness of the UK-China collaboration and the opportunity for researchers, with visits to London (Sept 2017), Manchester (October 2017) and Edinburgh (Oct 2017). |
| Output indicator 1.3 Global meetings, discussions and other knowledge sharing opportunities are held and directly supported by GAMRIF, which bring together researchers from LMIC organisations |
Year 3 (2018 to 2019) |
≥ 30 LMIC research organisations engaged Milestone: GAMRIF supports 2 international meetings with participants from LMIC research organisations |
Complete. Based on recorded information, approximately 43 researchers from LMICs were engaged across 3 international events: - a partnering webinar for the UK-China project was also held in May 2018. This engaged 7 researchers from China - Wilton Park Workshop held with IDRC for Inno-Vet AMR Project in April 2018. This event engaged 18 representatives from LMIC institutions - Partnering visit to Argentina in September 2018. This event recorded 18 individuals from Argentina who had confirmed registration, however many more individuals attended the workshop than were recorded |
| Output indicator 2.1 The number of bilateral research projects between researchers from the UK and a bilateral partner that receive funding towards an agreed upon project |
Year 3 (2018 to 2019) |
14 projects | In progress. It was anticipated that 14 projects from bilateral research calls would have been selected and would have received financial support for the first quarter of work. While all 14 projects were selected, due to unanticipated delays, there has been lag in project start time and 2 projects were not expected to start work in the 2018 to 2019 financial year. |
| Output indicator 2.4 Successful research competitions are run and as a result GAMRIF funding for bilateral partnerships is allocated to selected projects |
Year 3 (2018 to 2019) |
7% of bilateral funding disbursed to selected research projects Milestone: Individual research competitions from each bilateral delivery partner have been completed, with all successful projects selected |
In progress. Due to delays (as discussed above), at the end of the 2018 to 2019 financial year, GAMRIF/DHSC had accrued for 3% of bilateral spending. All projects within bilateral research competitions were approved by DHSC for funding at the end of the 2018 to 2019 financial year. |
| Output indicator 3.1 The number of research projects within global initiatives that receive funding towards an agreed upon project |
Year 3 (2018 to 2019) |
21 projects | Complete. At the time of writing the annual review, 27 research projects from global initiatives had been approved for funding by DHSC. |
| Output indicator 3.2 The number of case studies from GIs that directly showcase how GAMRIF funds have supported innovation AMR in a LMIC |
Year 3 (2018 to 2019) |
n/a Milestone: 2 research competitions are run, that provides pump-priming funding. |
In progress. The first pump-priming research call was completed with successful projects currently underway. The second call is currently open and is on schedule for projects to start in 2019 to 2020. |
| Output indicator 4.1 The number of pilot implementations of GAMRIF funded AMR innovations in selected LMIC trial sites |
Year 3 (2018 to 2019) |
3 pilot sites finalised | Complete. Within this output, 5 pilot countries (with multiple sites) were selected. |
| Output indicator 4.2 The number of study reports that share results from product development towards a global audience |
Year 3 (2018 to 2019) |
1 study report 3 diagnostic target product profiles (TPPs) published |
In progress. The study report that was intended for publication has since been delayed to early 2019 to 2020. |
| The delivery partner requested for TPPs to be published in future project years, which was agreed by the GAMRIF team. | |||
| Output indicator 4.3 Funded innovations achieve milestones that advance the product down the commercial pipeline |
Year 3 (2018 to 2019) |
2 new products achieve new milestones | Complete. Three products continued development, and achieved milestones set in their original proposal. The increase in the number of products (to 3 instead of 2) is due to the addition of a grant to FIND during 2018 to 2019. |
Evidence of managing risks: key points
The following are the existing risks on the GAMRIF risk register as of March 2019, which score 12 or above based on their likelihood of occurrence and the impact on the project.
Table 2: GAMRIF risks that are rated 12 or above as of March 2019
| Risk | RAG rating | Mitigation action | Residual risk | Status/update |
|---|---|---|---|---|
| Financial/delivery: current funding profile could become unrealistic, as setting up a research fund takes time and 5 years is short for scientific research. This could lead to slippage in the overall funding profile and research delivery results, which may affect overall HMT ODA target contribution from GAMRIF. | A/R | 1. Work packages have provided clear financial forecasts with current estimates still within budgets. GAMRIF team to work closely with delivery partners to strengthen financial and programme forecasting 2. Agree select work packages on the basis of tranche payments in advance in Q1 and Q3 helping towards the 85% target 3. Ensure the Fund can pursue value for money repurposing options to continue meeting HMT ODA targets as well as global health security objectives |
A | All delivery partners have provided financial forecasts and programme delivery timelines, and the GAMRIF team continues to work with delivery partners to ensure forecasts are accurate as the projects unfold. WPs 4, 5 and 6 were also designed with tranche payments in Q1 and Q3. Finally, GAMRIF has repurposed £6 million since inception towards programmes within GAMRIF’s mandate, with £5 million to FIND and £1 million to the BactiVac Network. |
| Delivery: research programmes are inherently risky, and some science is expected to fail or face delays. This could lead to under-delivery against LogFrame targets and overall GAMRIF objectives to support AMR product development for LMICs. | A/R | 1. The project team should have regular updates with delivery partners to encourage progress and require reporting against milestones to be able to identify slippage early 2. Flexibility and responsiveness is required within the GAMRIF team to avoid or mitigate slippage when the risk is identified 3. LogFrame and results tracking should be designed to capture progress on a range of outputs, not only scientific results |
A | All work packages have provided clear milestones and targets with current estimates still within budgets. The GAMRIF team maintains regular reporting for early notice of changes to expected results. The LogFrame was also designed to capture success in the progress of the programme that is likely to occur regardless of research results (for example, through capturing partnering events). |
| Operational: team members are reassigned from GAMRIF to other departmental priorities due to EU Exit risk, thus impacting GAMRIF’s delivery | A/R | 1. Responsible team leader is having conversations with relevant people to emphasise GAMRIF’s personnel needs for the programme | A | No team members were reassigned in March 2019. However, this remains a risk until a clearer outcome under Brexit is realised. |
| Financial/delivery: bilateral projects that use the Newton Fund model require processes and deadlines to align across countries, which can be challenging. Difficulties could lead to underspends or under delivery across specific bilateral work packages. | R | 1. Work closely with delivery partners, particularly IUK and BBSRC who have experience with bilateral calls 2. Work closely with British Embassies in partner countries, who can help provide information and support the research process 3. Ensure that processes in the UK are established so that research can start promptly when all milestones have been met for project start-up 4. Explore possibility of a no cost extension to allow for late start and delays to projects |
A | The GAMRIF team stays in close contact with delivery partners who are administering bilateral calls, and as a result can make appropriate adjustments to timelines and spend forecasts and seek approvals if needed. The team also has regular contact with appropriate embassies. |
| Financial: delivery partner(s) may be new to working with ODA funds, and may have trouble administering funds within the period of the agreement. This could cause underspends within relevant work packages, with fewer products being developed than originally envisioned. | A/R | 1. Ensure research applicants properly incorporate ODA considerations in their project proposals 2. Support delivery partners to facilitate more applications from a range of researchers that may be LMIC focused 3. Support delivery partners to increase their understanding of ODA and methods of administration 4. Support delivery partner to increase in-house capacity for to oversee ODA funding |
G | GAMRIF has supported its delivery partners to increase their ODA capacity through providing ‘teach-ins’, connecting partners to ODA learning days (including those run by DFID), and through feeding into tools and communications that will be used by the partners during the lifetime of the partnerships. Substantial positive improvements have been seen. |
| Delivery: administering research competitions and monitoring the research requires appropriate staff resources. If delivery partners lack capacity, this could affect timeliness in running research competitions and managing awardees, which could impact overall success of relevant work packages. | A/R | 1. The GAMRIF team should not unnecessarily add processes or towards workloads 2. Where concerns have been identified, and when appropriate, communicate need for increased resource as part of the agreement between GAMRIF and delivery partners 3. Monitor recommendations from due diligence work that support additional hiring |
