Drivers of the higher COVID-19 incidence, morbidity and mortality among minority ethnic groups, 23 September 2020
Updated 20 May 2022
Executive summary
Ethnicity is a multi-dimensional concept which includes culture, language, religion, migrant status and physical appearance (race), with considerable diversity within and between ethnic minority groups.
- In the first 6 months of the pandemic ethnic minority groups have experienced:
- a) higher prevalence of SARS-CoV-2 infection (high confidence)
- b) higher incidence of COVID-19 disease (high confidence)
- c) higher COVID-19 mortality than White ethnic groups (high confidence)
- These patterns may be different in coming months, highlighting the importance of ongoing investigations by ethnicity and social characteristics.
Different dimensions of ethnicity could each affect COVID-19 risks through a variety of mechanisms, leading to risks potentially being different along different stages of the disease: from exposure, developing symptomatic disease, disease severity and to long term consequences of the disease.
Some ethnic minorities who are admitted to hospital may also experience poorer outcomes, such as death and critical care admissions, compared to White ethnic groups (medium confidence).
Modifiable social factors such as poverty and occupation make a large contribution to the greater burden of COVID-19 in ethnic minorities (high confidence).
Some evidence suggests ethnic minorities working in the same occupation as White ethnic groups experience greater COVID-19 risk. Difficulty in accessing personal protective equipment is a suggested and potentially modifiable reason for this in healthcare workers (low confidence). These social determinants of health are likely to be driven by broader issues of structural racism, namely the social forces which lead to inequalities between ethnic and racial groups.
Some comorbidities associated with severe COVID-19 are more common in some ethnic minority groups which may contribute to the ethnic inequalities seen. Evidence on obesity, diabetes, and cardiovascular disease to suggest they contribute to the differences in COVID-19 risk between South Asian ethnic groups and White ethnic groups (medium confidence). Obesity is an important comorbidity that is potentially modifiable in the medium-term.
Other factors, including the appropriateness of public health messages for reaching all communities and the accessibility of health services, are likely to explain some of the greater burden among ethnic minorities.
The evidence suggests genetics alone cannot explain the higher number of severe cases and deaths since ethnic minorities are very genetically diverse (high confidence).
Greater exposure to the virus, via infective dose or contact frequency, contributes to higher levels of clinical disease, as reflected by hospital admissions. It is unclear if this fully accounts for the higher levels of clinical disease in ethnic minority groups.
The relative importance of different pathways that cause ethnic inequalities in COVID-19 is not well understood. Focusing on understanding the immediately modifiable pathways such as occupation and healthcare access should be an urgent priority.
Datasets do not currently include adequate information on different stages of the disease, detailed clinical information and the social determinants of health, limiting insights that can be gained from detailed quantitative analysis.
This paper summarises the available evidence on the epidemiology of ethnic inequalities in COVID-19 and potential explanations for differences observed. We use a conceptual model that highlights potential pathways at each of the steps from exposure to the virus, through infection to the development of severe disease.
Background
There is now substantial evidence from the first 6 months of the pandemic in the UK that people from minority ethnic backgrounds have been disproportionately affected. Data from the Office for National Statistics (ONS) found a higher risk of death from minority ethnic groups when compared to the White group and data from Public Health England (PHE) has shown higher risks of COVID-19 death amongst minority ethnic groups during the pandemic, after accounting for age and sex differences. [footnote 1] [footnote 2] An analysis of 17 million primary care and death records found people from Black and South Asian ethnicities had an approximate 2-fold increased risk of death compared with White ethnicities, after taking into account differences in age and sex. [footnote 3]
The causes of ethnic inequalities in COVID-19 are unclear. There is likely to be an interplay of social, economic, biological and pre-pandemic health risks that vary across ethnic groups. This is because ethnicity is a multi-dimensional concept. Ethnicity can be defined as the “social group a person belongs to, and either identifies with or is identified with by others, as a result of a mix of cultural and other factors including language, diet, religion, ancestry, and physical features traditionally associated with race”. [footnote 4] Ethnicity is therefore a complex concept which includes country of birth, language, religion, culture and physical appearance, and these characteristics are themselves influenced by where an individual lives, their ancestry and, to a limited extent, their genetic background. [footnote 5] These dimensions of ethnicity are an important consideration when considering research findings. This paper aims to explain the role of sociological, behavioural, and biological factors driving the unequal impacts among minority ethnic groups in the UK.
Conceptual model to understand COVID-19 ethnic inequalities
Many studies to date have focused on highlighting the unequal impact of COVID-19 on minority ethnic groups, with limited understanding of the mechanisms and pathways that have led to these differences. We outline below potential mechanisms and pathways and then highlight the current evidence of how these may lead to the observed differences in health outcomes from COVID-19 between ethnic groups. A comprehensive understanding of the role of each of these factors and the relationships between them can inform targeting and developing policy interventions, as well as future research.
The differences in health outcomes from COVID-19 between ethnic groups could occur at multiple points from exposure to the virus, through infection to the development of severe disease. Diderichsen et al propose a framework widely used in studying health inequalities that highlights potential pathways at each of these steps, Figure 1. [footnote 6] [footnote 7] At each step, ethnic inequalities could develop through social, economic and biological mechanisms. A framework to understand ethnic inequalities in COVID-19 is first described, with the evidence related to each stage and potential explanations provided later in the document.
Firstly, minority ethnic groups could experience greater exposure to the virus, seen in pathway 1, representing differential exposure. For example, due to the working in occupations with high contact with potentially infected persons or living in overcrowded housing. Secondly minority ethnic groups may be at greater risk of infection after having come in contact with the virus, for example due to differences in immune response and nutritional status, which in itself could be related to stress or environmental conditions such as air pollution, represented by pathway 2, differential susceptibility to infection. Some minority ethnic groups may be more likely to develop disease once infected, pathway 3, representing differential vulnerability to disease. Of those with disease, some minority ethnic groups may be more likely to develop severe disease, complications and potentially die. This is seen in pathway 4, representing differential disease consequences. This could for example be due to differences in underlying co-morbidities or differences in access to health care between ethnic groups.
Ethnic inequalities may also arise due to other pathways during the pandemic; however, these are out of scope for this paper. Additional pathways are outlined below.
- Pathway 5: differential effectiveness of COVID-19 control measures. For example, the public health responses taken to control the pandemic could be less effective amongst some ethnic groups compared to others. However, existing evidence suggests lockdown measures may have been more effective in reducing COVID-19 mortality for some ethnic minority groups. [footnote 8]
- Pathway 6: differential social consequences of COVID-19 disease. For example, COVID-19 disease may lead to chronic impairments that result in job loss and future loss of earning due to poor health.
- Pathway 7: differential consequences of pandemic control measures. For example, lockdown leading to unemployment and health service disruption that disproportionately effects some ethnic groups.
