Ban lifted on use of UK plasma to manufacture life-saving albumin treatments
Leading scientists at the independent Commission on Human Medicines (CHM) have confirmed that albumin, a critically important medicine for the NHS, can now be safely derived from UK plasma donors.
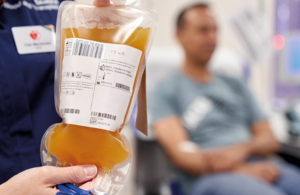
Human albumin (HA) treatments are made of plasma proteins from human blood. They are given to thousands of critically ill patients every year to replace blood loss from trauma such as severe burns or injuries, and those suffering from conditions such as liver disease or sepsis.
However, until recently, the use of UK-donated plasma to manufacture plasma-derived medicinal products has been banned as a safety precaution against the spread of Creutzfeldt Jakob Disease (vCJD). The NHS instead relied exclusively on imported plasma-derived products, primarily from the USA.
In 2020, the ban on using UK-sourced plasma to manufacture immunoglobulins, another type of plasma-derived medicinal product, was lifted.
The MHRA can now confirm that, following further review of the evidence, the CHM has also recommended lifting the ban on treating patients with UK albumin.
Patient case study: Lorna Stephenson, mum of five
Lorna Stephenson, aged 54, a married mum of five from Tyseley in southeast Birmingham, received albumin after myasthenia gravis, a rare, long-term condition that causes muscle weakness, left her in a coma.
Her own plasma - which contained antibodies that were attacking her own body - was removed. She received donor albumin to replace the vital albumin that she lost.
Lorna now receives an albumin exchange every 12 weeks at NHS Blood and Transplant’s specialist Therapeutic Apheresis Services unit in Birmingham.
She said: “Albumin plasma exchanges saved my life when I was in a coma.
“I still receive them and they ease my illness though I still can’t work as a beauty therapist again. I am not slurring my speech any more. I am able to do little things like comb my hair.
“I welcome the news that we can get albumin from UK donors. Without it, what would people like myself do?”
All organisations authorised to collect blood and blood components, and manufacturers of plasma-derived medicines, must continue to abide by the same robust safety standards and risk mitigation measures adopted for immunoglobulin manufacture such as the use of leucodepletion (filtering the white blood cells from donated blood to reduce the risk of adverse reactions), high-risk donor deferral and the ability to trace donations between donor and patient.
Medicines in the UK are reviewed and evaluated by expert teams at the MHRA and CHM before they can be approved for UK use. Any medical products made from UK-derived plasma will be evaluated to the same criteria as those made from non-UK plasma.
NHS Blood and Transplant collects plasma in two ways: through its three dedicated plasma donor centres in Birmingham, Reading and Twickenham, and by recovering plasma from regular whole blood donations. The plasma from both sources can now be used to make albumin for the NHS.
Dr Alison Cave, MHRA Chief Safety Officer, said:
We are committed to ensuring that all patients have access to safe, effective medical products and I am delighted that our work in this area continues to bear fruit.
I am so pleased that we have been able to support the lifting of this ban by examining the safety evidence and that there is now the potential to produce life-saving treatments from plasma that has been donated in the UK.
Patient case study: Olivia Price, critical care nurse
Critical care nurse Olivia Price, 32, of Birmingham city centre, received an albumin infusion during treatment for liver failure caused by the rare condition biliary atresia.
Fluid was leaking from her veins and building up in her abdomen – a condition known as ascites - putting her at risk of more complications.
More than four litres of fluid needed to be drained. Olivia received the albumin infusion to replace the albumin she had lost.
Olivia, who went on to receive a liver transplant in 2018, said: “Ascites is caused by pressure building up in your portal vein between the liver and spleen. The build-up of pressure causes fluid to leak out of the portal vein into the abdominal cavity.
“Albumin prevents rapid re-accumulation of fluid in the abdominal cavity. It also improves circulation and renal function by increasing blood volume.
“So, albumin helped me through the complications of liver failure – it enabled me to go on to be well enough to have a liver transplant.
“I am glad we can again use plasma from UK donors to make albumin. Plasma donors will be helping even more people with every gift.”
Neil O’Brien, Health Minister, said:
Albumin is a vital medicine for the NHS – used to treat conditions like liver disease or sepsis, and which helps thousands of critically ill patients suffering from blood loss every year.
This treatment has proven to be a lifesaver for people like Lorna Stephenson and Olivia Price, and now this ban is being lifted, it has the potential to help many more people receiving treatment on the NHS.
More plasma donation means we will be able to create more important medicines to care for people who have weakened immune systems, cancer and other diseases. If you’re able to, please come forward and donate.
Gerry Gogarty, Director of Plasma for Medicines at NHS Blood and Transplant, said:
This is another opportunity for the UK to reduce its reliance on imported plasma products, on which so many patients depend.
Human albumin is a protein which is used to treat ascites, peritonitis, hepatorenal syndrome, burns and liver disease and can be derived either from direct plasma donations or from plasma recovered from standard blood donation”.
Our donors won’t see any changes but every precious blood or plasma donation can now save even more lives.
We always need donors and you can register to donate blood at www.blood.co.uk/plasma.
David Webb, NHS England Chief Pharmaceutical Officer for England, said:
This crucial treatment helps thousands of critically ill patients every year and this robust assessment will enable the NHS to use UK-donated plasma again.
The use of locally sourced plasma treatments will help save more lives and the NHS will continue to work with partners to secure a supply chain of medicines that patients rely on.
Notes to editors
-
Since the introduction of leucodepletion (removal of white blood cells) in 1999 there have been no reports of transfusion-transmission of vCJD, and vCJD: https://onlinelibrary.wiley.com/doi/full/10.1111/vox.13416
-
To make use of the blood plasma, a process called fractionation must be undertaken to separate out the albumin protein and other parts in a way that enables a clinical application for patients. Currently, a commercial process is underway to establish the capability to fractionate UK plasma, that will lead to NHS patients benefitting from this additional source of treatments.
-
Filming and interviews:
The Birmingham Plasma Donor Centre will be open and available for filming on Monday 26 June, 8am to 3.30pm.
Olivia Price can be available to interview at her home address in Birmingham city centre on Monday.
Lorna Stephenson is also available at home in Birmingham on Monday.
To film at the centre or with the case studies, please contact NHS Blood and Transplant in advance on 01923 367 600 and pressoffice@nhsbt.nhs.uk. -
Pictures of both case studies are available and b-roll footage of plasma donation can be accessed here: https://we.tl/t-ONK4iAEBkB.
-
The CHM report will be published in due course. For any detailed queries, please contact the MHRA on 0203 080 7651 and newscentre@mhra.gov.uk.