G | GAMRIF has seen increased hiring where this risk has been raised and will continue to monitor this process, and be aware of workloads among delivery partners. |
| Delivery: products funded by GAMRIF are not accompanied by appropriate stewardship and access plans, and as a result future health innovations are not accessible in LMICs. | A/R | 1. Work with other stakeholders who have interests in this area (for example, Wellcome Trust) to support appropriate stewardship and access planning for research projects 2. Consider how different mechanisms may be used to integrate LMIC considerations, particularly for product access |
A/G |
GAMRIF is engaging with a stewardship and access working group that is developing tools for use by CARB-X-funded projects to support their fulfilment of this contractual requirement. The team has also provided support to GARDP for a workshop on this issue. In addition, GAMRIF is also supporting delivery partners to help researchers engage with experts on regulatory issues. |
Evidence of relationship management with delivery partners and stakeholders: key points
The GAMRIF team has regular meetings with delivery partners, ranging from fortnightly informal calls to formal quarterly review meetings. This has helped the project team to flag risks and issues as they arise and has helped the team to support delivery partners where needed to manage challenges. In most cases, team meetings have been continued with relative consistency, but the team has increased the frequency when there are specific risks that may need mitigation. In addition, the GAMRIF team has taken up opportunities to arrange face-to-face meetings with delivery partners when needed, which has been helpful where working sessions are required or where there are issues that require additional time between the GAMRIF team and delivery partners. The frequency of meetings with the GAMRIF project team and delivery partners are included below:
- WP1: currently as required (can be up to weekly), but in periods where risk mitigation is needed, meetings are regular either on a weekly or fortnightly basis
- WP2: fortnightly
- WP3: fortnightly
- WP4: currently scheduled fortnightly when required, and in less active times meetings are arranged as needed
- WP5: quarterly
- WP6: quarterly
- WP7: quarterly
Scheduled meetings cover both technical and financial needs with additional finance discussions scheduled with individuals in finance departments when required. For example, this could include at the end of the financial year or when additional work is needed to discuss and review forecasts.
In the case of WP2 with CARB-X, GAMRIF also attends Joint Oversight Committee meetings, which are governance meetings with other funders and core CARB-X personnel. These are attended by either the Deputy Director for Global Health Security or the Head of Global AMR Strategy and Delivery (who are both eligible to exercise the GAMRIF vote). Where not possible due to scheduling, the GAMRIF Project Lead attends these meetings and reports back to the 2 voting members.
As GAMRIF moves through the implementation phase, the financial forecasts may become less predictable due to changing research processes and different needs for specific projects. There may be a need to increase the frequency of finance meetings. To assess needs for formal finance meetings moving forward, the GAMRIF team intends to monitor the accuracy of financial forecasts over the first 2 quarters of research project implementation.
Significant changes to the assumptions made in the business case
Key points
When GAMRIF was established, there were key scientific and investment criteria that were developed by the EAB. These formed the guiding principles that have since provided direction for GAMRIF’s management. It is important to note that these recommendations remain for GAMRIF and continue to provide overarching guidance for project-level changes or decisions. These assumptions are outlined below:
Scientific criteria
- topics must directly address the development, transmission and management of drug-resistant infections
- individual projects must be directly and primarily relevant to needs of people in LMICs (that is, ODA-eligible)
- the scientific topics must focus on areas which have been neglected by other public and private funders
- projects must focus on innovation that creates meaningful new products or processes – this is not for iterative or duplicative research
- the portfolio as a whole – and, where appropriate, individual work packages – should take a ‘One Health’ approach
Investment criteria
- work packages should complement not duplicate other UK Government projects, generating synergies where possible
- projects should seek to leverage (or have the potential to leverage) funding from other international donors
- where possible, eligibility for funding should be as "global" as possible with investment made in the best research in the world, irrespective of location
- separate work packages should target specific outcomes or "challenges" – rather than a single broad investigator-led competition
- where possible, funding should be available and accessible to underfunded researchers including small and medium enterprises and researchers in LMICs
Since the business case was developed, GAMRIF has largely maintained the same trajectory as approved. However, there have been a 2 key changes: the increase in work packages or projects and the suspension of the project board.
First, GAMRIF has engaged in repurposing activities that support the GHS programme in meeting financial targets in a cost-effective, value-for-money manner. This resulted in a new £5 million grant to FIND to develop diagnostics for drug-resistant gonorrhoea over 2 years (a new project in WP5), and £1 million to the BactiVac Network over one year (a new WP). This advances GAMRIF’s specific objectives, while supporting ODA financial targets and the health security mandate across GHS.
Second, as GAMRIF has moved into the implementation phase, it was found that the project board was no longer the most appropriate governance mechanism. This board was created to provide guidance on key decisions for the GAMRIF project team during the initiation and set-up of grants, which was achieved. As the project moved into implementation it was found that a different mechanism(s) would be needed which responds to the varied delivery approaches across the 7 work packages. Alternatives are being explored by the GAMRIF team with guidance from the GHS Programme Board and the programme’s senior responsibility owner.
Project management: list of recommendations for reporting year
1.i Review Logframe indicators and milestones following this Annual Review, to ensure that indicators are appropriate for the work packages as well as the data already collected, ensuring that indicators and milestones can be tracked on a regular basis
1.ii Monitor the accuracy of financial forecasts from 1/4/2019 to 1/10/2019, and reassess if more frequent, formal finance meetings with delivery partner(s) are required
1.iii Develop appropriate project-level governance where this does not currently exist for GAMRIF work packages during the implementation period. Initiate the chosen governance structure by the end of Q2 2019 to 2020
Finance
Risk rating: Amber (Medium)
Evidence of meeting ODA funding eligibility
Key points
During GAMRIF’s set-up phase, each MOU or grant agreement with delivery partners was developed to include specific text that recognises that the funding provided by DHSC is strictly classed as ODA. This requires delivery partners to make reasonable efforts to ensure that the funds disbursed from GAMRIF are clearly used for activities that can be classified as ODA-eligible according to the rules laid out by the Organisation for Economic Cooperation and Development (OECD). These grant agreements also require delivery partners to alert the GAMRIF team should a risk arise that would cause funds to be spent on non-ODA-eligible activities.
This has proven to be a useful text as it has allowed the GAMRIF team to explore and mitigate potential risks for ODA funding, particularly in the areas of early-stage R&D where it is more challenging to link the scientific outputs with specific benefits to LMICs. Examples of mitigations that have been applied include:
- requiring ODA justifications from projects and research funding applicants to explicitly describe the ODA compliance of the proposed research
- requiring or encouraging the participation of LMIC partners to help ensure the project remains context-specific and of direct benefit to LMICs and to support resource-strengthening and capacity-building within in-country research institutions
- including ODA eligibility in the scope of research calls, and subsequently as a part of the scientific evaluation criteria for independent reviewers. This ensures downstream funding remains ODA-eligible
Furthermore, due to the diversity of GAMRIF delivery partners, the funds (irrespective of funding source, including non-DHSC funds) disbursed by some delivery partners would be classified as ODA funding in their entirety due to the nature of the organisation or design of the call. This includes organisations that exclusively work in the development space. These partnerships have required less support in terms of ODA compliance. In parallel, other work packages have required more oversight to ensure ODA compliance. As GAMRIF partnerships were set up to be diverse and to support research in under-funded areas of AMR R&D for LMICs, by nature there are only a limited number of possible delivery partners. GAMRIF has worked closely with some partners to ensure that projects are ODA eligible, specifically where this is a new consideration for the deliver partner.