All of these mechanisms arise from the wider social context that drive ethnic and other social inequalities, such as power relations and structural racism. Structural racism has been defined as “the macrolevel systems, social forces, institutions, ideologies, and processes that interact with one another to generate and reinforce inequities among racial and ethnic groups”. [footnote 9] The term draws attention to the way inequalities may arise not from the intended actions of individuals but rather from broader societal mechanisms. For example, historical experiences of minority ethnic groups and long-term discrimination may lead to a higher proportion working in high-risk occupations, living in crowded higher risk housing conditions, and having fewer resources for health, such as education and income. [footnote 10] [footnote 11] These factors are likely to increase mental health problems such as psychosocial stress and harmful health behaviours including smoking, diet and lack of exercise, increasing co-morbidities that put people at greater risk of COVID-19 disease, see Figure 1. [footnote 12] [footnote 13] These social processes largely underpin biological risks that are corelated with some ethnic groups. [footnote 14] Due to structural racism, health care planning may not take into account different experiences, perceptions and expectations of ethnic minorities, and therefore health services may poorly meet the needs of some ethnic groups – further widening inequalities. [footnote 15] [footnote 16] [footnote 18] These are the factors that mediate the relationship between ethnicity and health outcomes and whilst they are often statistically “controlled or adjusted” for in epidemiological studies, they are in fact important explanations of ethnic inequalities and not ‘confounding factors’. [footnote 18] Interventions to address the excess risk of poor outcomes from COVID-19 in ethnic minorities therefore likely requires a multi-faceted approach to address the mechanisms working across the pathways identified in Figure 1. The current evidence indicating which of these pathways are important in driving ethnic inequalities in COVID-19 is outlined below.
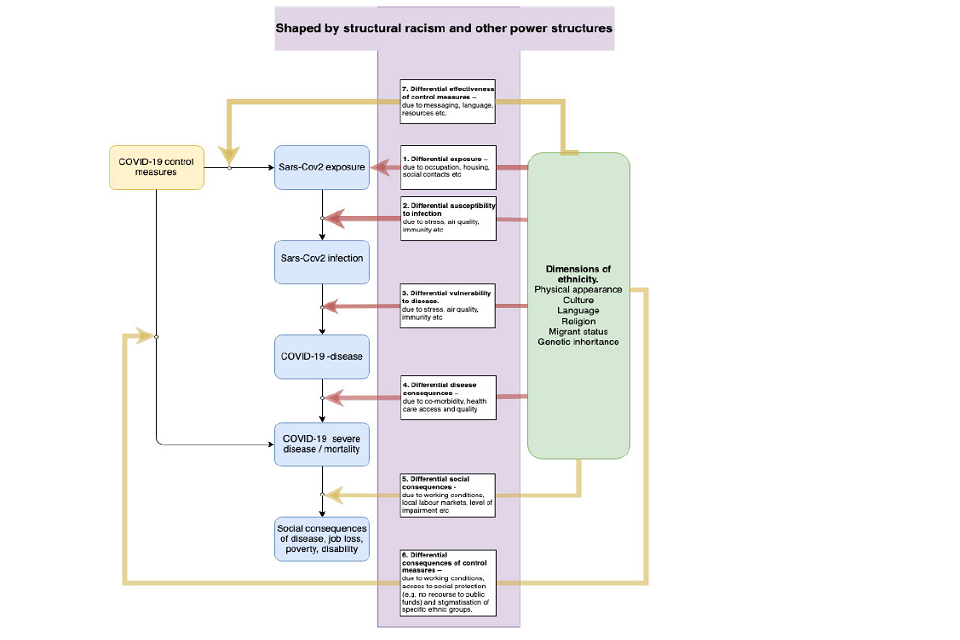
Flow chart showing how factors shaped by structural racism (shaded in purple box) feed into COVID-19 consequences. These include: SARS-CoV-2 exposure and infection; COVID-19 (severe) disease and mortality; and social COVID-19 consequences.
Figure 1: A framework for understanding ethnic inequalities in COVID-19, adapted from Diderichsen et al. [footnote 6] Ethnic inequalities can arise through seven different pathways, with pathways 1 to 4 impact on the direct health impacts of COVID-19 disease. Differential effectiveness of COVID-19 control measures (7) is the focus of another SAGE subgroup paper. Differential social consequences of experiencing disease (5) and differential consequences of pandemic control measures (6) are outside the scope of this paper but are under-researched areas. The main text summarises epidemiological evidence related to pathways 1 to 4. Note that the total burden of ethnic inequalities in COVID-19 harms, such as deaths, is likely to reflect causes that occur along pathways 1 to 4 plus 7 cumulatively. COVID-19 control measures (7) include all government, public health and health system responses taken to control the pandemic. Each of the pathways reflects potential targets for intervention to reduce ethnic inequalities in health.
Differential exposure to the virus and susceptibility to infection (Pathways 1 and 2)
Exposure to SARS-CoV-2 amongst some minority ethnic groups is likely to be higher, but direct data is lacking. This includes, for example, data on social contacts between individuals who are infected and non-infected, by ethnicity. Furthermore, it is possible that differences in exposure could be due to differences in the frequency of exposure or differences in the dose of virus that occurs at exposure, or both. As noted in the later section on potential explanations, we highlight potential reasons such as occupational and housing-related exposures. Having been exposed to the virus, individuals may develop infection, which could be asymptomatic or symptomatic (Pathway 2). If the infected individual develops symptoms, then this is called disease (Pathway 3), which may be mild or severe or fatal (Pathway 4).
