Biplane cardiovascular X-ray system (Floor Mounted) - risk of interruption in treatment/procedure
(Philips) Motorised geometry control system will reboot if users attempt simultaneous powered and manual movement of the C-arm. (MDA/2013/045R)
Reissued – revised information on cause of possible interruption.
CAS deadlines
Action underway: 25 July 2013, action complete: 12 August 2013
Note: These deadlines are for systems to be in place to take actions.
Device

An image of a Biplane cardiovascular X-ray system.
Biplane cardiovascular X-ray system:
Allura Xper FD10/10 Floor Mounted.
Manufactured by Philips.
All systems are affected.
Problem
Risk of interruption in treatment/procedure due to an inability to move the table as the motorised geometry control system will reboot if users attempt simultaneous powered and manual movement of the C-arm.
The reboot will take approximately 90 seconds during which time the table top is free floating. Motorised movements (height, tilt, and cradle) are not possible. Basic imaging will be available.
We have received a report of this issue leading to delay during emergency treatment (introduction of a chest drain during cardiac arrest).
The problem may arise if a user is performing a manual swing movement of the C-arm at the same time as another user requests a “short” motorised movement via the tableside module.
The movement affected is the Beam Swing only (movement D in image below).
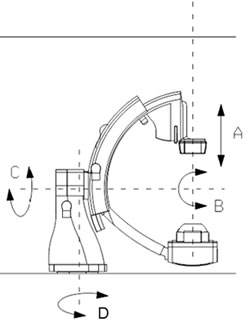
During the restart the table top is free floating, allowing it to be moved manually in a transverse as well as a longitudinal direction. Motorised movements (height, tilt, and cradle) are not possible during this restart period.
Action
- Identify affected devices.
- Inform users of the issue.
Action by
Superintendent diagnostic radiographers.
Distribution
This MDA has been sent to:
- Care Quality Commission (CQC) (headquarters) for information
- Clinical commissioning groups (CCGs)
- HSC trusts in Northern Ireland (chief executives)
- Local authorities in Scotland (equipment coordinators)
- NHS boards and trusts in Wales (chief executives)
- NHS boards in Scotland (equipment coordinators)
- NHS England area teams for information
- NHS trusts in England (chief executives)
Onward distribution
Please bring this notice to the attention of relevant employees in your establishment.
Below is a suggested list of recipients.
Trusts
CAS and SABS (NI) liaison officers for onward distribution to all relevant staff including:
- Cardiologists
- Cardiology departments
- Cardiology nurses
- Cardiology, directors of
- Cardiothoracic departments
- Cardiothoracic surgeons
- Cardiothoracic surgery directors
- Coronary care departments
- Coronary care nurses
- Day surgery units
- Health and safety managers
- Medical directors
- Medical physics departments
- Radiographer superintendents
- Radiographers
- Radiologists
- Radiology departments
- Radiology directors
- Risk managers
- Vascular surgeons
Independent distribution
Establishments registered with the Care Quality Commission (CQC) (England only)
This alert should be read by:
- Hospitals in the independent sector
- Independent treatment centres
Please note: CQC and OFSTED do not distribute these alerts. Independent healthcare providers and social care providers can sign up to receive MDAs directly from the Department of Health’s Central Alerting System (CAS) by sending an email to: safetyalerts@dh.gsi.gov.uk and requesting this facility.
Manufacturer contact
Manufacturer
Philips Customer Care Service Centre
Philips Healthcare
Philips Centre
Guildford Business Park
Guildford
GU2 8XH
Tel: 0870 532 9741
Email: ph.cvuk.support@philips.com
Feedback
If you have any comments or feedback on this Medical Device Alert please email us at: dts@mhra.gsi.gov.uk.
England
If you are in England, please send enquiries about this notice to the MHRA, quoting reference number MDA/2013/045R or 2013/001/024/401/008.
Technical aspects
David Grainger or Ann Seeruttun
Medicines and Healthcare Products Regulatory Agency
Floor 4, 151 Buckingham Palace Road
London SW1W 9SZ
Tel: 020 3080 7199 / 7161
Fax: 020 8754 3965
Email: david.grainger@mhra.gsi.gov.uk or ann.seeruttun@mhra.gsi.gov.uk
For clinical aspects please contact the MHRA clinical team on 020 3080 7248 or 020 3080 7032
How to report adverse incidents
Please report via our website: Reporting adverse incidents involving medical devices
Further information about CAS can be found on the CAS website
Northern Ireland
Alerts in Northern Ireland will continue to be distributed via the NI SABS system.
Enquiries and adverse incident reports in Northern Ireland should be addressed to:
Northern Ireland Adverse Incident Centre
Health Estates Investment Group
Room 17
Annex 6
Castle Buildings
Stormont Estate
Dundonald BT4 3SQ
Tel: 02890 523 704
Fax: 02890 523 900
Email: NIAIC@dhsspsni.gov.uk
How to report adverse incidents in Northern Ireland
Please report directly to NIAIC, further information can be found on the NIAIC website
Further information about SABS can be found on the SABS website
Scotland
Enquiries and adverse incident reports in Scotland should be addressed to:
Incident Reporting and Investigation Centre
Health Facilities Scotland
NHS National Services Scotland
Gyle Square
1 South Gyle Crescent
Edinburgh EH12 9EB
Tel: 0131 275 7575
Fax: 0131 314 0722
Email: nss.iric@nhs.net
Wales
Enquiries in Wales should be addressed to:
Improving Patient Safety Team
Medical Directorate
Welsh Government
Cathays Park
Cardiff CF10 3NQ
Tel: 029 2082 3922
Email: Haz-Aic@wales.gsi.gov.uk
Download documents