For example, the GAMRIF team has worked closely with the CARB-X executive and delivery team to develop their internal capacity to assess initial and on-going ODA compliance of GAMRIF-funded research projects to demonstrate their ODA eligibility. CARB-X’s mandate is to advance global innovations to combat bacterial infections, and the organisation works across global markets, not limited to LMICs. An unintended impact of GAMRIF funds has been new opportunities for product developers to research solutions specifically for LMIC settings. The GAMRIF team will continue to support CARB-X in improving processes and ensuring the ongoing ODA eligibility of selected projects as they are executed. As these are not traditional markets for most product developers, it is likely that without GAMRIF funding, these considerations would not have been specifically addressed otherwise.
To ensure that ODA fund disbursement remains consistent with the OECD guidance, the GAMRIF team has worked with the CARB-X team to increase their capacity to attract and fund ODA-eligible projects, and more broadly to increase ODA knowledge across the organisation. For example, work included considerations on how ODA funding opportunities are advertised to potential CARB-X grantees, the joint development of an ODA justification form as a part of research applications, the appointment of a CARB-X ODA consultant, and presentations and webinars about ODA development for potential applicants. It is expected that GAMRIF will continue to support this process moving forward.
GAMRIF has also supported wider delivery partner learnings on ODA. A GHS ODA Transparency event was hosted on July 2018, where GAMRIF partners were invited to learned more about ODA eligibility, and specifically International Aid Transparency Initiative (IATI) requirements. While GAMRIF has progressed its own IATI reporting, the GAMRIF team has been considering how to best support delivery partners in improving their own IATI requirements as they begin to disburse substantial funding to researchers during the implementation phase on DHSC’s behalf.
Evidence of meeting the target given by HMT on annual returns
Overview of how the project is contributing towards the cross-government requirement to spend 0.7% of GNI on ODA-eligible activities each calendar year
Following some very early changes to the project’s shaping, GAMRIF had no programme budget initially allocated for the 2017 to 2018 financial year. However, the 2017 to 2018 financial year required a small payment of £0.8 million for WP1 as a part of project initiation, which was allocated from departmental ODA funding underspend.
GAMRIF’s 2018 to 2019 HMT agreed budget was £4 million. Spend against this budget is 98%.
Table 3: GAMRIF spend against profiling for 2018 to 2019 financial year, and expenditure against an original 2018 to 2019 HMT £4 million budget
| Profiled as per business case* (£m) | Actual end of FY, including accruals (£m) | Variance against profile (£m) | % spend | |
|---|---|---|---|---|
| WP1: UK-China | 1 | 0.57 | -0.43 | 57% |
| WP2: CARB-X | 2 | 0.64 | -1.36 | 32% |
| WP3: IDRC | 0.4 | 0.27 | -0.13 | 68% |
| WP4: UK-Argentina | 0 | 0.14 | 0.14 | 114% |
| WP5: FIND | 1.5 | 1.04** | -0.46 | 69% |
| WP6: GARDP | 1 | 1 | 0 | 100% |
| WP7: BactiVac | 0 | 0.25** | 0.25 | 125% |
| Total | 5.9 | 3.91 | -1.99 | 66% |
* As per recommended practice followed by the GHS programme, the GAMRIF budget was over-profiled to compensate for the risks of likely underspending across the GHS and wider DHSC ODA budget during the financial year.
** Includes funding repurposed from underspend elsewhere in the GHS portfolio, which ensures cost-effective spending within health security objectives.
Key points
While GAMRIF was officially launched in the 2016 to 2017 financial year and the business case was approved in March 2018, no programme budget was allocated from HMT until the 2018 to 2019 financial year. This was the result of funds being ‘re-allocated’ back to HMT following a reduction in GNI, and an overall change in the cross-government ODA spending target. This also shifted the GAMRIF budget forward 1 year to allow necessary time to set up the GAMRIF portfolio as a part of the expected inception phase and ensure the strongest scientific scope and full ministerial support. The 2017 to 2018 financial year had a small overspend due to the start of the partnership with IUK within WP1, and as a result of no funds being profiled for this financial year. All other GAMRIF spend to date has been made during the 2018 to 2019 financial year.
In 2018 GAMRIF had the opportunity to review the portfolio and explore additional opportunities for investment with existing and new partners in order to support GHS’s obligation to support the UK Government requirement to spend 0.7% of GNI on ODA-eligible activities. This ensured that underspend from elsewhere in the GHS programme could be reallocated to valuable global health security initiatives, representing good value for money. In 2018, GAMRIF committed an additional £5 million to FIND over 2 years to develop diagnostic tools for drug-resistant gonorrhoea. GAMRIF also agreed a £1 million grant over 1 year to the BactiVac Network, which supports bacterial vaccine research. From this grant, £0.25 million was paid in 2018 to 2019, with £0.75 million remaining for the 2019 to 2020 financial year.
In 2018 to 2019, GAMRIF spent 98% of its budget; as previously discussed this included repurposed programme-wide funds from underspent budgets in the wider GHS portfolio to FIND and BactiVac. It is also important to note that spend profiles with individual delivery partners were developed after the business case was approved by HMT. Therefore, spend profiles with individual delivery partners may have been readjusted within the yearly spend profiles as per final agreements. Overall, there have been underspends or overspends of over 10% when compared to the business case in some work packages:
- in WP1, the £0.43 million underspend was due to delays in timelines with the UK/China research call
- in WP2, GAMRIF funding is allocated across CARB-X calls for projects that are in-scope in terms of their technical focus and ODA eligibility. Fewer projects than expected emerged in the first year of this partnership, resulting in an underspend of £1.36 million when compared to the business case. However, it is important to note that the final MOU spending profile differed, with the spend in this financial year expected to be £1 million instead of £2 million. This means the underspend was smaller. As such, mitigating actions have been taken with the delivery partner that are intended to increase the number of quality ODA-eligible applicants, and substantial work is underway to increase the reliability of spending projections. Furthermore, several projects receiving GAMRIF funding are expected to begin in the first half of 2019 to 2020
- while Table 3 shows an underspend against the profile for WP3 (£0.13 million) and an overspend for WP4 (£0.14 million), funding disbursement remains on track with the agreed payment schedule in the grant agreement/MOU with the delivery partner. Spending profiles for both WP3 and WP4 differ slightly from the budget set out in the original programme business case, which was approved before the spending profiles were finalised with the delivery partner.
- WP5 remains on schedule with the agreed payment schedule in the grant agreement with the delivery partner. The deviation (£0.46 million underspend) from the spend profile reflects a different payment schedule than originally predicted in the programme business case.
- WP6 spent on budget which was expected given the nature of the 12 month grant to GARDP. WP7 shows as a £0.25 million ‘overspend’ due to the development of this agreement occurring after the original 2018 to 2019 profile being set, however spend was to profile.
The 2017 to 2018 and 2018 to 2019 financial years also included salary costs for a project officer based in the British Embassy to Beijing to support to development of the UK-China partnership. Due to the nature of this work, it was funded by programme funds.
Finally, GAMRIF has worked closely with partners to develop, improve and implement financial forecasting processes. This allows GAMRIF to be reactive to changing budget needs. Moving into the 2019 to 2020 financial year, strong forecasting will become even more important as individual research projects fully launch and may encounter unforeseen changes that affect work package budgets. This will be a priority for both the GAMRIF team and delivery partners, as GAMRIF will be operating with a larger budget of £15 million per year for the next 3 years (that is, in 2019 to 2020, 2020 to 2021, and 2021 to 2022) and accounting for the associated larger grant disbursements.