Overall, there are few data available on SARS-CoV-2 infection across ethnic groups. However, the recent representative ONS national infection survey found evidence that ethnic minorities were more likely to have experienced the infection at some point in the past. [footnote 19] Those from ethnic minorities had a higher proportion of positive serology (antibody) tests compared to White groups, Figure 2. This suggests that these minority ethnic groups have experienced greater contact with the virus (pathway 1), however it could also indicate that they were more susceptible to infection when exposed (pathway 2). A study of over 100 thousand people in the UK (the REACT2 study), again designed to be representative, found higher antibody prevalence amongst all ethnic minorities studied. [footnote 20] Similar patterns of findings have also been found within the UK Biobank study, with statistical adjustment for age, sex, deprivation, region and urban-rural status leading to the associations remaining largely unchanged. [footnote 21]
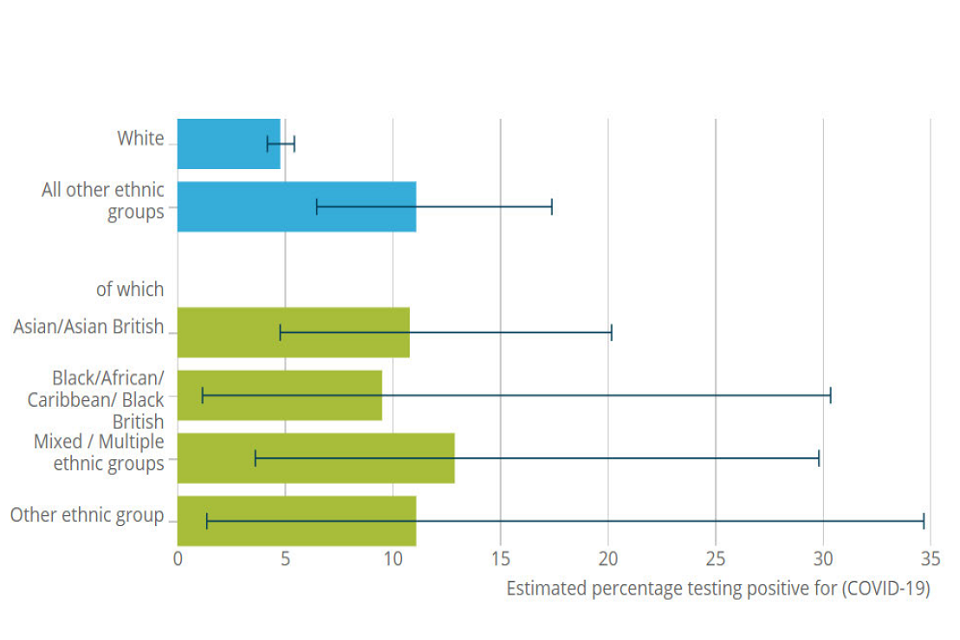
Horizontal bar chart. Estimated percentage of people testing COVID-19 positive. Top two bars (in blue) respectively show: white and all other ethnic groups. The latter is further subdivided by ethnicity into four categories, shown below blue bars in green
Figure 2: Serology test results by ethnicity in a representative population. Source: ONS infection survey. [footnote 7]
Differential vulnerability to disease (Pathway 3)
We can be confident that there are inequalities among ethnic groups in the chance of experiencing COVID-19 disease, as evidenced by test results and hospitalisations data from several studies (high confidence). These studies however are not able determine whether these differences have arisen due to differences in exposure to the virus (pathway 1), differences in susceptibility to infection (pathway 2) or differences in the risk of developing symptomatic disease amongst those infected (pathway 3). It is therefore unclear whether the findings summarised below reflect pathway 3 or pathways 1 and 2 earlier in the transmission process, with the ONS infection survey suggesting that at least part of the differences arising in these earlier pathways. An early study from primary care settings in Oxford found patients from a Black background had a 4-fold increased risk of getting the disease, defined here as test positive, compared to patients from a White background, once accounting for differences in comorbidities such as hypertension and diabetes.[footnote 22] Similar findings are found in national studies; for example, findings from a UK Biobank cohort of nearly 400,000 people, found people from a Black and South Asian background, in particular those from a Pakistani background, had a 3-fold increased risk of testing positive compared to those from a White background.[footnote 23] The chance of being tested did not substantially differ across ethnic groups, suggesting bias due to differential counting of cases did not explain the findings. A second systematic review and meta-analysis of 60 published studies examined the relationship between ethnicity and clinical outcomes, such as hospitalisation, finding people from some minority ethnic backgrounds experienced disease more commonly than White ethnic groups, after accounting for other explanations such as age and sex differences. [footnote 24]
The recently available OPENSAFELY study extended an analysis of COVID-19 mortality outcomes to study other outcomes along the disease pathway. They found important ethnic inequalities in testing positive, being admitted to intensive care and dying while the chance of actually being tested showed minimal ethnic differences among ethnic groups, suggesting results are unlikely to be due to differential testing. To differentiate vulnerability to the disease from greater exposure, data should be analysed to consider how risk changes across different stages of the disease process. For example, comparing the risk of reporting symptomatic disease amongst those with evidence of infection.
Differential disease consequences (Pathway 4)
Evidence of ethnicity as the sole cause of poorer clinical outcomes when compared to people of White background is limited. A systematic review examining hospitalisation and-or need to be put on a ventilator in intensive care found little evidence of a relationship between ethnicity and poorer clinical outcomes. [footnote 25] However, a large cohort study of nearly 35,000 UK hospitalisations found a 30% increased relative risk of critical care admission and ventilation among people from South Asian, Black or Minority ethnic groups and this relationship was still present after accounting for age and sex. [footnote 26] After accounting for some potential explanations of this increased risk (such as co-morbidities like diabetes), these minority ethnic groups were still more likely to require critical care and ventilation than White groups (medium confidence). However, it is possible that some differences in presentation could not be fully accounted for that could arise from modifiable factors such as awareness of optimal healthcare seeking behaviours.
Explanation and mechanisms
The evidence presented thus far suggests that there are a range of individual-level and societal-level mechanisms, working across the pathways outlined above that may be responsible for driving ethnic inequalities in COVID-19 outcomes. However, there is limited evidence that robustly studies the mechanisms through which such disproportionate outcomes arise among minority ethnic groups.
Socioeconomic position
Socioeconomic circumstances are known to strongly shape health in general and to be important contributors to ethnic inequalities in health. The ONS investigated ethnic differences in COVID-19 mortality using linked data bringing together information from the census (which provided ethnicity and socioeconomic variables) and deaths. [footnote 2] [footnote 8] It showed that several minority ethnic groups were at more risk of COVID-19 death than those from White backgrounds – for example, Black males had a 3.3 times greater risk than the White ethnic group. Males from a Black background had a 2-fold increased risk of death after taking into account age, sex, geography, socioeconomic position (assessed using the Index of Multiple Deprivation, highest educational attainment, occupation and tenure status), multigenerational household and household characteristics. Similar patterns were seen for Bangladeshi, Pakistani and Indian ethnic groups (Figure 3). Risks were lower once socioeconomic position was included together with other factors. [footnote 2] This suggests that socioeconomic position is a very important contributor to the risk of death. Studies have not looked specifically at causality, but these factors related to socioeconomic position could act on any of the pathways 1 to 4 outlined above. It is worth noting that these socioeconomic measures may not be up to date as they were from the 2011 census. Changes in circumstances since this time could not therefore be incorporated and this is likely to lead to an underestimation of the explanatory power of these factors. [footnote 27]
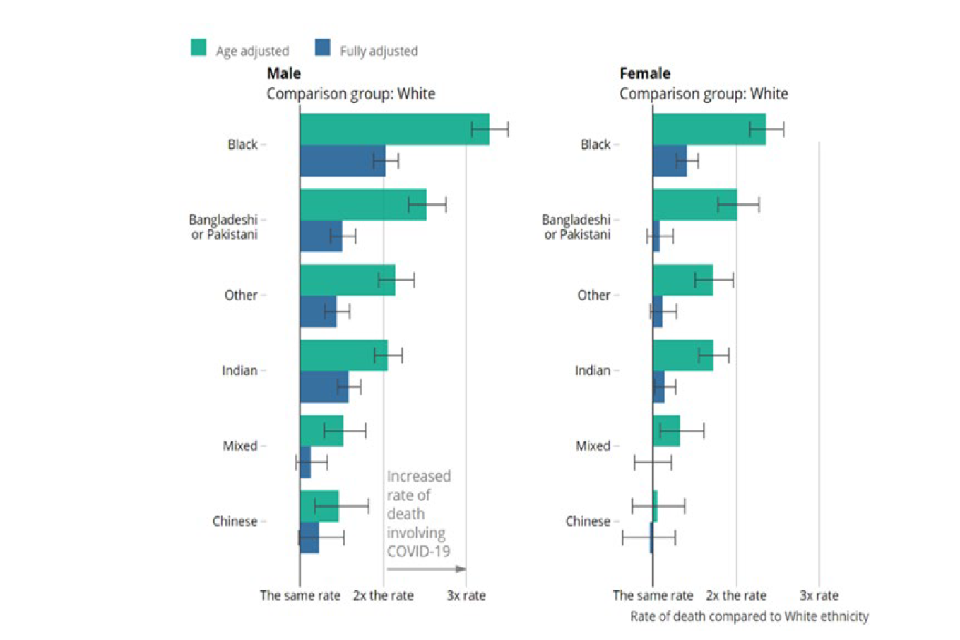
Two horizontal bar charts mapping COVID-19 death rate, split into six ethnic group categories. Data split by gender (left and right charts respectively show male and female data). Green and blue bars represent age and fully adjusted data, respectively.