Evidence of progress and actions to meet IATI transparency standards
Our self-assessed score as of March 2019 against the IATI transparency standards was 80 to 100% (very good).
Key points
Starting in January 2019, the GAMRIF team has made considerable strides to improve IATI transparency standards and is working to achieve a 65% target at a minimum within the first half of the 2019 calendar year. It should be noted that the GHS programme-wide transparency self-assessment currently indicates an average score of 55% (54%-57%) for the 6 work packages for which GAMRIF has already published IATI data. For context, the GHS self-assessment average score is currently 61%, with a minimum of 48% and a maximum of 79%. The team continues to build on these initial efforts, undertaking several steps with the goal of ensuring a rating of 65% or higher across all work packages. This includes the following work:
- before 2019, none of GAMRIF’s projects had been published to IATI. The first publication to IATI was undertaken in February 2019. This included descriptive data on WPs 1 to 6, including organisational, financial, location and sector information for each as well as their key objectives and approaches
- the GAMRIF team is in the process of publishing the programme business case as well as MOUs/grant agreements with delivery partners. The aim of this work is to improve project transparency and to a standard that would receive an IATI score of 65% or higher. GAMRIF efforts will be independently scrutinised as part of the upcoming ‘Publish What You Fund’ exercise later in 2019
Finance: list of recommendations for reporting year
2.i Continue to work closely with CARB-X to support their ODA eligibility processes and to increase quality applications that fall within the GAMRIF scope, scaling up efforts as needed to achieve spending targets
2.ii Strengthen financial forecasting processes to ensure that processes are robust and can provide timely data on changes expected to the budgets for GAMRIF research projects
2.iii Ensure appropriate coverage of published documents and data across GAMRIF portfolio, and that the quality of public documentation is sufficient to achieve a score of at least 65% against the IATI standard
2.iv Increase partner awareness of IATI standards and available resources to encourage partners to publish their data on Aidstream
Theory of change
Risk rating: Green (Low).
Evidence to show theory of change indicators
Key points
Specific indicators for GAMRIF are outlined in the programme LogFrame (Section 2). However, the GAMRIF theory of change looks at the activities, outputs, outcomes, and impact of the project. While these are not measured as specific indicators, GAMRIF has progressed along the expectations of the theory of change, as outlined below. The full theory of change is included in Annex B.
From April 2017 to March 2019, GAMRIF has progressed along the activities, outputs and outcomes in the theory of change. Assuming correctness of this theory of change, this means that GAMRIF is on track to contribute to the intended impact.
Progress to date against activities
Research and development: Research projects are funded to conduct innovative research across ‘One Health’ approaches to AMR
To date, the GAMRIF portfolio includes 5 research projects that investigate environmental health, 26 projects that investigate animal health, and 24 projects that include human health (with some of the projects falling into more than one of these categories). The research calls also integrated a focus on innovative science, which has supported a cutting-edge portfolio.
Engagement and policy: Research projects funded in collaboration with international partners
GAMRIF’s international funding partners for competition-specific projects include China’s MoST, Wellcome Trust, US Department of Health and Human Services Biomedical Advanced Research and Development Authority (BARDA), Germany’s Federal Ministry of Education and Research (BMBF), Bill & Melinda Gates Foundation, Argentina’s National Scientific and Technical Research Council (CONICET) and Ministry of Health and Canada’s International Development Research Centre (IDRC). Some of GAMRIF’s delivery partners are also funded by other governments and organisations that provide them with core funding, making the overall programme of work possible (for example, additional funders to FIND or GARDP).
Progress to date against outputs
Encouragement of international partners to research innovative concepts tacking AMR in LMICs
Innovative concepts addressing AMR have been central to GAMRIF’s overall portfolio, for example through finding a partner to pursue environmental health, an area that has historically held low interest for funders. Within calls, GAMRIF has also encouraged researchers to demonstrate their scope for innovative science. For example, the UK-China call included traditional Chinese medicines (for example, using traditional Chinese medicines and herbs in drugs) in the scope.
High-quality research that aims to reduce the need for antibiotics through alternative medicines and vaccine development
The scope of WP2 is vaccines and alternatives for humans, and the scope of WP3 is vaccines and alternatives for animals. This is also included in the scope of WP1. That call is, however, wider than alternative medicines and vaccines, with the call also including diagnostic technologies. WP7 also provides funds exclusively towards bacterial vaccine research.
Reduce the use of antibiotics in farming of food- producing animals
WP1 will support the reduction in the need for antibiotics through researching growth promoter alternatives to be used in animal feed, as well as through diagnostics and vaccine research.
WP3 aims to develop innovative veterinary solutions focusing on product development, and to reduce the therapeutic (prevention and control) and non-therapeutic (growth optimisation) use of antibiotics in terrestrial and aquatic animals being raised for food, while still protecting animal health and welfare. The final portfolio will also investigate livestock and aquaculture farming contexts.
All projects under WP4 consider farm practice. The first project in WP5 on diagnostic connectivity also considers animal health in the technology development to support better clinical decision making for antibiotic use in animals.
Reduce the environmental pollution of resistant bacteria to antibiotics
WP1 will support the reduction in the need for antibiotics through researching growth promoter alternatives to be used in animal feed, as well as through diagnostics and vaccine research.
WP3 aims to develop innovative veterinary solutions focusing on product development, and to reduce the therapeutic (prevention and control) and non-therapeutic (growth optimisation) use of antibiotics in terrestrial and aquatic animals being raised for food, while still protecting animal health and welfare. The final portfolio will also investigate livestock and aquaculture farming contexts.
All projects under WP4 consider farm practice. The first project in WP5 on diagnostic connectivity also considers animal health in the technology development to support better clinical decision making for antibiotic use in animals.
Improved measurements of clinical data and its uptake into national level surveillance
WP5 will support improved clinical decision-making, as well as improved connectivity to national surveillance systems. The development of diagnostic tools for drug-resistant gonorrhoea will also improve accurate diagnosis, and therefore more effective treatment, of this disease.
Progress to date against outcomes
International focus and funding in tackling AMR in LMICs through research and development is increased
To date, DHSC (through GAMRIF) has allocated up to £57 million towards international research and product development to tackle AMR in LMICs. The programme has also leveraged an additional £41 million for this work from other funders. The partnership with Argentina also supported the UK Government to hold discussions on the importance of AMR ahead of the G20 meeting in Argentina in 2018.
Innovative solutions tested and moved up Technology Readiness Level through the R&D Pipeline
This has not been measured at this point in the project but will be captured going forward (for example, in the Performance Management Framework under WP3).
Improved supply of appropriate and affordable products & tools for combatting AMR available to LMICs
It is too early in the programme to provide data on specific outcomes. However, GAMRIF continues to support the principle of access to affordable innovations for people in LMICs. For example, GAMRIF has been supporting the access and stewardship planning that is a contractual requirement for all CARB-X product developers. Additionally, the InnoVet-AMR project was designed to require researchers to develop commercialisation plans for LMIC markets.
Behaviour change in industry and clinical practice in LMICs from: research evidence into economic incentives, food security evidence and clinical trial rollouts
The GAMRIF portfolio supports projects that are implementing clinical trials in LMIC settings, as well as supporting research that may produce results around food security evidence (for example, transmission in farming environments, through WP4).
While GAMRIF does not expect to fund any research, evidence outputs on economic incentives directly, it does support the work of DHSC in this area where appropriate, particularly through the team leader’s (Head of Global AMR Strategy & Delivery) shared role which spans GAMRIF and DHSC’s AMR diplomacy work.
The research produced due to GAMRIF funding has the potential to impact behaviour change in industry and clinical practice (for example, through improving investment into LMIC product development or antibiotic stewardship), but the work funded through GAMRIF could also affect a wider group of stakeholders such small-scale farmers. This output is correct but could use broader language to capture other stakeholder groups.