Figure 3: Rate of COVID-19 death by ethnic group and sex relative to the White group in England and Wales between 2 March to 15 May 2020. Source ONS. Fully adjusted factors include region, population density, area deprivation, household composition, socioeconomic position, highest qualification held, household tenure, multigenerational household flags and occupation indicators (including key workers).
Occupation
The risk of exposure to the virus and viral dose is likely to vary according to occupation, modifiable risk factor; for example, those working in public-facing roles are likely to have a greater potential for viral contacts due to increased social mixing. Some minority ethnic groups are overrepresented in health and social care and other key public sectors (Figure 4).
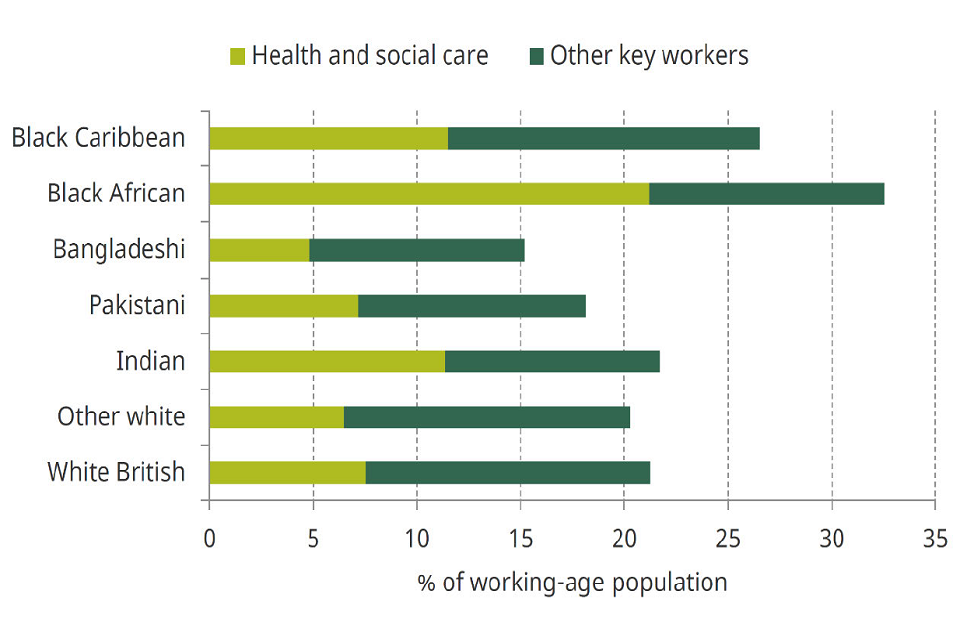
Horizontal bar chart showing percentage of working-age population divided into 7 ethnic group categories. For each bar, light and dark green respectively represent percentage of population that are health or social care workers versus other key workers.
Figure 4: Percentage of the working-age population in key worker roles (light green health and social care roles, dark green other key workers) broken down by ethnic group. Source: Institute for Fiscal Studies May 2020. [footnote 27] This is particularly evident for people from a Black ethnic minority, where more than a quarter are employed in key worker positions. Differences also emerge within groups, for example, people from a Black African background make-up less than 3% of the employed population but account for 7% of nurses and contributed to the largest proportion of deaths among NHS staff. [footnote 28]
It is also possible that the impact of working in a specific occupation may differ for minority ethnic groups compared to the White British majority. In frontline healthcare workers in the UK and USA, ethnic minorities experienced a greater risk of a positive test and reporting inadequate access to effective personal protective equipment. [footnote 29] Similarly, an analysis of UK Biobank data suggested an increased risk of being a positive hospitalised COVID-19 case among non-White frontline essential workers compared to White essential workers. [footnote 30]
In addition to higher levels of unemployment which affects socioeconomic conditions, some minority ethnic groups experience higher risks of under-employment and insecure employment (for example, the ‘gig economy’, self-employment, zero hours contract care workers). [footnote 31] There is therefore a risk that loss of earnings resulting in financial hardship is more likely among some minority ethnic groups which could act as a barrier to self-isolation when individuals experience COVID-19 symptoms or are asked to quarantine. This may in turn lead to secondary spread from individuals not self-isolating due to financial concerns. The higher representation of some minority ethnic groups in at-risk occupations and insecure employment is likely to increase their risk of exposure to the virus compared to the White British majority (pathway 1 above).
Household circumstances
There is good evidence that both aerosol and droplet transmission of the virus occurs more frequently indoors than outdoors [footnote 32] [footnote 33] , in particular from confirmed cases to household members who might be mixing when sharing bathrooms, bedrooms and other household living spaces. [footnote 34] It is likely that during the peak viral shedding period, people living in overcrowded housing (more than one person per bedroom) are at greater risk of infection. Data from the ONS English Housing Survey shows overcrowding is higher among minority ethnic groups compared with the white group: 24% and 16% people of a Bangladeshi and Black African background live in overcrowded housing, compared with 2% of those from a White British background (Figure 5). [footnote 35]
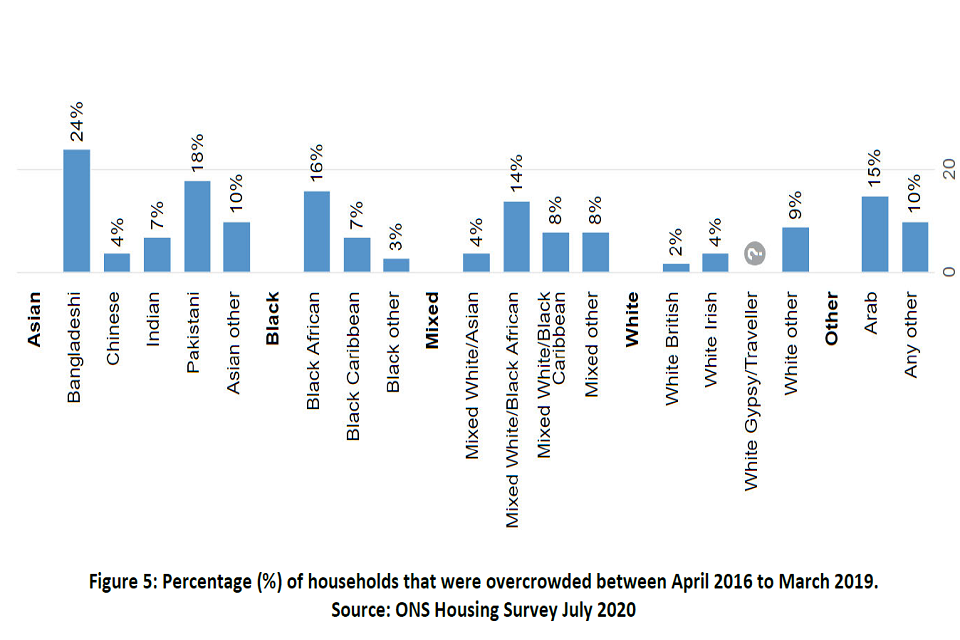
Bar chart showing percentage of overcrowded households from April 2016 to March 2019 for different ethnic groups. Greatest overcrowding is 24 per cent, seen for Bangladeshi ethnic groups. Least overcrowding is seen for White British, at 2 per cent.