Impact
The intended impacts for the GAMRIF project include:
- prevention and reduction of the likelihood of public health emergencies and AMR
- early detection of threats in order to save lives
As the intended impact from the overall GAMRIF theory of change, these impact statements remain accurate and continue to be the aim of the overall GAMRIF project.
Evidence to show project’s theory of change assumptions remain accurate
Key points
The GAMRIF theory of change made the following assumptions:
1. Budget available to fund research requirements
The budget allocated to GAMRIF from HMT remains available for the lifetime of the project, which is not expected to change. However, in order to avoid a dip in activity and any loss in momentum, it will be important for the project team to integrate final project years with an overlap in future Spending Review-funded programming.
There has also been an increase in GAMRIF-programmed budget for the current Spending Review period due to ODA-fund repurposing, to ensure funding remained directed towards health security investments (for example, a second project in WP5). It is expected that funds will remain available for these commitments, however this assumption remains important for GAMRIF’s success. In the unlikely chance that funds committed through repurposing activities become unavailable, this would prevent activities from occurring and could have serious reputational damages.
2. Research proposals received meet expectations of content and standard
This assumption is still correct and has largely been realised. Barring WP2 and WP7, all the calls in the GAMRIF portfolio have now closed, with research projects selected by high-quality scientific peer review processes and with ODA assurance undertaken by delivery partners and the GAMRIF team. The only outstanding calls are for an additional round of pump-priming projects under the WP7 with the BactiVac Network and new CARB-X funding round(s) (WP2). Early indications suggest a sufficient number of proposals will be received. For all calls that have been completed in 2018 to 2019, there were more proposals received than funds available, meaning that the best available high-quality projects with innovative science were selected for GAMRIF funding.
3. Suitable process put in place for administering, delivering and monitoring grants
This continues to be an assumption under the GAMRIF theory of change. Ensuring appropriate processes has been a key part of GAMRIF delivery since the project’s inception. The team will continue to improve these processes between GAMRIF and delivery partners, as well as through liaising with other teams in DHSC (e.g the Grants Hub) to ensure processes remain suitable.
4. Global institutional (including WHO) leadership on agenda and funding
5. Additional funding committed and invested from new donors
The above 2 assumptions remain relevant. GAMRIF supports the UK’s continued leadership to advocate for action against AMR, as renewed in the publication in January 2019 of the most recent National Action Plan on AMR (2019-2024). However, GAMRIF’s £50 million contribution is much smaller than required by the field. The 2016 O’Neill review called for US$2bn per year, and a funding landscape near this amount requires sustained global leadership on AMR. With the high cost of R&D, GAMRIF needs to continue to share project costs with other funders, as the potential to fund much-needed innovative science with a wide range of projects would greatly decrease without additional funding from other sources.
6. Researchers develop access, affordability and stewardship plans
This remains an accurate assumption. Appropriate stewardship and access (S&A) plans (where affordability is embedded within access) for antimicrobial products is challenging, with little public information available on existing S&A plans due to commercial confidentiality. It will be important for GAMRIF to remain active in these conversations to ensure that plans balance company and LMIC needs. GAMRIF is currently engaged in working groups on these topics with delivery partners.
7. Research gained is reliable and sufficient to progress through the Technology Readiness Level (TRL) Pipeline
This assumption remains important to produce expected outputs. To ensure that the science in the GAMRIF portfolio has potential to progress across TRLs, all research competitions utilised technical expert panels to support selection of strong science that will meet this assumption.
8. Evidence found is appropriately shared and accessed across the field
This assumption also remains. Results will not have been realised at this stage of the GAMRIF project. However, GAMRIF has encouraged early planning for knowledge-sharing of both positive and negative results (for example, the Performance Monitoring Framework for WP2 measures publications) and obligations to make these results available in open-access format have also been embedded where possible in grant agreements.
9. Methods developed are realistically feasible and affordable for LMICs
As with Assumption 6, this assumption is also important for GAMRIF to achieve the intended outcomes in the theory of change. Engaging key LMIC stakeholders in S&A planning, as well as commercialisation plans, should help realise this assumption.
10. LMICs remain stable and engaged, and ensure tools and advancements are utilised
This is an important assumption for any programme that engages with LMICs (or realistically any other country). Instability or limited engagement of partner countries or countries of impact has the potential to destabilise research projects, affect funds available towards research, or impact in-country implementation of tools and advancements. GAMRIF will need to continue to monitor stability and consider mitigations should evidence of country instability or poor engagement arise. To promote engagement, GAMRIF will leverage the benefits of having a single team leader across the project and the AMR diplomacy work. This enables support to be provided to researchers through introductions to in-country teams, either delivering the Fleming Fund or as part of the FCO/BEIS Science and Innovation Network.
11. Improved control of drug resistance infection
12. Slowing of emergence and transmission of drug resistance infections
13. Improved food security both on national and global scale
The above 3 assumptions remain important to the desired change from the GAMRIF project, but are not isolated to the project alone. Better control and reduced transmission/emergence of drug-resistant infections, as well as improved food security, relies on progress across global public health and the agri/aquaculture sectors that extends beyond GAMRIF’s scope.
Theory of Change: lst of recommendations for reporting year
3.i Develop an approach for measuring TRLs or advancement of innovations across the ‘One Health’ portfolio
3.ii Update the theory of change behaviour change outcome to more accurately reflect areas where GAMRIF is expected to affect behaviour change
3.iii Work across HMG and with external partners to ensure that research and products will be accompanied by appropriate plans/activities to support access in LMIC settings. This includes ensuring that conditions regarding stewardship, access, commercialisation and policy translation will be upheld
3.iv Ensure stronger alignment of the GAMRIF theory of change with the LogFrame, to include the comparable impact and intermediate outcomes of the GAMRIF project
External engagement
Risk rating: Amber/Green (Medium/Low)
Evidence of use and success of the communication strategy
Key points
As of March 2019, GAMRIF has an early version of a communications plan in draft. Progress was paused on the basis of feedback from the GHS Programme Board, which requested an initial focus on programme-wide rather than project-specific communications strategies. As general practice, the GAMRIF team has adopted an approach to amplify and support partner communications as well as generating its own content where appropriate.
The GAMRIF team has been actively involved in partner communications to support press releases in the writing as well as circulation. Partner press releases are often published in support with news stories on GOV.UK. From April 2017 to March 2019, GAMRIF was involved in 5 communications through GOV.UK (for details see Annex A), while working with all partners to support much wider communications; for example, through producing a joint press release with FIND, hosted on their website.
To build its profile, GAMRIF remains active on the GHS twitter account, for example by sharing stories and successes from delivery partners and providing updates on events where GAMRIF team members are in attendance.
During the second half of the 2018 to 2019 financial year, it became increasingly apparent to the project team that GAMRIF did not have sufficient visibility across the research community. This suggests the approach to ‘amplify and support’ falls short for GAMRIF. More public information and active branding is required to ensure that GAMRIF is known within the AMR research landscape, and that achievements are shared and attributed to the DHSC-led funding. This is important for many reasons, but notably to recognise the leadership that GAMRIF/DHSC has brought in previously underinvested areas of R&D, to ensure visibility for the unique research projects within the GAMRIF portfolio, to support their development into the future, and to ensure that the project remains collaborative and aligned across the research landscape.
As a result, the GAMRIF team is committed to enhancing its strategy to increase GAMRIF’s visibility. This will increase both international stakeholder and the UK public’s understanding of DHSC’s work in ODA-funded R&D through GAMRIF. The team has been working with the DHSC communications team to develop a GAMRIF webpage on GOV.UK, as well as to have a GAMRIF logo approved. These are both key foundations for communications and branding, and the team will consider how these, among other communications tools, could be built and launched over the 2019 to 2020 financial year.
Furthermore, in the process of developing this annual review, the team has also learned that better communication indicators and tracking processes are required to properly record the communications activities in which GAMRIF has been involved. As such, the team will look to develop a communications monitoring process as a part of a larger strategy. An enhanced communications strategy with an evaluation plan could also help the GAMRIF team understand the effectiveness of different communications to best direct efforts in the final 2 years of the initial GAMRIF portfolio.
Evidence of external engagement
Overview of engagement across stakeholders.
GAMRIF’s key stakeholders are divided as per the following sections.
Delivery partners
GAMRIF’s delivery partners are integral to the project’s success, and therefore GAMRIF strives to maintain strong, mutually-beneficial relationships with them all. Formal relationship management with delivery partners has been described in Section 4. However, there are also broader and more informal engagement methods to maintain positive and productive working relationships with partner organisations, and to support their wider business needs where appropriate. A good example of this is where GAMRIF can use its convening powers to create links between networks with similar interests.
The GAMRIF team has supported several events in partnership with delivery partners in the past, which have been necessary to advance GAMRIF objectives, but also help to increase visibility for GAMRIF and delivery partners. From April 2017 to March 2018 these included a scoping and partnering mission to China alongside Innovate UK (IUK), a scoping meeting with IDRC, and a partnering mission to Argentina with BBSRC. In addition to events directly related to GAMRIF work packages, GAMRIF team members also remain active in attending wider AMR events and academic conferences to maintain contact with delivery partners and other stakeholders through face-to-face meetings. These events also come with strong learning opportunities. Some examples of these events include the BactiVac Annual Networking Meeting, a Human and Animal Antimicrobial Resistance and Vaccines Workshop hosted by IUK, the 12th Berlin Conference on Life Sciences Novel Antimicrobials and AMR Diagnostics 2019, among other events.
Strategic stakeholders
Along with its own funding, GAMRIF was developed with the aim to leverage additional investment into AMR R&D through interacting with international organisations, partnerships and global fora. As a result, GAMRIF has relationships with a wide range of strategic and international stakeholders across the AMR R&D landscape. GAMRIF maintains routine engagement with strategic stakeholders such as: the AMR research funders across the UK landscape through the Medical Research Council (MRC)-hosted AMR Funders’ Forum; other funders such as Wellcome Trust and the Bill and Melinda Gates Foundation; strategic external stakeholders such as the Chair of GAMRIF’s EAB as well as other EAB members (particularly in the first 2 years of the GAMRIF Project); and strategic stakeholders across Government such as the Chief Medical Officer for England Dame Sally Davies, or the Veterinary Medicines Directorate in Defra.
The GAMRIF project has been resourced with a team leader (Head of Global AMR Strategy & Delivery) posting that is responsible for overseeing GAMRIF, as well as the UK’s international AMR diplomacy activities. As a result, GAMRIF’s core objectives have been represented in diplomatic and strategic discussions when relevant. This includes within the Global AMR R&D Hub and at Call to Action events held in both Berlin (2017) and Accra (2018). These inter-governmental, inter-organisational and multilateral fora can be leveraged as appropriate. By design, this maintains GAMRIF’s strategic stakeholder relationships in an efficient manner.
Cross-government stakeholders
As a ‘One Health’ fund, GAMRIF has extensive working relationships across government. This includes:
- DFID, which has been represented on the GAMRIF project board
- Defra, due to close working relationships with the Veterinary Medicines Directorate on animal health and product regulation
- BEIS, which has extensive experience with bilateral research partnerships through the Newton Fund, and has provided guidance for GAMRIF’s bilateral partnerships
- UKRI, as GAMRIF delivery partners include IUK, BBSRC and the Natural Environment Research Council (NERC). GAMRIF has also been an active member of the MRC-chaired AMR Funders Forum
Within DHSC, GAMRIF holds close working relationships with other projects in the GHS programme (for example, The UK Vaccines Network and Fleming Fund), the Global Health Research programme, as well as the domestic AMR team. These relationships ensure continuous learning, and synergies within the department.
Research community
The research community is a critical area of engagement for GAMRIF. GAMRIF has maintained attendance at academic conferences which ensures that the team continues to have a strong knowledge of the research landscape, which is critical to understand what remains to be ‘neglected’ or ‘underinvested’ areas of AMR R&D. This also ensures that GAMRIF can scope out potential collaborations and opportunities to leverage further funding for the future.
GAMRIF has also presented at academic conferences to increase awareness of the project and the research portfolio, such as at the_UK & International Veterinary Vaccinology Network Conference 2019_, where GAMRIF presented the initial results of the InnoVet-AMR portfolio. Through these event attendances, GAMRIF maintains close connections with research institutions, academia, UKRI and other stakeholders in the research community. Between April 2017 to March 2019, GAMRIF has attended over 20 events with the research community, both nationally and internationally. This extensive engagement is made possible through the Scientific and Research Project Coordinator posting, with this individual also routinely feeding into strategic discussions on AMR across GHS and DHSC due to technical and research landscape knowledge.
Industry stakeholders
GAMRIF was developed to engage with industry to support tangible solutions for AMR. This engagement is largely via delivery partners who fund industry partners (for example, CARB-X and Innovate UK). However, industry partners are frequently in attendance at events with the wider research community, as discussed in the above paragraph and the Head of Global AMR Strategy and Delivery attends the UK Government-Industry AMR partnership conversations. GAMRIF’s attendance at these events allows the team to remain aware of the work by and views of industry in AMR R&D.
Public audience
As discussed in Section 12, GAMRIF remains active through the GHS twitter account and has released 5 GOV.UK public communications, while supporting the news releases of partner organisations. These are the primary ways that GAMRIF has engaged with the public. As results from individual research projects become available in future years, GAMRIF may be able to garner some public interest through blogging, video stories, vignettes, and other media. This could help increase public awareness for AMR, better represent LMIC stakeholder needs in product development and advocate for issues such as S&A.
External engagement: list of recommendations
4.i Publish a GOV.UK landing page and a GAMRIF logo for early 2019 to 2020
4.ii Formalise a communications strategy for the GAMRIF project that will guide systematic communications and engagement activities up to the end of the programme in 2021/22, including a communications monitoring strategy and branding strategy
4.iii Leverage research results as appropriate for public interest stories to:
a) continue advocating for increased donor investment into AMR
b) increase public knowledge to maintain political momentum and support stewardship for new technologies and innovations for AMR
Lessons identified
Key points
A common theme across this annual review is the importance of strong relationship management and engagement with delivery partners and key stakeholders (for example, other funders or points-of-contact in British embassies). Regular meetings with delivery partners have been critical for risk management, to discuss and improve financial forecasting, and to collaborate on activities such as events with the research community. Good engagement with other stakeholders also ensures that GAMRIF has a voice in larger discussions that could impact the success of work packages, but also influence factors that could affect the project’s theory of change.
Conducting this annual review has required the GAMRIF project team to compile information from the past 2 years, and in many cases has shed a light on areas where information management and data tracking could be improved. This review has also helped the team to pinpoint how efforts to improve the project’s knowledge management should be best directed to strike a balance between recording important information without becoming overburdened by this effort. This has also helped the GAMRIF team to improve the LogFrame, by realising where targets were under-achieving or not reflective of earlier years in the programme. As GAMRIF refines programme monitoring, the team will review this document with a critical eye to ensure the most important data is captured that will support measurement of the project outcome.