Figure 5: Percentage of households that were overcrowded between April 2016 to March 2019. Source: ONS Housing Survey July 2020.
Older people living in multi-generational housing may be at risk of contracting potentially severe disease from household members. [footnote 36] ONS Census data shows that Asian and Black ethnic minority populations are more frequently living in households with multiple generations, for example people with children living with grandparents, compared to White groups. Approximately 21.7% of Asian ethnic minorities and 14.1% of Black minorities live in multi-generational households compared to only 6.8% from a White group. [footnote 37] There was little difference among the Asian ethnic minority groups, whilst within the Black ethnic groups, Black African groups more often lived in multi-generational households compared with Caribbean. The combination of overcrowded housing and multi-generational households among minority ethnic groups could lead to greater exposure to infection (pathway 1 above), particularly amongst the older generations, when compared to White groups. [footnote 36] However, the most recent OPENSAFELY study found statistical adjustment for a proxy measure of household size led to very small changes in the observed relationships between ethnicity and COVID-19 outcomes, suggesting (but not confirming) that this is unlikely to be an important mechanism.
Neighbourhood circumstances
Ethnic minorities tend to live within urban areas and more densely populated areas where viral transmission may be greater due to more contacts with infected persons. Using census data for England alongside information on COVID-19 deaths, a study found that differences in population density and local authority of residence made an important contribution to ethnic inequalities [footnote 2] , which likely due to the increased risk of exposure (pathway 1). It is also possible that the frequency of social contacts between households could differ across ethnic groups, even after accounting for population density, but studies directly assessing this during the pandemic have not been conducted to our knowledge.
Modifiable factors such as air pollution which differs across neighbourhoods has been linked to increased risks of COVID-19, probably due to increased susceptibility (pathway 2) and it is known that some ethnic minorities often live in areas with higher levels of air pollution within the UK. [footnote 38] [footnote 39] [footnote 40]. However, evidence is currently contradictory about its importance, with some studies finding that air pollution does not appear important when population density is accounted for. [footnote 41] Other modifiable factors such as stress, including racism, has also been linked to the impacts of air pollution on lung health being exacerbated. [footnote 42] It is therefore possible that air pollution could lead to even greater susceptibility in minority ethnic groups, than might be expected on the basis of solely the levels of air pollution observed.
Comorbidities and health-related behaviours
Chronic health conditions from before the pandemic might contribute to ethnic inequalities in COVID-19. Comorbidities which impair a person’s immune system (such as autoimmune conditions, cancers and diabetes) could influence the chance of developing COVID-19 disease following SARS-CoV-2 exposure (differential susceptibility – pathway 2) and some comorbidities (such as respiratory conditions) have been shown to affect COVID-19 disease prognosis (differential disease consequences – pathway 3). Similarly, modifiable health-related behaviours (such as smoking and diet) are associated with increased COVID-19 risks, with evidence that these relationships are casual. [footnote 43]
Unfortunately, there are few studies which have investigated the complex relationships between comorbidities and COVID-19. In the OPENSAFELY study, the risk for ethnic differences in COVID-19 death slightly changed after accounting for a large number of comorbidities and some behaviour-related risk factors, suggesting they only explain a small part of these inequalities. In the UK Biobank study, even after accounting for comorbidities at baseline, South Asians were still more than twice as likely to be hospitalised compared to the White British group. When investigating disease outcomes amongst people admitted to hospital, the ISARIC-CCP study identified diabetes as a potentially important factor for the poorer outcomes of South Asians, accounting for 18% of the greater risk of death. Many of the studies to date have assumed similar effects of comorbidities and health-related behaviours across ethnic groups. However, it is possible that the same comorbidity may result in differing impacts. For example, obesity could lead to greater COVID-19 risk in some ethnic groups. [footnote 44]
Unequal health care access
Before the pandemic, evidence suggests modifiable barriers to healthcare are experienced by many ethnic minorities, but they vary across different parts of the health system and impact different ethnic groups in different ways. For example, Scottish data suggested that while secondary care was largely equitable, primary health care met the needs of the Pakistani ethnic group less well. [footnote 45] Differences in healthcare access and quality between ethnic groups, will potentially lead to differences in the severity of COVID-19 outcomes (pathway 3 - differential disease consequences). Modifiable barriers to healthcare can arise across different settings, with potential for the impact of differential care to accumulate. These are illustrated in Figure 6. It is important to note that differential healthcare reflects not only the actions of the individual person but also the design of the health system, which may not be meeting the needs of ethnically diverse communities. There are limited data on access to healthcare during COVID-19. However, the ISARIC-CCP study found amongst people who had been admitted to hospital, ethnic minorities had a similar delay between the onset of symptoms and their hospital admission. [footnote 26] Published studies have been limited on the accessibility of primary care, testing settings and those who were not admitted to hospital. Migrants (including those with ‘no recourse to public funds’) may be a specific group at risk of experiencing barriers to healthcare.
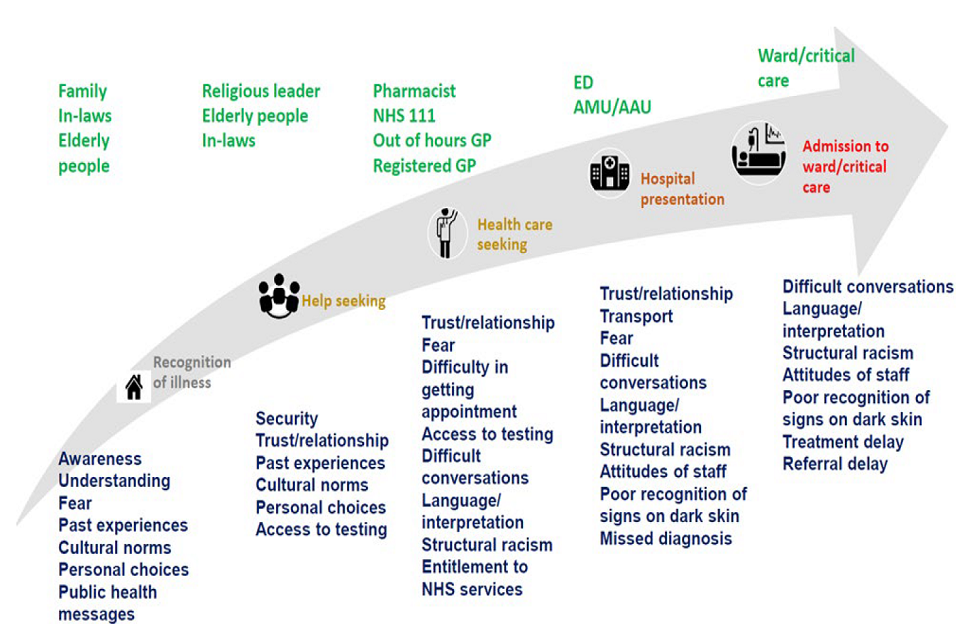
Infographic on stages of seeking health advice and care, from illness recognition to ward or critical care admission. Green writing above summarises key people involved at each stage. Blue writing below summarises barriers and factors delaying stages.
Figure 6: Trajectory of health seeking and health care, and factors which may influence delays in presenting to healthcare or receiving appropriate care, or in having care escalated due to progressive disease. ED=Emergency Department, AMU= Acute Medical Unit, AAU= Acute Admissions Unit. Individual or family lack of awareness or understanding of COVID-19 symptoms, cultural norms, or mistrust in health care professionals, may lead to delays in seeking health advice. Other factors such as fear, mistrust, language barriers, difficulty in getting an appointment, and lack of entitlement to NHS services, might lead to further delays in seeking medical help. Once the individual arrives at the hospital, language barriers, miscommunication, fear, and mistrust, might contribute to the individual not conveying relevant information to the health care professional or the health care professional not receiving the relevant information. In addition, structural racism, and poor recognition of signs in dark skin (such as cyanosis due to low oxygen in the blood), could lead to further delays in admission or escalation of care.
Psychosocial stress
Social threats in day-to-day life due to individuals’ experiences of racism (either structural or as a result of another’s actions) can lead to psychosocial stress which has direct biological effects. [footnote 33] Stress has been associated with less effective immune functioning, with evidence that this impact might differ across ethnic groups. [footnote 46] It is therefore possible that stress-related pathways could increase susceptibility to the virus but direct evidence in relation to COVID-19 remains limited though is developing. [footnote 47] There are early indications that the adverse mental health consequences of the pandemic might also be disproportionately affecting ethnic minorities (Figure 7). However, evidence on ethnic inequalities in the indirect health consequences of the pandemic on non-COVID-19 health outcomes remain limited.
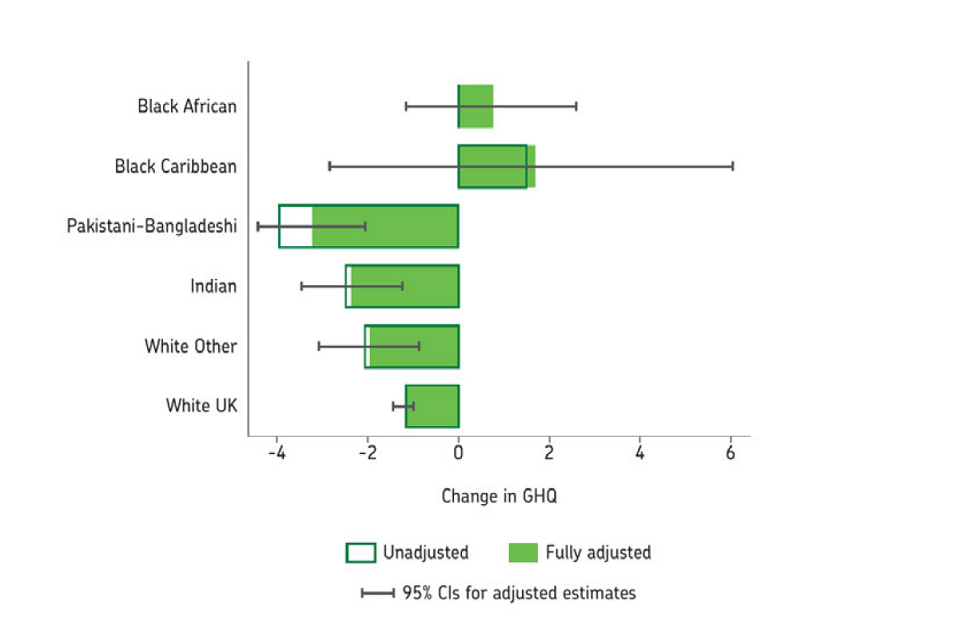
Box plots of mental health status change, pre- and during-pandemic. Black African; Black Caribbean; Pakistani-Bangladeshi; Indian; White Other and White UK ethnic groups. Hollow and filled green boxes respectively represent unadjusted and adjusted data.
Figure 7: Change in mental health status as measured by the General Health Questionnaire (GHQ), between two surveys conducted pre-pandemic (2017 to 2018) and during the pandemic (April to May 2020) shown by ethnicity. Negative change indicates worsening in mental health. Source: Understanding Society Working Paper August 2020.
Biological factors
The role of biological factors such as genetics has drawn considerable attention, however these are unlikely to explain the ethnic inequalities. The ACE receptor is a binding site for SARS‐CoV‐2 to the human cell, and genetic variations which alter how the virus binds to the cell, may confer resistance against or susceptibility to SARS‐CoV‐2 infection. [footnote 48] [footnote 49] Most ACE2 gene variants are rare, but three variants which affect how the virus binds to human cells are more common in African populations. A second genetic study spanning the whole human genome, highlighted some genes which confer susceptibility and confirmed that blood group A was associated with a higher risk than non-A blood groups. [footnote 49] Other genetic variations which are specific to certain races, called human leukocyte antigens, could also act as key determinants of susceptibility to, or protection from SARS-CoV-2 infection. [footnote 50] Multisystem inflammatory syndrome in children (MIS-C) is a hyperinflammatory syndrome associated with SARS-CoV-2, affecting heart, skin, mucous membranes, and guts. [footnote 51] Most studies report that children from minority ethnic backgrounds were over-represented, but reasons for this are unclear. [footnote 52]
Predisposing genetic factors may influence infection susceptibility, regulate a person’s immune response against SARS-CoV-2 (Pathway 2) and influence disease severity (pathway 3). [footnote 53] Genetic variations such as those that provide protection against malaria, tuberculosis and pneumococcal infection due to an enhanced host immune response, may be harmful for other conditions such as sepsis. [footnote 54] Tal et al. demonstrated that, compared to White Americans, people from a Black background, were more likely to develop a more vigorous inflammatory host response. This raises the possibility that when confronted with SARS-CoV-2, people from a Black minority ethnic group could be more prone to develop a damaging overreaction of the body’s immune system, the so called “cytokine storm” (53). However, whether this actually reflects genetic differences is unclear. For most other conditions where such ethnic differences in health are observed, they reflect underlying social differences which have been affected by ethnicity rather than genetic differences. [footnote 55] [footnote 56] [footnote 57] Vitamin D levels have been suggested as potentially contributing to ethnic differences in COVID-19 risk, given absorption could potentially differ by skin colour. However, both observational analysis and more causal genetic studies have not found a relationship between Vitamin D and COVID-19 disease, suggesting this is unlikely to be an important explanation. [footnote 58]
In summary, the overall evidence suggests genetic factors are not major contributors to ethnic inequalities but there are some specific genetic factors which may have a small influence on COVID-19 risk. More definitive genetic studies could subsequently determine variants which make individuals more vulnerable to infection and progression towards unfavourable outcomes or make them more or less likely to respond to different drugs. [footnote 59]
Current evidence gaps
Since the start of the pandemic evidence indicates marked ethnic inequalities in COVID-19, with risks arising through several mechanisms. The most important factors are likely to be related to the social determinants of health, including socioeconomic position, occupation and housing circumstances, which are modifiable. These are in turn likely to impact on comorbidities and health-related behaviours. It is important to stress that not all these factors are modifiable; for example, we can’t change an individual’s ancestry, culture, religion or country of birth, but we can change their occupational risks, employment contracts, housing circumstances, health-related behaviours and differential healthcare experiences. In summary, the risks of getting the infection primarily relate to more systemic and structural issues (such as exposure at work or in the home, social contacts, stress, immunity, pollution), and risks of developing more severe infection relate to pre-existing co-morbidities, and access to health care (including optimisation of control of comorbidities).