As this is the first annual review for GAMRIF, this has been an opportunity to reflect about the structure of the fund, and how that impacts both challenges and successes. The mix of bilateral partnerships, global initiatives, and product development partnerships (PDPs) across work packages has been advantageous for building in some flexible arrangements with delivery partners, as well as varying GAMRIF-team resource demands across the different types of partnerships. Working across bilateral partnerships and with partners who are new to administering ODA funding has created some of the largest resource demands and is linked with the spend profile. However, these partnerships are expected to produce some highly innovative projects. This is a learning on the time required for project design and initiation, should GAMRIF seek similar partnerships in the future.
This review also highlights the importance of good engagement and communications, and that this needs to be measured and evaluated to ensure that efforts are best directed.
Overall project delivery and recommendations
Project management RAG rating
-
Average RAG rating during the annual review period (1 April 2017 to 31 March 2019): Amber (medium)
-
End of the annual review period (31/3/2019): Amber/Green (medium/low)
Finance RAG rating
- Amber (medium)
Theory of Change
- Green (low)
External engagement
- Amber/Green (medium/low)
Overall delivery confidence rating
- Amber/Green (medium/low)
List of recommendations
Project management
1.i Review Logframe indicators and milestones following this Annual Review, to ensure that indicators are appropriate for the work packages as well as the data already collected, ensuring that indicators and milestones can be tracked on a regular basis
1.ii Monitor the accuracy of financial forecasts from 1/4/2019 to 1/10/2019, and reassess if more frequent, formal finance meetings with delivery partner(s) are required
1.iii Develop appropriate project-level governance where this does not currently exist for GAMRIF work packages during the implementation period. Initiate the chosen governance structure by the end of Q2 2019 to 2020
Finance
2.i Continue to work closely with CARB-X to support their ODA eligibility processes and to increase quality applications that fall within the GAMRIF scope, scaling up efforts as needed to achieve spending targets
2.ii Strengthen financial forecasting processes to ensure that processes are robust and can provide timely data on changes expected to the budgets for GAMRIF research projects
2.iii Ensure appropriate coverage of published documents and data across GAMRIF portfolio, and that the quality of public documentation is sufficient to achieve a score of at least 65% against the IATI standard
2.iv Increase partner awareness of IATI standards and available resources to encourage partners to publish their data on Aidstream
Theory of Change
3.i Develop an approach for measuring TRLs, or advancement of innovations across the ‘One Health’ portfolio
3.ii Update the theory of change behaviour change outcome to more accurately reflect areas where GAMRIF is expected to affect behaviour change
3.iii Work across HMG and with external partners to ensure that research and products will be accompanied by appropriate plans/activities to support access in LMIC settings. This includes ensuring that conditions regarding stewardship, access, commercialisation and policy translation will be upheld
3.iv Ensure strong alignment of the GAMRIF theory of change with the LogFrame, to include the comparable impact and intermediate outcomes of the GAMRIF project
External Engagement
4.i Publish a GOV.UK landing page and a GAMRIF logo for early 2019 to 2020
4.ii Formalise a communications strategy for the GAMRIF project that will guide systematic communications and engagement activities up to the end of the programme in 2021/22, including a communications monitoring strategy and branding strategy
4.iii Leverage research results as appropriate for public interest stories to:
a) continue advocating for increased donor investment into AMR
b) increase public knowledge to maintain political momentum and support stewardship for new technologies and innovations for AMR
Annex A: additional data
Table 7a: timelines of competitions: call/RFP details
| WP | Name | Open | Webinar | Close |
|---|---|---|---|---|
| WP1: UK-China | UK-China collaboration to tackle AMR | 3 Apr 2018 | 18 Apr 2018 | 6 Jun 2018 |
| WP2: CARBX | Round 2a1 (vaccines and biotherapeutics) and Round 2a2 (non-traditionals) | 1 Jun 2018 | Jul 2018 ODA focussed | Oct 2018 |
| WP3: InnoVet-AMR | Developing innovative veterinary solutions for the fight against antimicrobial resistance | 25 Jun 2018 | 31 Jul 2018 | 12 Sep 2018 |