Direct evidence about exposure to the virus is currently limited, but indirect evidence about likely exposure suggests this may be important. Improving our understanding of if and how behaviours differ by ethnicity (such as following public health recommendations) may be warranted. A major gap is a lack of understanding on what actually causes the inequality, and the relative importance of different pathways to each other. For example, is occupation twice as important as health behaviours, or differential healthcare five times more important as housing? Addressing these questions therefore requires more causal approaches to analysing data, such as G-methods including marginal structural models, g-formula and structural nested models. [footnote 60] [footnote 61] Similarly, nearly all analyses to date have assumed that these factors have the same effect across all ethnic groups, but this is at best simplistic, as our conceptual model illustrates, and issues of structural racism may mean this is not the case. [footnote 62] [footnote 63] The accessibility and adequacy of healthcare across ethnic groups, including for testing and health protection services, remains unclear. Uncertainty also remains about the extent to which different ethnic groups experience adverse consequences of the disease too. Lastly, some genetic influences on COVID-19 risks are emerging, but these are very unlikely to be important explanations of the differences between ethnic groups.
The conceptual model (Figure 1) provides a starting point for guiding future analyses, highlighting the data requirements needed to investigate different causal pathways. If the causal mechanisms are to be unravelled successfully, more granular data are needed to capture the complexities of ethnicity and socioeconomic position. It is unlikely that any single dataset will be able to provide a comprehensive understanding of this issue, so coordinated analyses across datasets are likely to be necessary. This may involve focusing on different pathways within different analyses. Further research involving specific occupational risks, in-depth ethnographic study of previous life events (in first generation immigrants versus later generation immigrants), and ethnographic studies of the patient healthcare journey with COVID-19, may provide valuable insights. In addition, interventions to address the excess risk of poor outcomes from COVID-19 in ethnic minorities therefore likely requires a multi-faceted approach to address the mechanisms working across the pathways identified in Figure 1.
References
-
Public Health England. Disparities in the risk and outcomes of COVID-19 [Internet]. 2020 Aug p.92. ↩
-
ONS. Coronavirus (COVID-19) related deaths by ethnic group, England and Wales - Office for National Statistics [Internet]. 2020 [cited 2020 Sep 13]. ↩ ↩2 ↩3 ↩4
-
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. ↩
-
Bhopal R. Glossary of terms relating to ethnicity and race: for reflection and debate. J Epidemiol Community Health. 2004 Jun 1;58(6):441–5. ↩
-
Bhopal RS. Migration, Ethnicity, Race, and Health in Multicultural Societies [Internet]. Migration, Ethnicity, Race, and Health in Multicultural Societies. Oxford University Press; [cited 2020 Sep 18]. ↩
-
Diderichsen F, Hallqvist J, Whitehead M. Differential vulnerability and susceptibility: how to make inequalities. Int J Epidemiol. 2019 01;48(1):268–74. ↩ ↩2
-
Diderichsen F, Andersen I, Manuel C, Andersen A-MN, Bach E, Baadsgaard M, et al. Health Inequality -determinants and policies. Scand J Public Health. 2012 Nov 1;40(8_suppl):12–105. ↩ ↩2
-
Ayoubkhani D, Nafilyan V, White C, Goldblatt P, Gaughan C, Blackwell L, et al. Ethnic minority groups in England and Wales - factors affecting the size and timing of elevated COVID-19 mortality: a retrospective cohort study linking Census and death records. medRxiv. 2020 Aug 4;2020.08.03.20167122. ↩ ↩2
-
Gee GC, Ford CL. Structural Racism and Health Inequities. Bois Rev Soc Sci Res Race. 2011 Apr;8(1):115–32. ↩
-
Karlsen S, Nazroo JY, Smith NR. Ethnic, Religious and Gender Differences in Intragenerational Economic Mobility in England and Wales. Sociology. 2020 Jun 15;0038038520929562. ↩
-
Nazroo JY, Bhui KS, Rhodes J. Where next for understanding race/ethnic inequalities in severe mental illness? Structural, interpersonal and institutional racism. Sociol Health Illn. 2020;42(2):262–76. ↩
-
The Marmot Review. Fair Society, Healthy Lives - The Marmot Review: Strategic Review of Inequalities in England post-2010. London; 2010. ↩
-
Mackenbach JP, Valverde JR, Bopp M, Brønnum-Hansen H, Deboosere P, Kalediene R, et al. Determinants of inequalities in life expectancy: an international comparative study of eight risk factors. Lancet Public Health. 2019 Oct 1;4(10):e529–37. ↩
-
The British Academy. What factors make a community more vulnerable to COVID-19? A summary of a British Academy workshop. London; 2020 Sep. ↩
-
Mead N, Roland M. Understanding why some ethnic minority patients evaluate medical care more negatively than white patients: a cross sectional analysis of a routine patient survey in English general practices. BMJ 2009 Sep 17;339. ↩
-
Chauhan A, Walton M, Manias E, Walpola RL, Seale H, Latanik M, et al. The safety of health care for ethnic minority patients: a systematic review. Int J Equity Health [Internet]. 2020 Jul 8;19. ↩
-
VanderWeele TJ, Robinson WR. On causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiol Camb Mass. 2014 Jul;25(4):473–84. ↩ ↩2
-
ONS. Coronavirus (COVID-19) Infection Survey: characteristics of people testing positive for COVID-19 in England, August 2020 [Internet]. 2020 [cited 2020 Sep 13]. ↩
-
Ward H, Atchison CJ, Whitaker M, Ainslie KEC, Elliott J, Okell LC, et al. Antibody prevalence for SARSCoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv.2020:2020.08.12.20173690. ↩
-
UK Biobank. UK Biobank SARS-CoV-2 Serology Study- Weekly Report. 2020 Jul. ↩
-
Lusignan S de, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, et al. Risk factors for SARSCoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020 Sep 1;20(9):1034–42. ↩
-
Niedzwiedz CL, O’Donnell CA, Jani BD, Demou E, Ho FK, Celis-Morales C, et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med. 2020 29;18(1):160. ↩
-
Raharja A, Tamara A, Kok LT. Association Between Ethnicity And Severe Covid-19 Disease: A Systematic Review And Meta-analysis. medRxiv. 2020 Aug 14;2020.08.12.20157271. ↩
-
Pan D, Sze S, Minhas JS, Bangash MN, Pareek N, Divall P, et al. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. EClinicalMedicine [Internet]. 2020 Jun 1;23. ↩
-
Harrison EM, Docherty AB, Barr B, Buchan I, Carson G, Drake TM, et al. Ethnicity and Outcomes from COVID-19: The ISARIC CCP-UK Prospective Observational Cohort Study of Hospitalised Patients [Internet]. Rochester, NY: Social Science Research Network; 2020 May [cited 2020 Sep 13]. Report No: ID 3618215. ↩ ↩2
-
Fewell Z, Davey Smith G, Sterne JAC. The Impact of Residual and Unmeasured Confounding in Epidemiologic Studies: A Simulation Study. Am J Epidemiol. 2007 Sep 15;166(6):646–55. ↩ ↩2
-
Platt L, Warwick R. Are some ethnic groups more vulnerable to COVID-19 than others? [Internet]. The Institute for Fiscal Studies; 2020 May [cited 2020 Sep 13] p. 27. ↩
-
Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo C-G, Ma W, et al. Risk of COVID-19 among front-line-health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020 Sep 1;5(9):e475–83. ↩
-
Mutambudzi M, Niedzwiedz CL, Macdonald EB, Leyland AH, Mair FS, Anderson JJ, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120,075 UK Biobank participants [Internet]. Occupational and Environmental Health; 2020 May [cited 2020 Sep 13]. ↩
-
Labour market economic commentary - Office for National Statistics [Internet]. [cited 2020 Sep 13]. ↩
-
Morawska L, Tang JW, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020 Sep 1;142:105832. ↩
-
Nishiura H, Oshitani H, Kobayashi T, Saito T, Sunagawa T, Matsui T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv. 2020 Apr16;2020.02.28.20029272. ↩ ↩2
-
Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020 Apr 20;369. ↩
-
ONS. Overcrowded households - English Housing Survey [Internet]. 2020 [cited 2020 Sep 13]. ↩
-
Haroon S, Chandan JS, Middleton J, Cheng KK. Covid-19: breaking the chain of household transmission. BMJ [Internet]. 2020 Aug 14;370. ↩ ↩2
-
2011 Census - Office for National Statistics [Internet]. [cited 2020 Sep 13]. Available from: https://www.ons.gov.uk/census/2011census ↩
-
Wu X, Nethery RC, Ma MBS, Braun D. Exposure to air pollution and COVID-19 mortality in the UnitedStates.20. ↩
-
Travaglio M, Yu Y, Popovic R, Selley L, Leal NS, Martins LM. Links between air pollution and COVID-19 in England. medRxiv. 2020 Jun 6;2020.04.16.20067405. ↩
-
Fecht D, Fischer P, Fortunato L, Hoek G, de Hoogh K, Marra M, et al. Associations between air pollution and socioeconomic characteristics, ethnicity and age profile of neighbourhoods in England and the Netherlands. Environ Pollut. 2015 Mar 1;198:201–10. ↩
-
Bray I, Gibson A, White J. Coronavirus disease 2019 mortality: a multivariate ecological analysis in relation to ethnicity, population density, obesity, deprivation and pollution. Public Health. 2020 Aug;185:261–3. ↩
-
Astell-Burt T, Maynard MJ, Lenguerrand E, Whitrow MJ, Molaodi OR, Harding S. Effect of air pollution and racism on ethnic differences in respiratory health among adolescents living in an urban environment. Health Place. 2013 Sep;23:171–8. ↩
-
Mark PJ, Gkatzionis A, Walker V, Grant A, Wootton RE, Moore LSP, et al. Cardiometabolic traits, sepsis and severe covid-19 with respiratory failure: a Mendelian randomization investigation. medRxiv. 2020 Jun 20;2020.06.18.20134676. ↩
-
Sattar N, Ho FK, Gill JMR, Ghouri N, Gray SR, Celis-Morales CA, et al. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes Metab Syndr. 2020;14(5):1149–51. ↩
-
Katikireddi SV, Cezard G, Bhopal RS, Williams L, Douglas A, Millard A, et al. Assessment of health care, hospital admissions, and mortality by ethnicity: population-based cohort study of health-system performance in Scotland. Lancet Public Health. 2018;3(5):e226–36. ↩
-
Dowd JB, Palermo T, Chyu L, Adam E, McDade TW. Race/ethnic and socioeconomic differences in stress and immune function in The National Longitudinal Study of Adolescent Health. Soc Sci Med 1982. 2014 Aug;115:49–55. ↩
-
Hussain M, Jabeen N, Raza F, Shabbir S, Baig AA, Amanullah A, et al. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J Med Virol. 2020;92(9):1580–6. ↩
-
Benetti E, Tita R, Spiga O, Ciolfi A, Birolo G, Bruselles A, et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur J Hum Genet. 2020 Jul 17;1–13. ↩
-
Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. medRxiv. 2020 Mar 27;2020.03.11.20031096. ↩ ↩2
-
Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020 Jun 17;0(0):null. ↩
-
Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020 Sep 1;4(9):669–77. ↩
-
Vepa A, Bae JP, Ahmed F, Pareek M, Khunti K. COVID-19 and ethnicity: A novel pathophysiological role for inflammation. Diabetes Metab Syndr. 2020;14(5):1043–51. ↩
-
Ovsyannikova IG, Haralambieva IH, Crooke SN, Poland GA, Kennedy RB. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol Rev. 2020;296(1):205–19. ↩
-
Netea MG, Wijmenga C, O’Neill LAJ. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol. 2012 May 18;13(6):535–42. ↩
-
Krieger N. Stormy Weather: Race, Gene Expression, and the Science of Health Disparities. Am J Public Health. 2005 Dec 1;95(12):2155–60. ↩
-
Krieger N. Epidemiology and the People’s Health: Theory and Context [Internet]. Epidemiology and the People’s Health. Oxford University Press. ↩
-
Yudell M, Roberts D, DeSalle R, Tishkoff S. Taking race out of human genetics. Science. 2016 Feb 5;351(6273):564–5. ↩
-
Association of 25 hydroxyvitamin D concentration with risk of COVID-19: a Mendelian randomization study, medRxiv. ↩
-
Lippi G, Lavie CJ, Henry BM, Sanchis-Gomar F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med. 2020 Aug 27;58(9):1415–22. ↩
-
VanderWeele T. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford, New York: Oxford University Press; 2015. 728 p. ↩
-
Naimi AI, Cole SR, Kennedy EH. An introduction to g methods. Int J Epidemiol. 2017 Apr 1;46(2):756–62. ↩
-
Jackson JW, VanderWeele TJ. Decomposition Analysis to Identify Intervention Targets for Reducing Disparities. Epidemiol Camb Mass. 2018;29(6):825–35. ↩
-
VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiol Camb Mass. 2014 Sep;25(5):749–61. ↩