| WP4: UK-Argentina | UK-Argentina Joint Call on AMR in the Environment | 1 Nov 2018 | 5 Nov 2018 | 13 Dec 2018 |
| WP5a: FIND (AMR Cx) | RDT-reading mobile app for AMR surveillance | 12 Mar 2019 | n/a | 10 Apr 2019 |
| WP6: GARDP | n/a | n/a | n/a | n/a |
| WP7: BactiVac | Round 2 pump-priming call | 15 Aug 2018 | n/a | 14 Oct 2018 |
| WP7: BactiVac | Round 3 pump-priming call | 11 Feb 2019 | n/a | 5 May 2019 |
Table 7b: timelines of competitions: scientific review/assessment
| WP | Date | Reviewer |
|---|---|---|
| WP1: UK-China | Jun-Jul 2018 (UK) Aug to Sep 2018 (China) | Innovate UK assigned assessors (UK) MoST assigned assessors (China) |
| WP2: CARBX | Dec 2018 (2a1) Jan 2019 (2a2) |
CARB-X Scientific Advisory Board (AdBoard) |
| WP3: InnoVet-AMR | 25 to 26 Oct 2018 | Scientific Advisory Committee (SAC) |
| WP4: UK-Argentina | 25-26 Mar 2019 | Joint UK-Argentina Scientific Panel |
| WP5a: FIND (AMR Cx) | Apr 2019 | FIND assigned internal and external reviewers |
| WP6: GARDP | n/a | n/a |
| WP7: BactiVac Round 2 pump-priming call | Nov-Dec 2018 | Members of the Network Management Oversight Board (NMOB) |
| WP7: BactiVac Round 3 pump-priming call | Jun-Jul 2019 | Members of the Network Management Oversight Board (NMOB) |
Table 7c: final project selection
| WP | Date | Approver |
|---|---|---|
| WP1: UK-China | Nov 2018 | MoST and Innovate UK/DHSC via email |
| WP2: CARBX | 29 Jan 2019 (2a1) 5 Mar 2019 (2a2) |
Joint Oversight Committee (JOC) |
| WP3: InnoVet-AMR | Nov 2019 | Governance Steering Committee (GSC) |
| WP4: UK-Argentina | Mar 2019 | CONICET/BBSRC/DHSC Funders’ Forum |
| WP5a: FIND (AMR Cx) | 1 May 2019 | FIND Science Advisory Committee (SAC) |
| WP6: GARDP | n/a | n/a |
| WP7: BactiVac Round 2 pump-priming call | 21 Jan 2019 | Network Management Oversight Board (NMOB) |
| WP7: BactiVac Round 3 pump-priming call | Aug 2019 | Network Management Oversight Board (NMOB) |
Table 7d: project start
| WP | Project start |
|---|---|
| WP1: UK-China | Jan-Mar 2019 |
| WP2: CARBX | Upon finishing contracting |
| WP3: InnoVet-AMR | Mar/April 2019 |
| WP4: UK-Argentina | 1 Aug 2019 |
| WP5a: FIND (AMR Cx) | 1 September 2019 |
| WP6: GARDP | n/a |
| WP7: BactiVac Round 2 pump-priming call | 1 Mar 2019 |
| WP7: BactiVac Round 3 pump-priming call | 1 Oct 2019 |
Figure 1: project status RAG rating by month (trend format)
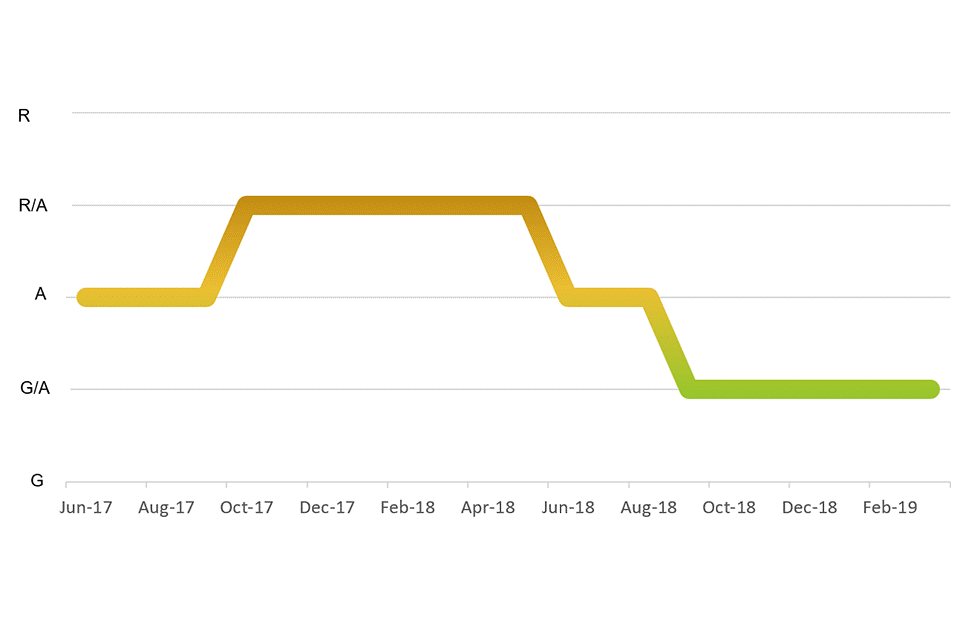
The graph shows RAG status as follows:
- June 2017: Amber
- August 2017: Amber
- October 2017: Red/Amber
- December 2017: Red/Amber
- February 2018: Red/Amber
- April 2018: Red/Amber
- June 2018: Amber
- August 2018: Amber
- October 2018: Green/Amber
- December 2018: Green/Amber
- February 2019: Green/Amber
Table 8: GOV.UK news releases during GAMRIF Year 1, 2 and 3
| Title | Headline | Date | URL |
|---|---|---|---|
| "Tackling drug resistance: UK-China funding competition opens" | A competition has opened that will make up to £10 million available to UK academic and industrial organisations to work in a consortium with Chinese partners. | 28 March 2018 | www.gov.uk/government/news/tackling-drug-resistance-uk-china-funding-competition-opens |
| "DHSC joins global fight to tackle antimicrobial resistance in animals" | 12 April 2018 | https://healthmedia.blog.gov.uk/2018/04/12/dhsc-joins-global-fight-to-tackle-antimicrobial-resistance-in-animals/ | |
| "£30 million of funding to tackle antimicrobial resistance" | The UK government has announced that it will be committing over £30 million of funding to the global fight against antimicrobial resistance (AMR). | 22 May 2018 | www.gov.uk/government/news/30-million-of-funding-to-tackle-antimicrobial-resistance |
| "UK & Argentina join forces to combat antimicrobial resistance" | 22 May 2019 | https://healthmedia.blog.gov.uk/2018/05/22/uk-argentina-join-forces-to-combat-amr/ | |
| "Government promises more funding to fight superbugs" | The government has agreed an extra £10 million to fight antimicrobial resistance (AMR) at home and abroad. | 19 November 2018 | www.gov.uk/government/news/government-promises-more-funding-to-fight-superbugs |
Annex B: theory of change
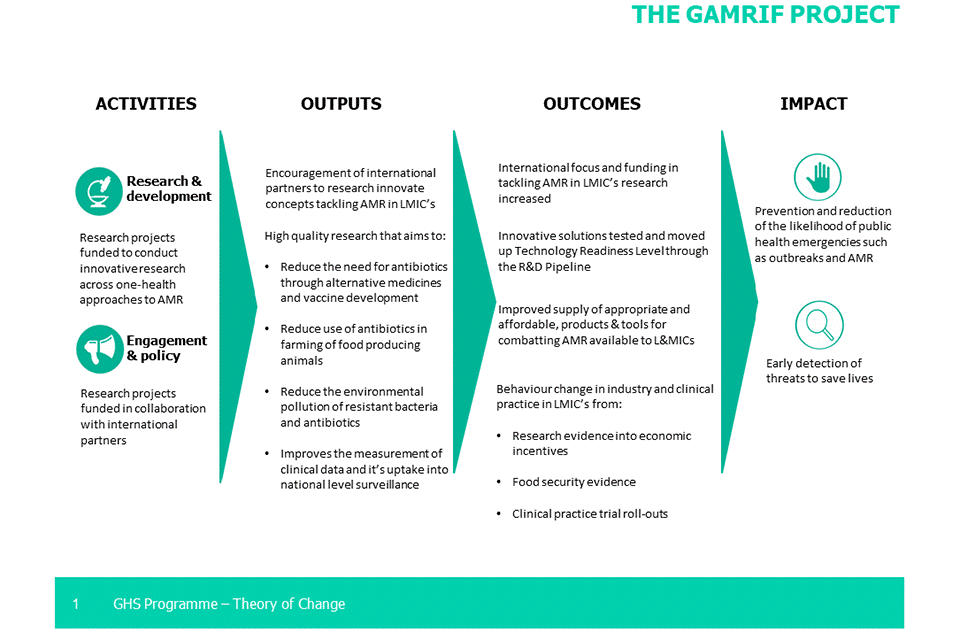
The GAMRIF theory of change. The diagram shows how activities under research and development as well as engagement and policy should contribute towards the intended impact to detect, prevent and reduce AMR.
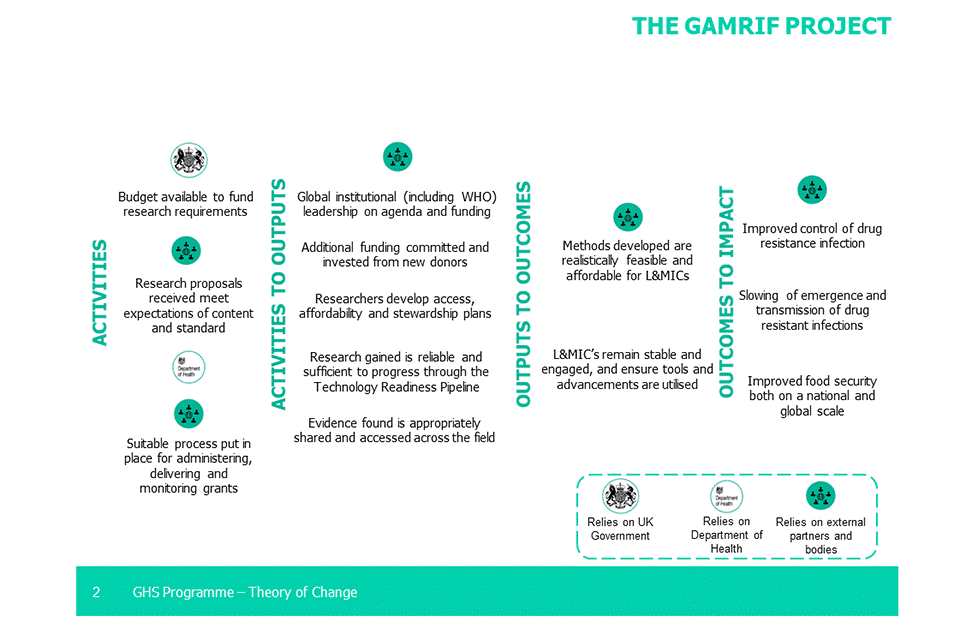
The diagram shows assumptions underlying GAMRIF’s theory of change. This also shows that GAMRIF has strong linkages with other stakeholders in DHSC, across HMG and externally.
-
Please note that Quarter 3 2018 to 2019 marked the milestone when all research calls had been opened, but in the case of CARB-X and BactiVac, we anticipate participating in more than 1 round of calls. ↩
